Abstract
Co-cultures of Bacillus subtilis and Kluyveromyces marxianus were investigated in submerged fermentation for xylanase production at shake-flask scale. Xylanase production markedly increased when arabinose, xylose, or hazelnut shells were used as the single carbon source. Maximal xylanase of 49.5 IU/mL was achieved with 4% B. subtilis, 4% K. marxianus, 40% solid load of hazelnut shells, and pH 7.0. Overall, xylanase was enhanced by 4.4-fold compared to initial un-optimized monoculture production and 2.8-fold compared to initial un-optimized co-cultured production, after optimization by response surface method.
Keywords: Xylanase, Optimization, Co-culture, Submerged fermentation, Bacillus subtilis, Kluyveromyces marxianus
Introduction
The global food and beverage enzyme market is estimated to reach $2.3 billion by 2018 with an annual growth rate (CAGR) of 8.0% from 2013 to 2018. Among all, carbohydrases account for almost 70% of the enzyme market with diverse applications in textile, paper and pulp, food and beverage industries (Butt et al. 2008), and are expected to reach 3.7 billion dollars by 2019 (Market Research Reports 2014). Carbohydrases are categorized based on their specificity towards natural glycoside substrates such as xylanases, cellulases, mannanases, and pectinases (Gusakov et al. 2011). Xylanase (endo-1,4-β-xylohydrolase) is a hydrolytic enzyme, which is involved in depolymerization of xylan (Rajoka 2007).
Xylan is the second abundant polysaccharide after cellulose occupying 20–40% of the plant biomass and is used for d-xylose production after isolation from birchwood or other hemicellulosic sources (Da Silva and Afschar 1994). Forestry, agricultural practices, breweries, paper and pulp, and food industries generate large amounts of xylan-rich biomass (Okafor et al. 2007).
Xylanases are widely used in vast industrial processes such as bio-bleaching of kraft pulp, digestibility of animal feed (Bhat 2000), juice extraction and clarification, and improving dough properties for baking (Bajpai 1999; Jia et al. 2011). To meet the high demands, xylanases were produced from bacteria (Azeri et al. 2010), mold (Cao et al. 2008; Chapla et al. 2010), actinomycetes (Thomas et al. 2013), and yeast (Xin and He 2013) by submerged (SmF) and solid state (SSF) fermentation methods. The SSF is advantageous over SmF due to higher production rate, lower effluent generation, and simpler fermentation equipment design (Renge 2012); however, the industrialized use of SSF still suffers from the complex product purification process, long fermentation period (e.g., 5–6 days), the heterogeneity of fermentation media, and enzyme loss in the solid residues (Gupta et al. 2008). Therefore, the SmF method is more extensively practiced for production of xylanase and other by products.
A number of studies is available in literature on monoculture xylanase production by Aspergillus species, Bacillus species or Kluyveromyces species. As per co-culturing studies, Gutierrez-Correa and Tengerdy (1998) reported 35–45% higher xylanase production than single cultures using fungal cultures of Trichoderma reesei LM-UC4E1 with either Aspergillus niger ATCC 10864 or A. phoenicis QM 329. Garcia-Kirchner et al. (2002) reported improved protein content, cellulase, and xylanase production using co-cultures of Penicillium sp. CH-TE-001 and (A) terreus CH-TE-013 compared to individual culture of each strain. Jeyakumar and Rajesh (2012) reported xylanase production with co-cultures of Bacillus polymyxa and Cellulomonas uda grown on sugarcane leaf extract under SmF. Unlike published studies, co-culture of a yeast and bacterium culture [i.e., K. marxianus and (B) subtilis] was considered in our study. Xylanase production with fungal cultures usually takes 5–6 days for attaining maximal level, whereas this period is shorter (2 days) with K. marxianus and B. subtilis (Rajoka 2007; Irfan et al. 2012). Thus, this can provide cost-effective option for xylanase production.
Another key role in economics of xylanase production belongs to carbon source; therefore, low cost alternatives have to be considered for large-scale processes. To decrease the cost of the xylan, lignocellulosic substrates like wheat bran, sugarcane bagasse, rice straw, corn cobs, etc., were used for production of xylanase (Milagres et al. 2004; Dobrev et al. 2007). Gupta et al. (2001) reported an improved xylanase production (fivefold) by Staphylococcus sp. SG-13 in a poplar wood medium over xylan alone as carbon source. Additionally, Ghanem (1992) reported that mixed culture of T. reesei and K. marxianus was more efficient for single cell protein production (51%) from beet pulp than a monoculture of T. reesei (49%). Therefore, in this study, hazelnut shells as an agricultural waste and a low cost lignocellulosic substrate for xylanase production was used for the first time. The world’s annual production of hazelnuts (Corylus avellana L.) nearly averages 1 million tons. Hazelnut fruits have a hard, smooth shell with about 27.5% hemicellulose by weight. Today, approximately 66% of hazelnut is produced only in the Black Sea region of Turkey and nearly 453,150 tons of hazelnut shells are generated (Pütün et al. 1999).
Therefore, the aim of the present work was to assess and optimize culture conditions of xylanase production by SmF method using co-cultures of Kluyveromyces marxianus and Bacillus subtilis. The conventional “change one-factor-at-a-time” method and response surface method (RSM) were employed for initial screening and optimization, respectively. The xylanase production by co-cultures of B. subtilis and K. marxianus was also briefly compared to monoculture counterpart using synthetic medium and then the effects of fermentation time, inoculum size, and type of carbon sources on xylanase production were studied to determine the major factors that remarkably affect the xylanase production via the co-culturing strategy and optimize the fermentation conditions.
Materials and methods
Hazelnut shells
Hazelnut shells were obtained from Ordu, a city in the Black Sea Region of Turkey. Hazelnut shells contained 24.20% cellulose, 28.20% hemicellulose, 34.64% lignin, 9.70% moisture, and 1.13% ash (Uzuner and Cekmecelioglu 2014). In this study, hazelnut shells were first ground into fine particles (1 mm) and then stored until use.
Dilute acid hydrolysis
Ground hazelnut shells at the pre-specified solid loadings were hydrolyzed using 100 mL of 0.6 M H2SO4 solution for 30 min at 130 °C in an autoclave (Uzuner and Cekmecelioglu 2014). After hydrolysis, the solid fraction of the slurry was separated by vacuum filtration and then pH of the supernatant was adjusted to the desired pH levels.
Microorganisms and inoculum preparation
Bacillus subtilis NRRL B-4219 and K. marxianus NRRL Y-8287 was obtained from ARS culture collection, Northern Regional Research Laboratory (NRRL), Peoria, IL, USA.
To prepare the inoculum, both microorganisms were grown in media containing (per 50 mL) 0.02 g of yeast extract, 0.002 g of MgSO4, 0.046 g of K2HPO4, 0.01 g of KH2PO4 and 0.5 g of d-xylose at 35 °C and 130 rpm for 24 h. The pre-specified volume of 24-h grown cultures (each maintained at 6 × 107 CFU/mL) was used for inoculation of the fermentation medium (Tables 1, 2) according to literature, in which the total inoculum was reported to be in the range of 2–5% (Gutierrez-Correa and Tengerdy 1998; Garcia-Kirchner et al. 2002; Ahamed and Vermette 2008; Nadia et al. 2010; Irfan et al. 2013).
Table 1.
Initial screening of factors for xylanase production
| Total Inoculum amount (mL) | Microorganism | Xylanase production (IU/mL)* | |
|---|---|---|---|
| B. subtilis | K.marxianus | ||
| 1.0 | – | 1.0 | 11.23 ± 0.02 |
| 1.0 | 1.0 | – | 12.11 ± 0.05 |
| 1.0 | 0.5 | 0.5 | 17.93 ± 0.04 |
| 2.0 | – | 2.0 | 13.69 ± 0.5 |
| 2.0 | 2.0 | – | 15.63 ± 0.1 |
| 2.0 | 1.0 | 1.0 | 19.65 ± 0.4 |
*Reported in replicates
Table 2.
Coded and uncoded independent variables used in response surface optimization
| Variables | Coded levels | ||
|---|---|---|---|
| − 1 | 0 | + 1 | |
| Uncoded levels | |||
| Inoculum amounts of B. subtilis (mL/100 mL) | 1.0 | 2.5 | 4.0 |
| Inoculum amounts of K. marxianus (mL/100 mL) | 1.0 | 2.5 | 4.0 |
| pH | 4.0 | 5.5 | 7.0 |
| Hazelnut shell concentration (%w/v) | 10.0 | 25.0 | 40.0 |
Xylanase production
Xylanase enzyme was produced by inoculating the fermentation medium (50 mL per 250 mL flasks) with 24-h grown cultures of B. subtilis and K. marxianus and incubating at 35 °C and 130 rpm for 24 h by submerged fermentation method. Initial pH of media was adjusted to 6.5 for all trials. The fermentation media consisted of (g/50 mL), yeast extract 0.01, MgSO4 0.001, K2HPO4 0.023, KH2PO4 0.005 and predefined amounts of carbon source. After fermentation, liquid medium was centrifuged at 2000×g at 15 °C for 15 min to obtain cell-free broth, which contains the crude enzyme.
Xylanase activity
Xylanase activity was determined using 1% birchwood xylan solution in 100 mL acetate buffer with a pH of 5.0 as the substrate in xylanase assay. A half milliliter of diluted enzyme sample was mixed with 0.5 mL of the substrate solution and incubated at 50 °C and 130 rpm for 30 min. The liberated reducing sugars were measured at 550 nm with a spectrophotometer according to the DNS method (Miller 1959). One unit of the xylanase is defined as the amount of enzyme that releases 1 µmole of xylose equivalents per minute under assay conditions.
Initial screening for selection of important factors
To distinguish the effect of each culture, culture volume and co-culturing on xylanase production and to determine the range of design points for optimization, some initial trials were carried out at 35 °C, pH 6.5 and 130 rpm for 24 h (Table 1).
The effect of sole carbon source (xylose, glucose, arabinose, sucrose, xylan, ground hazelnut shell and hydrolyzed hazelnut shell) on xylanase production was also tested.
Response surface optimization
The individual and interaction effects of variables (inoculum size, pH and hazelnut shell concentration) were evaluated by Box–Behnken response surface methodology (RSM). The level of each factor is shown in Table 2 in coded and uncoded forms.
A second order polynomial regression model was developed as a predictive model for xylanase production as follows:
| 1 |
where Y is the predicted response (or xylanase activity), β0, βi, βii, and βij are constants coefficients of the model, Xi, Xj and i = 1 to 4; j = 1 to 4, i ≠ j represents the independent variables in coded form.
To define the significant terms and the coefficients of predictive model, analysis of variance (ANOVA) and regression analysis were performed by the statistical software MINITAB® 16.1 (Minitab Inc. State College, PA, USA). The optimum conditions for maximizing the enzyme production were determined by response optimizer in MINITAB® 16.1.
Analysis of variance (ANOVA) was used at α = 0.05 to identify any differences in sample measurements and the fitting of experimental data to the regression model was evaluated by root mean square error (RMSE) and mean absolute error (MAE) values as illustrated in the following equations;
| 2 |
| 3 |
where Pi and Oi are predicted and experimental xylanase activity values, respectively, and N represents the number of data points.
Results and discussion
Initial screening
Xylanase production by single and co-cultures of B. subtilis and K. marxianus was first evaluated using inoculum levels in the range of 1.0–2.0 mL at 35 °C, pH 6.5 and agitation of 130 rpm (Table 1). When fermentation medium was inoculated with 1.0 mL of either K. marxianus or B. subtilis, xylanase activities of 11.23 ± 0.02 and 12.11 ± 0.05 IU/mL, respectively, were achieved after 24 h of fermentation (Table 1). Higher xylanase activity of 17.93 ± 0.04 IU/mL was produced when co-cultures of B. subtilis (0.5 mL) and K. marxianus (0.5 mL) were used. The highest xylanase (19.65 ± 0.4 IU/mL) was achieved at 2% inoculum, which contains 1 mL of K. marxianus and 1.0 mL of B. subtilis. The same inoculum size (2%) but in single culture form resulted in xylanase activities of 13.69 ± 0.5 and 15.63 ± 0.1 IU/mL, respectively, for K. marxianus and B. subtilis. The results in Table 1 also indicated that B. subtilis was a better xylanase producer under the same conditions. The study of Garcia-Kirchner et al. (2002), who also reported that single cultures of Penicillium spp. CH-TE-001 and Aspergillus terreus CH-TE-013 produced lower xylanase activities (1.45, 3.96 IU/mL) than co-culture of both microorganisms (5.02 IU/mL), supports the results of our study.
Effect of fermentation time
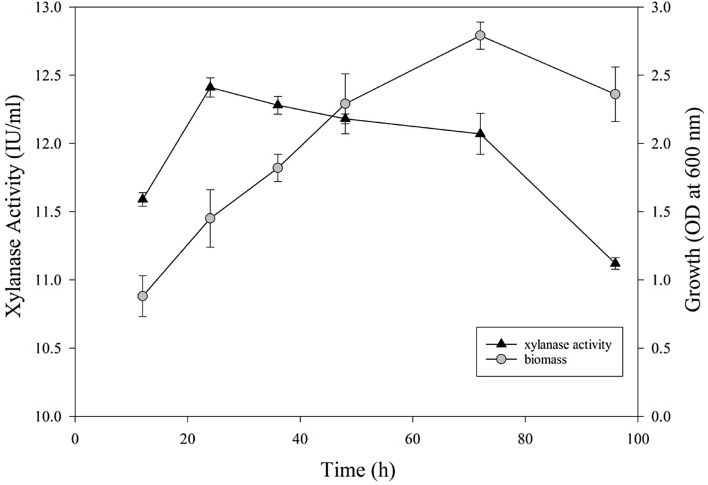
Xylanase production was measured after 12, 24, 36, 48, 72 and 96 h under constant substrate concentration (xylose, 2.0 g/50 mL), amount of inoculum, temperature, agitation, and pH (Fig. 1). The results indicated that the highest enzyme production (12.41 ± 0. 1 IU/mL) was achieved after 24 h at 35 °C, pH 6.5 and with agitation of 130 rpm. Afterwards, a decrease was observed in xylanase production. The biomass and xylanase productions were directly proportional up to 24 h, thereafter enzyme production ceased and biomass increased up to 72 h, and then decreased slowly (Fig. 1). This shows that xylanase production is growth associated in early stages only. Reduction in enzyme production at later stages when biomass growth is maximal might be due to the production of inhibitory metabolites that inhibit the enzyme production (Niladevi and Prema 2008). Additionally, the maximal xylanase (12.41 IU/mL) in co-culture is lower than the sum of single cultures due to these inhibitory metabolites. Bocchini et al. (2002) also reported the highest xylanase activity after 24 h of incubation (7.10 IU/mL) with B. circulans D1. In contrast, Ling Ho and Heng (2015) reported the highest xylanase activity of 11.968 ± 1.419 U/mL with B. subtilis in SmF using wheat bran and soybean hull after 48 h. Thus, microbial strain and carbon sources can affect the timing of maximal xylanase enzyme production.
Fig. 1.
Time-course of xylanase production vs. growth by co-cultures of B. subtilis and K. marxianus at pH 6.5, 35 °C and shaking at 130 rpm
Effect of sugar type
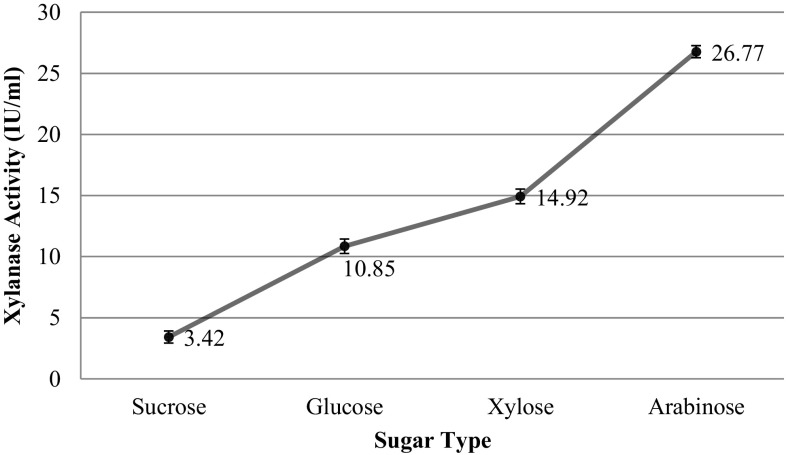
To study the effect of sugar type on xylanase production using co-cultures, different types of sugar were used in the medium at 35 °C, pH 6.5 and 130 rpm for 24 h. The results of these trials are plotted in Fig. 2. The highest xylanase activity was obtained with arabinose (26.77 ± 0.5 IU/mL) and xylose (14.92 ± 0.6 IU/mL) rich media, respectively. Rifaat et al. (2006) used Streptomyces chromofuscus to produce xylanase using different sugars, and reported the highest results with xylose (12.31 IU/mL) and glucose (10.26 IU/mL).
Fig. 2.
Xylanase production using different sugars at 35 °C, pH 6.5, 24 h, and 130 rpm
Effect of raw material type
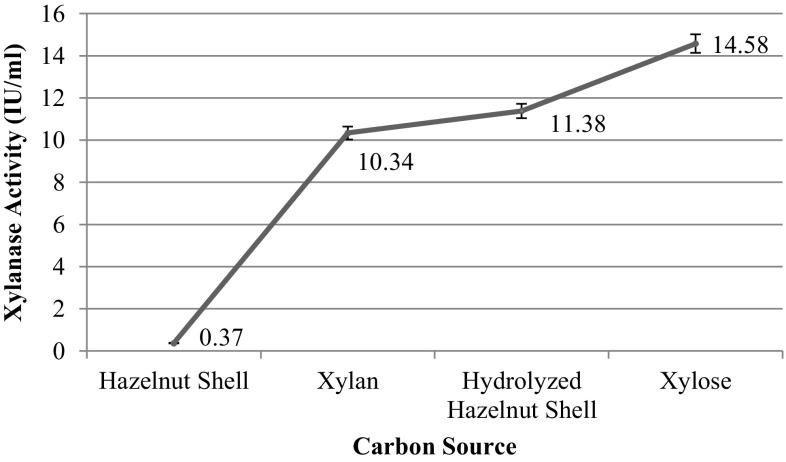
The xylanase production by co-cultures of B. subtilis and K. marxianus grown on different carbon sources is shown in Fig. 3. The highest xylanase production was obtained with xylose (14.58 ± 0.06 IU/mL), and followed by hydrolyzed hazelnut shell (11.38 ± 0.01 IU/mL). It was clear from Fig. 3 that hydrolysis of hazelnut shell is required, as xylanase production increased remarkably from 0.37 to 11.38 IU/mL when hazelnut hydrolysate was used as carbon source rather than ground hazelnut shells. The positive effect of hydrolysis was also shown by Rifaat et al. (2006), who produced xylanase using pretreated and untreated pulp. The xylanase activity resulted from hydrolyzed hazelnut shells (11.38 ± 0.01 IU/mL) is also competitive and showed that hazelnut shells are promising raw material for cost-effective xylanase production.
Fig. 3.
Effect of carbon source forms on xylanase production at 35 °C, pH 6.5 and 130 rpm after 24-h fermentation
Box–Behnken optimization
Preliminary experiments showed that the key factors affecting the xylanase production are inoculum size (mL/100 mL), solid load (% v/w) and pH. Also the upper level and lower levels of each variable were chosen according to the preliminary experiments. The Box–Behnken design was used in this experiment to identify the effects of individual factors and the interactions between factors. The experimental design by Box–Behnken response surface method (RSM) and experimental and predicted xylanase activity values are shown in Table 3. The highest xylanase activity of 42.65 ± 0.65 IU/mL was observed at 2.5% B. subtilis (B), 2.5% K. marxianus (K), 40% solid load, and pH 7.0, while the lowest value (3.65 ± 0.43 IU/mL) belonged to 2.5% B. subtilis, 2.5% K. marxianus, 10% solid load, and pH 4.0.
Table 3.
Box–Behnken experimental design and experimental vs predicted xylanase activity values for various combination of fermentation conditions
| Run no. | Ba,d (mL/100 mL) | Kb,d (mL/100 mL) | Solid (%w/v) | pH | Activity (IU/mL) | |
|---|---|---|---|---|---|---|
| Experimentalc | Predicted | |||||
| 1 | 2.50 | 4.00 | 25.0 | 7.0 | 17.01 ± 0.86 | 18.93 |
| 2 | 4.00 | 2.50 | 10.0 | 5.5 | 7.96 ± 0.43 | 8.84 |
| 3 | 1.00 | 1.00 | 25.0 | 5.5 | 21.75 ± 0.86 | 20.12 |
| 4 | 2.50 | 2.50 | 25.0 | 5.5 | 17.44 ± 0.86 | 17.83 |
| 5 | 4.00 | 2.50 | 25.0 | 7.0 | 24.12 ± 0.65 | 22.89 |
| 6 | 2.50 | 2.50 | 10.0 | 7.0 | 5.52 ± 0.28 | 2.84 |
| 7 | 2.50 | 2.50 | 10.0 | 4.0 | 3.65 ± 0.43 | 0.44 |
| 8 | 2.50 | 1.00 | 10.0 | 5.5 | 4.51 ± 0.43 | 7.52 |
| 9 | 2.50 | 1.00 | 25.0 | 4.0 | 9.25 ± 0.86 | 8.31 |
| 10 | 2.50 | 2.50 | 40.0 | 4.0 | 20.46 ± 0.86 | 21.72 |
| 11 | 4.00 | 2.50 | 40.0 | 5.5 | 40.49 ± 0.65 | 40.38 |
| 12 | 2.50 | 2.50 | 25.0 | 5.5 | 18.52 ± 0.65 | 17.83 |
| 13 | 1.00 | 4.00 | 25.0 | 5.5 | 17.01 ± 0.43 | 14.09 |
| 14 | 2.50 | 1.00 | 25.0 | 7.0 | 22.18 ± 0.43 | 20.87 |
| 15 | 1.00 | 2.50 | 25.0 | 7.0 | 13.56 ± 0.43 | 15.24 |
| 16 | 4.00 | 1.00 | 25.0 | 5.5 | 18.30 ± 0.43 | 19.80 |
| 17 | 2.50 | 2.50 | 40.0 | 7.0 | 42.65 ± 0.65 | 44.43 |
| 18 | 2.50 | 4.00 | 40.0 | 5.5 | 39.42 ± 0.43 | 37.01 |
| 19 | 2.50 | 2.50 | 25.0 | 5.5 | 17.44 ± 0.86 | 17.83 |
| 20 | 2.50 | 1.00 | 40.0 | 5.5 | 42.00 ± 0.86 | 41.54 |
| 21 | 1.00 | 2.50 | 25.0 | 4.0 | 4.73 ± 0.17 | 6.56 |
| 22 | 4.00 | 2.50 | 25.0 | 4.0 | 7.53 ± 0.43 | 6.45 |
| 23 | 2.50 | 4.00 | 10.0 | 5.5 | 7.10 ± 0.43 | 8.17 |
| 24 | 2.50 | 4.00 | 25.0 | 4.0 | 4.08 ± 0.43 | 6.37 |
| 25 | 1.00 | 2.50 | 40.0 | 5.5 | 36.40 ± 0.43 | 36.50 |
| 26 | 1.00 | 2.50 | 10.0 | 5.5 | 4.08 ± 0.43 | 5.18 |
| 27 | 4.00 | 4.00 | 25.0 | 5.5 | 21.75 ± 0.86 | 21.95 |
a B. subtilis
b K. marxianus
cResults are averages of three replicates
dGrowth medium contains 6 × 107 cfu/mL
The quadratic predictive model was then developed by excluding the insignificant terms as follows:
| 5 |
where Y is the response (xylanase activity), XB, XK represent inoculum size of B. subtilis and K. marxianus, respectively and XS is solid load and XpH indicates the pH levels.
The revised ANOVA results (Table 4) indicated that the quadratic model was found statistically significant (p < 0.05) at the 95% confidence level. Linear (p = 0.000), quadratic (p = 0.000), and interaction effects (p = 0.000) were highly significant. The determination coefficient (R2) of the model was calculated to be 0.95, indicating that the model could explain 95% of the variability in the response. The insignificant lack of fit (p = 0.099 > 0.05) also verified that the model fitted well to the experimental data. All the factors (inoculum size, pH and hazelnut shell concentration) showed significant effects (p < 0.05). Among all, pH was the most dominant factor on xylanase production having the largest positive coefficient (23.2478). Interactions between solid–solid, pH–pH, B-K, B-pH, and solid-pH showed significant effects (p < 0.05); while interactions between B-B, K-K, B-solid, K-solid, and K-pH were insignificant (P > 0.05). The constructed model was also evaluated with error analysis. Root mean square error (RMSE) and mean absolute error (MAE) values were calculated as 1.77 and 0.004, respectively. Low values of RMSE and MAE also showed that the model fitted well.
Table 4.
Revised ANOVA results and estimated regression coefficients for xylanase production without insignificant terms
| Term | Coefficient | p |
|---|---|---|
| Regression | 0.000 | |
| Linear | 0.000 | |
| Square | 0.000 | |
| Interaction | 0.000 | |
| Lack of fit | 0.099 | |
| Constant | − 45.1128 | 0.000 |
| B (XB) | − 5.75749 | 0.000 |
| K (XK) | − 2.920 | 0.031 |
| Solid (XS) | − 1.18142 | 0.000 |
| pH (XpH) | 23.2478 | 0.000 |
| Solid*solid | 0.0197502 | 0.000 |
| pH*pH | − 2.44184 | 0.000 |
| B*K | 0.909707 | 0.009 |
| B*pH | 0.861828 | 0.014 |
| solid*pH | 0.225751 | 0.000 |
*Result is significant when p < 0.05
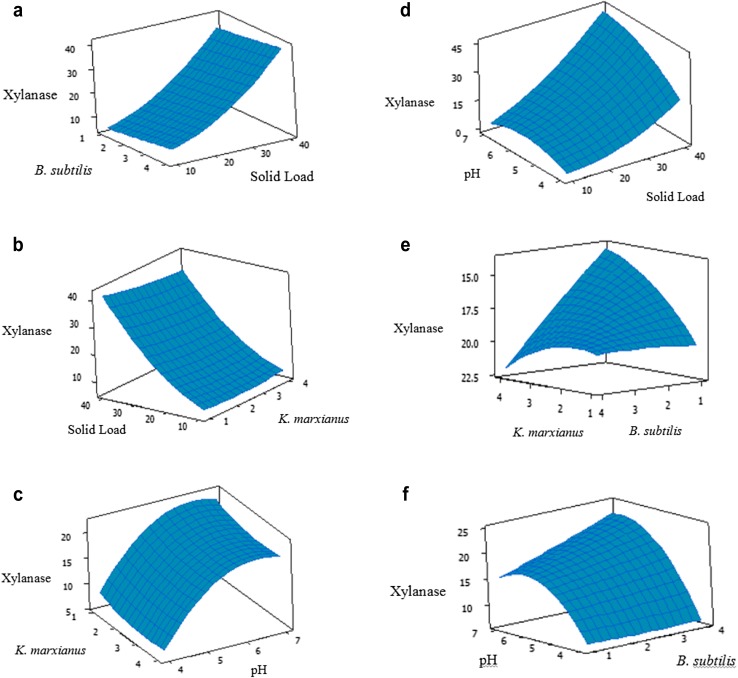
The response surface plots for the effects of inoculum size, hazelnut shell concentration and pH, and their interactions are presented in Fig. 4a–f. In Fig. 4a, b, it can be seen that xylanase activity increases slightly when inoculum size of either B. subtilis or K. marxianus increases, where the slope of B. subtilis is larger. However, xylanase activity increases sharply up to 40 IU/mL with increasing solid concentration from 10 to 40% (w/v). The increase in pH from 4 to 7 caused nonlinear increase in xylanase production from 8 to 20 IU/mL, respectively, where solid load was kept at mid-value (Fig. 4c). In Fig. 4d, the xylanase activity shows high dependency on varying pH and solid concentration.
Fig. 4.
Surface plots displaying the effect of a B. subtilis (%v/v) and solid load (%w/v), b solid load and K. marxianus, c K. marxianus and pH, d pH and solid load, e K. marxianus and B. subtilis, and f pH and B. subtilis on xylanase production (IU/mL) (the third variable is kept constant at mid-value)
In fermentation medium, pH affects various enzymatic reactions by affecting the transportation of different chemical products and enzymes through the cell membrane. Too low and high pH levels can also inhibit the xylanase activity. Providing the optimal solid concentration is important, as low concentration can cause lack of nutrients for microorganisms to live, whereas the high concentrations may yield overgrowth and cell death. Thus, our results also confirmed these facts, and the xylanase activity increased nonlinearly with increasing solid concentration and high xylanase production was observed around mid-values of pH (5–6) and decreased at higher and lower ends. Similar xylanase activity (41.6 IU/mL) at pH 7 with 10 g/100 mL of solid load was reported by Nadia et al. (2010) with Streptomyces lividans.
The effect of varied inoculum size of K. marxianus and B. subtilis on xylanase activity at mid-values of pH and solid load is shown in Fig. 4e. Xylanase activity increases with increasing inoculum size of K. marxianus up to 2% and decreases thereafter. The impact of varying inoculum size for B. subtilis is less certain. The pH of medium has a stronger effect on xylanase production compared to microbial load as observed again in Fig. 4f plotted at 25% solid load and 2.5% K. marxianus. However, microbial load is still important for fermentation process. At low microbial load levels, it can take too long to achieve maximum xylanase production due to insufficient use of fermentation medium or insufficient biomass may just result in low xylanase enzyme. The high microbial load levels may cause high biomass with low substrate remaining for xylanase production.
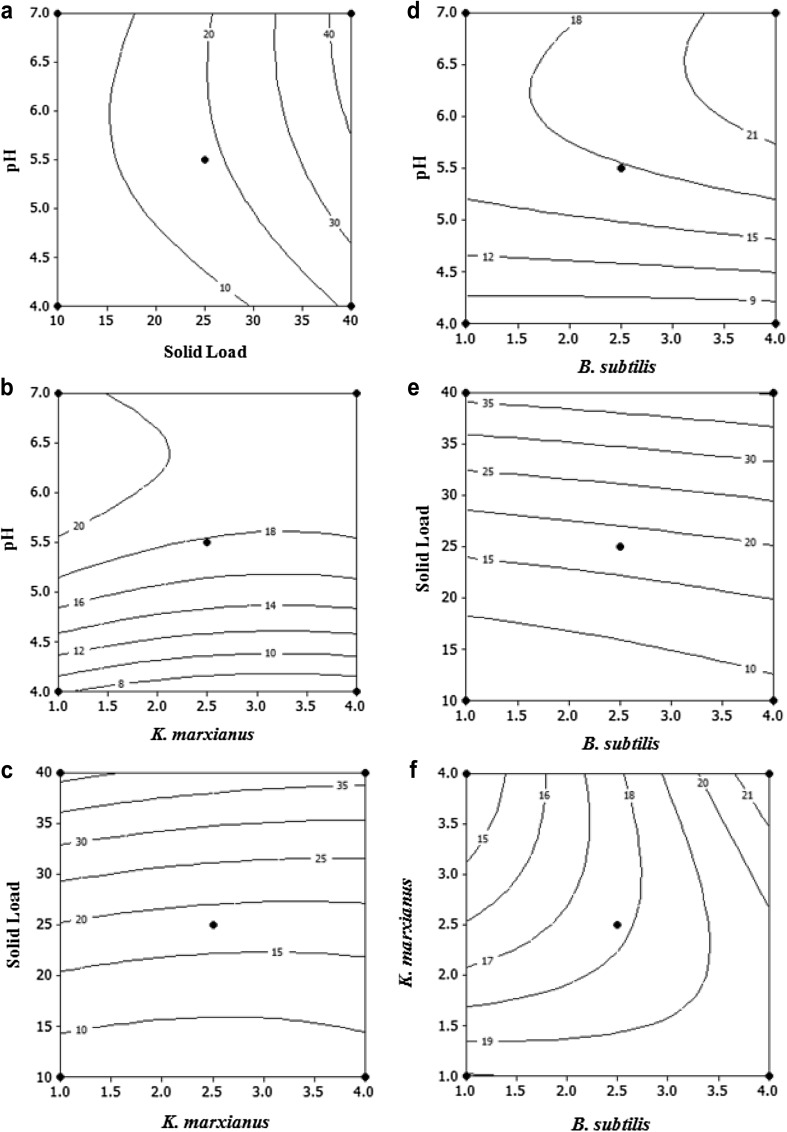
The effect of process variables can also be seen clearly from the contour plots in Fig. 5a–f. The highest enzyme activity of 40 IU/mL was observed with the combination of solid concentration (40.0%) and pH (7.0). The effect of B. subtilis, pH, and solid concentration on enzyme activity is also visible in the contour plots. At pH 7, xylanase activity abruptly increased from 10 to 40 IU/mL with increasing solid concentration from 12 (% w/v) to 39 (% w/v), but at pH 4, an increase in the solid concentration from 29 to 39% increased the xylanase activity smoothly from 10 to 20 IU/mL.
Fig. 5.
Contour plots displaying the effect of a pH and solid load, b pH and K. marxianus, c solid load and K. marxianus, d pH and B. subtilis, e solid load and B. subtilis and f K. marxianus and B. subtilis on xylanase activity (IU/mL) (the third variable is kept constant at mid value)
Optimum conditions and model verification
Optimal conditions for xylanase production were found as 4% B. subtilis, 4% K. marxianus, and 40% (w/v) solid load, and 7 pH, giving a maximal xylanase activity of 49.5 IU/mL as predicted by second order predictive model. For verification of the model, additional runs were carried out in triplicates. The experimental result (43.4 IU/mL) was found close to the predicted result (49.5 IU/mL) with low standard deviation (0.05) indicating high reproducibility. In the light of overall results, it can be said that the model is adequate at predicting xylanase production at a definite condition according to the R2 (0.95), lack of fit (p = 0.099 > 0.05), RMSE (1.77) and MAE (0.004). The results also implied that xylanase production has been increased by 340% (or 4.4-fold) compared to initial un-optimized single culture conditions and a 2.8-fold increase was achieved compared to initial un-optimized co-cultured xylanase production by RSM.
Evaluation of xylanase production before and after optimization
Xylanase can be produced by submerged or solid state fermentation by fungi and bacteria. Different carbon sources can be utilized by microorganisms, but it is more cost-effective to use agricultural residues instead of pure carbon sources. There are a number of reports (Table 5) describing co-culturing of two microorganisms including molds and bacteria for improved xylanase production; however, to the best of our knowledge, no study has been reported about co-culturing of B. subtilis and K. marxianus. Table 5 presents a summary of recent studies reporting xylanase production with single and mixed culture of mold and bacteria for comparison. Jeyakumar and Rajesh (2012) reported xylanase activity of 44.773 IU/mL with co-culture of B. polymyxa and C. uda at 50 °C, pH 8 after 48-h fermentation using sugarcane leaf extract as carbon source. Deshpande et al. (2008) produced xylanase (33.6 IU/mL) by co-cultures of T. reesei and A. niger using cellulose rich medium at 29 °C, pH 4.8 after 6 days of fermentation. In the light of these results, xylanase production with co-culture of B. subtilis and K. marxianus is remarkably advantageous with high activity and productivity values achieved in comparison to other studies (Jeyakumar and Rajesh 2012; Bocchini et al. 2002; Rifaat et al. 2006; Panda et al. 1987).
Table 5.
Xylanase production with single and mixed culture of microorganisms
| Organism | Substrate | Cultivation conditions | Xylanase activity | Reference |
|---|---|---|---|---|
| T. reesei D-1-6/Awenti Pt 2804 | Cellulose | Mixed culture pH 4.8, 29 °C 144 h |
33.6 IU/mL | Panda et al. (1987) |
| Penicillium sp. and A. terreus |
Sugarcane
Bagasse |
Mixed, 29 °C 180 rpm, 2 days | 5.02 IU/mL | Garcia-Kirchner et al. (2002) |
|
T. reesei
A. niger |
Water hyacinth | Mixed, SSF 30 °C, 85% RH, 10 days | 57.2 IU/g | Deshpande et al. (2008) |
| Streptomyces albus | Rice straw pulp | 28 °C 200 rpm, 5 days | 32.53 IU/mL | Rifaat et al. (2006) |
| S. chromofuscus | Rice straw pulp | 28 °C 200 rpm, 5 days | 43.01 IU/mL | Rifaat et al. (2006) |
| Bacillus subtilis | Wheat bran | 37 °C, 140 rpm, 24 h | 36.8 IU/mL | Irfan et al. (2013) |
|
Bacillus polymyxa
Cellulomonas uda |
Sugarcone leaf extract + xylan (0.2%) | pH:8, 50 °C, 2 days mixed culture | 44.77 IU/mL | Jeyakumar and Rajesh (2012) |
|
Bacillus subtilis
Kluyveromyces marxianus |
Hazelnut shell | pH:7, 35 °C, 24 h, mixed culture | 49.5 IU/mL | This study |
Conclusion
In summary, xylanase production by single and co-cultures of B. subtilis and K. marxianus was evaluated and co-cultured xylanase production was optimized by the Box–Behnken response surface method in this study. Xylanase production was enhanced by 4.4-fold (or 340%) after optimization compared to initial un-optimized single culture production. A 2.8-fold increase was also achieved after optimization by RSM compared to initial un-optimized co-cultured xylanase production. In conclusion, co-culturing of B. subtilis and K. marxianus is shown to enhance xylanase production.
Acknowledgements
This work was funded by the Scientific Council of Research at METU, (Project no: BAP-03-14-2011-001). The authors also acknowledge the Department of Food Engineering for providing the experimentation facilities.
Compliance with ethical standards
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to 3 Biotech and declare that they have no conflict of interest in the publication.
References
- Ahamed A, Vermette P. Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochem Eng J. 2008;42:41–46. doi: 10.1016/j.bej.2008.05.007. [DOI] [Google Scholar]
- Azeri C, Tamer U, Oskay M. Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr J Biotechnol. 2010;9:63–72. [Google Scholar]
- Bajpai P. Application of enzymes in the pulp and paper industry. Biotechnol Prog. 1999;15:147–157. doi: 10.1021/bp990013k. [DOI] [PubMed] [Google Scholar]
- Bhat M. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/S0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Bocchini D, Alves-Prado H, Baida L, et al. Optimization of xylanase production by Bacillus circulans D1 in submerged fermentation using response surface methodology. Process Biochem. 2002;38:727–731. doi: 10.1016/S0032-9592(02)00207-8. [DOI] [Google Scholar]
- Butt M, Tahir-Nadeem M, Ahmad Z, Sultan M. Xylanases and their applications in baking industry. Food Technol Biotechnol. 2008;46:22–31. [Google Scholar]
- Cao Y, Meng D, Lu J, Long J. Statistical optimization of xylanase production by Aspergillus niger AN-13 under submerged fermentation using response surface methodology. Afr J Biotechnol. 2008;7:631–638. [Google Scholar]
- Chapla D, Divecha J, Madamwar D, Shah A. Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochem Eng J. 2010;49:361–369. doi: 10.1016/j.bej.2010.01.012. [DOI] [Google Scholar]
- Da Silva S, Afschar A. Microbial production of xylitol from d-xylose using Candida tropicalis. Bioprocess Eng. 1994;11:129–134. doi: 10.1007/BF00518734. [DOI] [Google Scholar]
- Deshpande S, Bhotmange M, Chakrabarti T, Shastri P. and xylanase by Trichoderma reesei (QM 9414 mutant), Aspergillus niger and mixed culture by solid state fermentation (SSF) of water hyacinth (Eichhornia crassipes) Indian J Chem Technol. 2008;15:449–456. [Google Scholar]
- Dobrev G, Pishtiyski I, Stanchev V, Mircheva R. Optimization of nutrient medium containing agricultural wastes for xylanase production by Aspergillus niger B03 using optimal composite experimental design. Bioresour Technol. 2007;98:2671–2678. doi: 10.1016/j.biortech.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Garcia-Kirchner O, Muñoz-Aguilar M, Pérez-Villalva R, Huitrón-Vargas C. Mixed submerged fermentation with two filamentous fungi for cellulolytic and xylanolytic enzyme production. Appl Biochem Biotechnol. 2002;98–100:1105–1114. doi: 10.1385/ABAB:98-100:1-9:1105. [DOI] [PubMed] [Google Scholar]
- Ghanem KM. Single cell protein production from beet pulp by mixed culture. Qatar Univ Sci J. 1992;12:85–88. [PubMed] [Google Scholar]
- Gupta S, Kuhad R, Bhushan B, Hoondal G. Improved xylanase production from a haloalkalophilic Staphylococcus sp. SG-13 using inexpensive agricultural residues. World J Microbiol Biotechnol. 2001;17:5–8. doi: 10.1023/A:1016691205518. [DOI] [Google Scholar]
- Gupta S, Kapoor M, Sharma KK, et al. Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour Technol. 2008;99:937–945. doi: 10.1016/j.biortech.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Gusakov A, Kondratyeva E, Sinitsyn A. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem. 2011 doi: 10.1155/2011/283658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Correa M, Tengerdy R. Xylanase production by fungal mixed culture solid substrate fermentation on sugar cane bagasse. Biotechnol Lett. 1998;20:45–47. doi: 10.1023/A:1005379030092. [DOI] [Google Scholar]
- Irfan M, Nadeem M, Syed Q, Baig S. Effect of medium composition on xylanase production by Bacillus subtilis using various agricultural wastes. Am Eurasian J Agric Environ Sci. 2012;12(5):561–565. [Google Scholar]
- Irfan M, Nadeem M, Syed Q. Purification and kinetics study of thermostable cellulase free xylanase from Bacillus subtilis. Protein Pept Lett. 2013;20:1225–1231. doi: 10.2174/09298665113209990007. [DOI] [PubMed] [Google Scholar]
- Jeyakumar RM, Rajesh L. Effect of various physical parameters for the production of the enzyme xylanase from mixed culture of Bacillus polymyxa and Cellulomonas uda. Asian J Biomed Pharm Sci. 2012;2:72–74. [Google Scholar]
- Jia C, Huang W, Abdel-Samie M. Dough rheological, mixolab mixing, and nutritional characteristics of almond cookies with and without xylanase. J Food Eng. 2011;105:227–232. doi: 10.1016/j.jfoodeng.2011.02.023. [DOI] [Google Scholar]
- Ling Ho H, Heng KL. Xylanase production by Bacillus subtilis in cost-effective medium using soybean hull as part of medium composition under submerged fermentation (SmF) and solid state fermentation (SsF) J Biodivers Biopros Dev. 2015;2:143. [Google Scholar]
- Markets and Markets h (2014) Market research reports. Marketsandmarkets.com. Accessed 11 Dec 2014
- Milagres A, Santos E, Piovan T, Roberto I. Production of xylanase by Thermoascus aurantiacus from sugar cane bagasse in an aerated growth fermentor. Process Biochem. 2004;39:1387–1391. doi: 10.1016/S0032-9592(03)00272-3. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nadia H, Amal M, Abeer A. Xylanase production by Streptomyces lividans (NRC) and it’s application on waste paper. Aust J Basic. 2010;4:1358–1368. [Google Scholar]
- Niladevi KN, Prema P. Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour Technol. 2008;99(11):4583–4589. doi: 10.1016/j.biortech.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Okafor U, Okochi V, Onyegeme-okerenta B, Nwodo-Chinedu S. Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biochem Res. 2007;6:1710–1714. [Google Scholar]
- Panda T, Bisaria V, Ghose T. Effect of culture phasing and a polysaccharide on production of xylanase by mixed culture of Trichoderma reesei D16 and Aspergillus wentii Pt 2804. Biotechnol Bioeng. 1987;30:868–874. doi: 10.1002/bit.260300709. [DOI] [PubMed] [Google Scholar]
- Pütün A, Özcan A, Pütün E. Pyrolysis of hazelnut shells in a fixed-bed tubular reactor: yields and structural analysis of bio-oil. J Anal Appl Pyrolysis. 1999;52:33–49. doi: 10.1016/S0165-2370(99)00044-3. [DOI] [Google Scholar]
- Rajoka M. Kinetic parameters and thermodynamic values of β-xylosidase production by Kluyveromyces marxianus. Bioresour Technol. 2007;98:2212–2219. doi: 10.1016/j.biortech.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Renge V. Enzyme synthesis by fermentation method: a review. Sci Rev Chem Commun. 2012;2:585–590. [Google Scholar]
- Rifaat H, Nagieb Z, Ahmed Y. Production of xylanases by Streptomyces species and their bleaching effect on rice straw pulp. Appl Ecol Environ Res. 2006;4:151–160. doi: 10.15666/aeer/0401_151160. [DOI] [Google Scholar]
- Thomas L, Joseph A, Arumugam M, Pandey A. Production, purification, characterization and over-expression of xylanases from actinomycetes. Indian J Exp Biol. 2013;51:875–884. [PubMed] [Google Scholar]
- Uzuner S, Cekmecelioglu D. Hydrolysis of hazelnut shells as a carbon source for bioprocessing applications and fermentation. Int J food Eng. 2014;10:799–808. doi: 10.1515/ijfe-2014-0158. [DOI] [Google Scholar]
- Xin F, He J. Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Bioresour Technol. 2013;135:309–315. doi: 10.1016/j.biortech.2012.10.002. [DOI] [PubMed] [Google Scholar]