Abstract
Purpose
The Middle East and North Africa (MENA) region registers some of the lowest serum 25‑hydroxyvitamin D [25(OH)D] concentrations, worldwide. We describe the prevalence and the risk factors for hypovitaminosis D, completed and ongoing clinical trials, and available guidelines for vitamin D supplementation in this region.
Methods
This review is an update of previous reviews published by our group in 2013 for observational studies, and in 2015 for randomized controlled trials (RCTs) from the region. We conducted a comprehensive search in Medline, PubMed, and Embase, and the Cochrane Library, using MeSH terms and keywords relevant to vitamin D, vitamin D deficiency, and the MENA region, for the period 2012–2017 for observational studies, and 2015–2017 for RCTs. We included large cross-sectional studies with at least 100 subjects/study, and RCTs with at least 50 participants per arm.
Results
We identified 41 observational studies. The prevalence of hypovitaminosis D, defined as a 25‑hydroxyvitamin D [25(OH)D] level below the desirable level of 20 ng/ml, ranged between 12–96% in children and adolescents, and 54–90% in pregnant women. In adults, it ranged between 44 and 96%, and the mean 25(OH)D varied between 11 and 20 ng/ml. In general, significant predictors of low 25(OH)D levels were female gender, increasing age and body mass index, veiling, winter season, use of sun screens, lower socioeconomic status, and higher latitude.
We retrieved 14 RCTs comparing supplementation to control or placebo, published during the period 2015-2017: 2 in children, 8 in adults, and 4 in pregnant women. In children and adolescents, a vitamin D dose of 1000–2000 IU/d was needed to maintain serum 25(OH)D level at target. In adults and pregnant women, the increment in 25(OH)D level was inversely proportional to the dose, ranging between 0.9 and 3 ng/ml per 100 IU/d for doses ≤2000 IU/d, and between 0.1 and 0.6 ng/ml per 100 IU/d for doses ≥3000 IU/d. While the effect of vitamin D supplementation on glycemic indices is still controversial in adults, vitamin D supplementation may be protective against gestational diabetes mellitus in pregnant women. In the only identified study in the elderly, there was no significant difference between 600 IU/day and 3750 IU/day doses on bone mineral density. We did not identify any fracture studies.
The available vitamin D guidelines in the region are based on expert opinion, with recommended doses between 400 and 2000 IU/d, depending on the age category, and country.
Conclusion
Hypovitaminosis D is prevalent in the MENA region, and doses of 1000–2000 IU/d may be necessary to reach a desirable 25(OH)D level of 20 ng/ml. Studies assessing the effect of such doses of vitamin D on major outcomes, and confirming their long term safety, are needed.
Abbreviations: 25(OH)D, 25‑hydroxyvitamin D; ALKP, alkaline phosphatase; BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; Ca, Calcium; CARS, Childhood Autism Rating Scale; CDC, Centers for Disease Control; DEQAS, Vitamin D External Quality Assessment Scheme; DXA, dual-energy X-ray absorptiometry; ESCEO, European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; GDM, Gestational Diabetes Mellitus; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; ID LC-MS/MS, isotope dilution liquid chromatography - tandem mass spectrometry; IOM, Institute of Medicine; KSA, Kingdom of Saudi Arabia; PO4, phosphorus; RCT, randomized controlled trials; ROB, risk of bias; RR, relative risk; T2D, type 2 diabetes; LCMS/MS, liquid chromatography-tandem mass spectrometry; MENA, Middle East North Africa; OSTEOS, Lebanese Society for Osteoporosis and Metabolic Bone Disorders; PTH, parathyroid hormone; SDp, pooled standard deviation; UAE, United Arab Emirates; UVB, ultraviolet B; VDDR2, vitamin d dependent rickets type 2; VDR, vitamin d receptor; VDSP, Vitamin D Standardization Program; WM, weighted mean
Keywords: Middle East and North Africa, Hypovitaminosis D, Predictors, Vitamin D assays, Vitamin D guidelines
Highlights
-
•
Hypovitaminosis D is prevalent in the Middle East and North Africa.
-
•
Trials suggest that a vitamin D dose of 1000–2000 IU/day is needed.
-
•
Randomized trials are needed to develop rigorous and region-specific vitamin D guidelines.
1. Introduction
One of the earliest reports on low 25-hydroxyvitamin D [25(OH)D] concentrations in the Middle East was in a group of apparently healthy young university students from Saudi Arabia, in the early eighties (Sedrani et al., 1983). The vitamin D field has since witnessed an explosion in publications, with a larger number of systematic reviews and meta-analyses than original publications, in the last decade (Holick, 2007; El-Hajj Fuleihan et al., 2015; Ross et al., 2011; Holick et al., 2011; Van Schoor and Lips, 2017; Sperati et al., 2013; Keum and Giovannucci, 2014; Bolland et al., 2014; Mao et al., 2013; Ford et al., 2014; Stubbs et al., 2015; Avenell et al., 2014; Weaver et al., 2016; Zheng et al., 2013; Bjelakovic et al., 2014; Chowdhury et al., 2014; Lips, 2010).
The Middle East North Africa (MENA) region spans latitudes from 15 to 39° N. At such latitudes, it would be anticipated that vitamin D synthesis from the skin should be possible over 300/364 days of the year (Tavera-Mendoza and White, 2007). However, some of the lowest reported serum 25(OH)D levels, the metabolite that reflects vitamin D nutritional status, are from this region (Arabi et al., 2010; Bassil et al., 2013; Hoteit et al., 2014; El-Rassi et al., 2012). A recent systematic review of 195 studies, involving over 168,000 participants from 44 countries, revealed mean values <50 nmol/l (20 ng/ml) in 37% of studies, with higher proportions in the Middle East and Asia (Hilger et al., 2014). Environmental and lifestyle factors account for substantial variations in serum 25(OH)D levels, amounting to 7.5–37.5 nmol/l (3 to 15 ng/ml).
In this paper we describe 3 case presentations illustrating presentations for low vitamin D in the region. We then synthesize information based on a comprehensive literature search obtained from: reviews and studies on the prevalence of hypovitaminosis D, vitamin D randomized trials (completed and ongoing), vitamin D and overview guidelines, in the MENA region. The MENA countries, as defined by the World Bank, include: Algeria, Bahrain, Djibouti, Egypt, Jordan, Iran, Iraq, Kuwait, Lebanon, Libya, Malta, Morocco, Oman, Palestine/Israel, Qatar, Kingdom of Saudi Arabia (KSA), Syria, Tunisia, United Arab Emirates (UAE), and Yemen (MENA Countries Definition World Bank, n.d.). Details on literature search, abstract and title screen, eligibility criteria, calculation of weighted mean (WM) and pooled standard deviation (SDp), are presented in Appendix I (Arabi et al., 2010; Bassil et al., 2013; MENA Countries Definition World Bank, n.d.; Chakhtoura et al., 2017a, Chakhtoura et al., 2017b; Green, 2011; Weighted Mean Calculation, n.d.; Pooled Standard Deviation Calculation, n.d.).
2. Case presentations
2.1. Osteomalacia in a postmenopausal woman mimicking osteoporosis
A 52-year-old post-menopausal white female presented with worsening low back and hip pain of one year duration. She had difficulty getting up from the chair and climbing upstairs, and was unable to perform her daily activities. She reported avoiding dairy products, spent little time outdoors, had been veiled for many years, and was on no medications. Her physical exam was notable for diffuse musculoskeletal tenderness and inability to stand up from a sitting position. Her laboratory evaluation revealed hypocalcemia, calcium (Ca) of 6.9 mg/dl (normal 8.5–10.5), a normal albumin, hypophosphatemia, phosphorus (PO4) of 2.5 mg/dl (normal 2.9–5), an elevated alkaline phosphatase (ALKP) of 820 IU/l (normal 35–120), a 25(OH)D level of <5 ng/ml with a parathyroid hormone (PTH) level of 250 pg/ml (normal 10–65). Skull films revealed multiple lucent areas in the skull vault and parietal areas. Her bone mineral density (BMD) at the lumbar spine had a T-score of −2.46, and at the hip of −2.42. The biochemical profile was consistent with osteomalacia, although this diagnosis was not established by histomorphometry. Workup for malabsorption including stool fat, and a duodenal biopsy to rule out sprue, was negative. She was treated with calcium, 600 mg po tid, plus high-dose vitamin D 600,000 IU intramuscularly twice within 2 months, and had a rapid and impressive clinical improvement. Her difficulty with ambulation improved within 1 week of start of therapy. Her serum Ca and PO4 normalized within a month, but her ALKP and PTH took over a year to normalize. Her BMD showed increments of 40% at the spine and 35% at the hip at 4 months, 63% and 39% at 10 months, and 62% and 52% at 15 months, at these sites, respectively (Al-Ali and El-Hajj Fuleihan, 2000). Treatment of osteomalacia is extremely rewarding, with dramatic clinical improvement, accompanied by rapid increments and normalization of BMD, reflecting mineralization of osteomalacic bone. Such presentation has become relatively uncommon in Lebanon, but is still seen in the Gulf States, where concealed clothing style is the custom.
2.2. Rickets in infancy
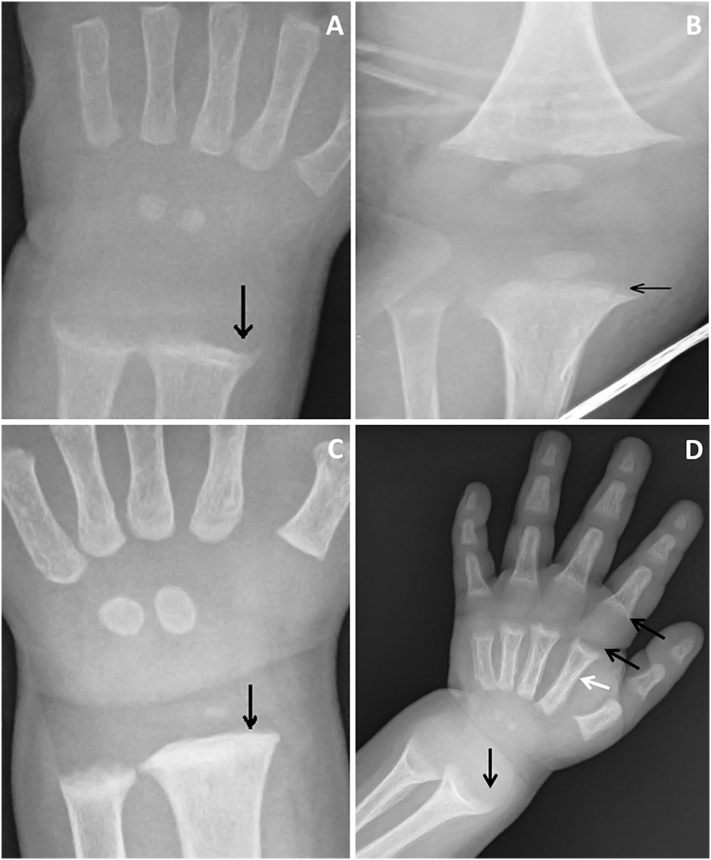
JAH is a 14-month old male admitted to the pediatric Intensive Care Unit at the age of 4 months for a severe lower respiratory infection requiring intubation. On routine blood chemistry found to have a serum Ca of 4.5 mg/dl (normal 8.5–10.5), PO4 was 4.2 mg/dl (normal for age 3.5–6.6), ALKP was 833 IU (normal for age 20–385) and a PTH was 357 pg/ml (normal 15–76). 25(OH)D concentration was <3 ng/ml and 1,25 (OH)2D3 was 105 pg/ml (normal 20–46). X-rays of wrists and knees showed changes of rickets (Fig. 1A, B). The patient had an excellent clinical, biochemical, and radiographic (Fig. 1C) response to vitamin D, with gradual rise in calcium to 10.5 mg/dl and 25(OH)D level to 40.4 ng/ml. The clinical and radiologic presentation may be similar or more severe (Fig. 1D) in patients homozygous for the H397P mutation in the ligand binding domain of the VDR, otherwise known as vitamin D resistant rickets, or vitamin D dependent rickets type 2 (VDDR2), where parents are heterozygous (Malloy and Feldman, 2010). In contrast however, such patients are refractory to even large doses of alfacalcidol and calcium, and may have alopecia. Due to frequent consanguinity in the Middle East, this condition is more prevalent than in western populations.
Fig. 1.
Four panel figure of radiographs from a 4-month old boy with nutritional rickets (A–C), and a 2 year old girl with vitamin D dependent rickets type 2 (D). Wrist (A), and knee (B) radiographs reveal cupping and fraying of the metaphysis of the long bones (black arrow). Wrist radiograph (C) post-treatment demonstrates healing at the level of the radius (black arrow) with persistent changes at the ulna. Radiographic changes, with severe cupping extending from the radius to the metacarpals and phalanges (D), may be more severe in patients with vitamin D dependent rickets type 2 (black and white arrows).
2.3. Asymptomatic low vitamin D level in a pregnant woman
A 32-year-old Lebanese pregnant lady was referred for evaluation and management of a low 25(OH)D level of 18 ng/ml, at 11 weeks of gestation. She is gravida 1 para zero, and has a non-significant past medical history. Early during her pregnancy, she was started on calcium carbonate 500 mg and cholecalciferol 600 IU twice a day, in addition to a prenatal multivitamin. She does not take dairy products. Upon presentation, she was completely asymptomatic. The patient was prescribed cholecalciferol 10,000 IU weekly throughout her pregnancy, and repeat 25(OH)D level at delivery was 32 ng/ml, with the same assay. Such presentation with borderline low 25(OH)D level is a common presentation, not only in pregnant women, but also in adolescents, adults, and elderly from the Middle East. The impact of such levels, as opposed to clear vitamin D deficiency, on major outcomes is unclear.
3. Prevalence of low 25(OH)D concentrations by age category and their predictors
In a previous review, we had noted that rickets and osteomalacia still occur in this sunny area (Bassil et al., 2013). We had also reported that hypovitaminosis D, defined as a serum 25(OH)D below 20 ng/ml, prevailed, with proportions between 30 and 90% depending on the age, gender, and country (Bassil et al., 2013). Prolonged breastfeeding without vitamin D supplementation and low dietary calcium intake were recognized risk factors for rickets and hypovitaminosis D in children. Advancing age, female gender, multiparity, clothing style, season, socio-economic status and urban living were predictors of hypovitaminosis D in adults (Bassil et al., 2013).
To update the above, we conducted a systematic search in Medline, PubMed, and Embase, using MeSH terms and keywords relevant to vitamin D, vitamin D deficiency, and focusing on the 20 countries of the MENA region, as per the World Bank definition (MENA, n.d.). We have limited the search to years 2012–2017, in view of our previous reviews on the topic, latest published in 2013 (Arabi et al., 2010; Bassil et al., 2013). The search resulted in 4012 articles that were screened by title and abstract, of which 182 were reviewed in full text, and 41 with a sample size of at least 100 participants were selected and summarized here-in (Appendix I, search methodology) (Arabi et al., 2010; Bassil et al., 2013; MENA, n.d.; Chakhtoura et al., 2017a, Chakhtoura et al., 2017b; Green, 2011; Weighted Mean Calculation, n.d.; Pooled Standard Deviation Calculation, n.d.). The studies included 1 from Algeria, 3 from Bahrain, 2 from Egypt, 10 from Iran, 3 from Jordan, 10 from KSA, 1 from Kuwait, 1 from Lebanon, 3 from occupied Palestine/Israel, 1 from Morocco, 1 from each of Qatar, Syria, and Tunis, and 3 from UAE. A unified assessment for the prevalence of hypovitaminosis D, by age categories, based on the studies selected, was challenging in view of the variability in the 25(OH)D cut-offs, season and assays used in the various studies (Appendix II) (Hoteit et al., 2014).
The weighted means for serum 25(OH)D levels were between 13 and 18 ng/ml in children from Iran, Lebanon, and KSA, between 11 and 13 ng/ml in pregnant women from KSA and Iran, and between 13 and 24 ng/ml in adults in these 3 countries (Fig. 2). Adults from Bahrain had the lowest weighted mean 25(OH)D level of 13 ng/ml (Fig. 2).
Fig. 2.
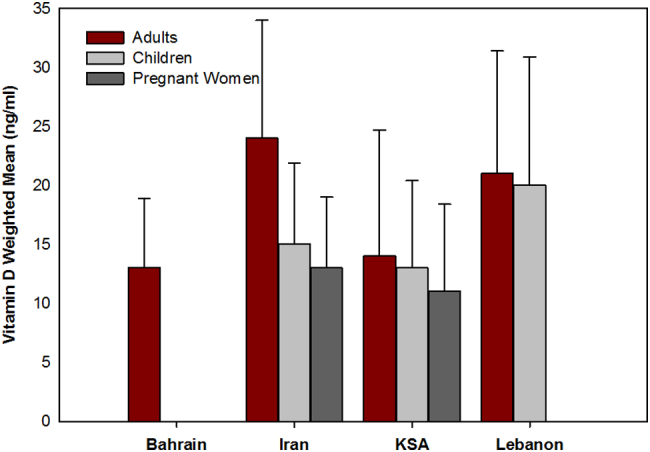
Weighted means (±pooled SD) for serum 25‑hydroxy-vitamin D levels by age group in 4 countries in the Middle East. In adults, the weighted means are based on 2 studies from each of Bahrain (N = 1150), Iran (N = 6842) and KSA (N = 4305) and one from Lebanon (N = 5091, spanning two periods, 2000–2004, and 2007–2008); in children the weighted means are based on 3 studies from Iran (N = 2809) and KSA (N = 5143) and one from Lebanon (N = 494, spanning the 2 same periods); and in pregnant women 3 from Iran (N = 351) and 2 from KSA (N = 1612).
In the pediatric age group, 90% of neonates in Bahrain and 94% in Jordan had a 25(OH)D below 20 ng/ml, while 65% in Saudi Arabia had a 25(OH)D level below 10 ng/ml (Appendix II) (Hoteit et al., 2014; Al-Mahroos et al., 2013; Khalesi et al., 2012; Khuri-Bulos et al., 2013; Fouda et al., 2017; Mohamed and Al-Shehri, 2013; Djennane et al., 2014; Shady et al., 2015; Ebrahimi et al., 2014; Kelishadi et al., 2014; Nikooyeh et al., 2017; Nichols et al., 2012; Al-Daghri et al., 2015; Al-Daghri et al., 2016; Al Shaikh et al., 2016; Kaddam et al., 2017; Sulimani et al., 2016; Korchia et al., 2013; Bezrati et al., 2016; Narchi et al., 2015; Rajah et al., 2012; Al-Sahlawi et al., 2014; Botros et al., 2015; Khalessi et al., 2015; Naseh et al., 2017; Al-Ajlan et al., 2015; Golbahar et al., 2013; Ardeshir Larijani et al., 2014; Esteghamati et al., 2014; Jaddou et al., 2012; Alfawaz et al., 2014; Ardawi et al., 2012; Tuffaha et al., 2015; Zhang et al., 2016; El Maghraoui et al., 2012; Saliba et al., 2012a; El-Menyar et al., 2012; Sayed-Hassan et al., 2014; Sridhar et al., 2016; Hosseini et al., 2014; Saliba et al., 2012b). Predictors of low neonatal vitamin D included maternal 25(OH)D level, maternal vitamin D supplementation, time spent outdoors, gestational-neonatal age, and season.
In pregnant and lactating women, 54–96% of subjects in studies from Bahrain, Egypt, Iran and KSA had a 25(OH)D level below 20 ng/ml, and the proportions ranged from 27 to 68% for a cut-off below 10–12 ng/ml, in the 3 studies from KSA and Iran (Appendix II) (Hoteit et al., 2014).
In children and adolescents, the proportion of subjects with a 25(OH)D level below 15–20 ng/ml ranged between 12%–62% in studies from Algeria, Iran, Bahrain, Egypt, Iran, occupied Palestine-Israel, Jordan, KSA, and Lebanon. The highest proportions were reported in studies from KSA and UAE reaching proportions of 96% and 99% for this cut off, respectively, and 33–66% for a cut-off below 10–12 ng/ml in these countries (Appendix II) (Hoteit et al., 2014).
By far, the largest number of selected studies was in adults, spanning an age range of 18–65 years, where the proportion of subjects with a 25(OH)D level below 20–25 ng/ml ranged between 37% and 96%, depending on the age group and country, including Bahrain, Egypt, Iran, Jordan, KSA, Kuwait, Lebanon and Syria. The highest proportion of 96% was reported in a population based household survey in the spring in 2039 young adults from Jordan where the Liquid Chromatography Mass Spectrometry (LCMS/MS) vitamin D assay was used. The proportion of subjects with 25(OH)D levels below 10–12 ng/ml varied between 31 and 67% in studies from Bahrain, Iran, Jordan, KSA, Morocco, and Syria (Appendix II) (Hoteit et al., 2014).
In the elderly, the prevalence of levels below 20 ng/ml was between 38% and 77% in elderly from Egypt, Iran, and Lebanon, and 65% in KSA for a level range of 0–28 ng/ml for KSA (Appendix II) (Hoteit et al., 2014).
Consistent predictors of low 25(OH)D levels across the lifecycle included increasing age, female gender, high weight or body mass index (BMI), low physical activity, low intake of calcium or vitamin D supplements, concealed clothing, low sun exposure duration, winter season, lower education or socio-economic status, and urban residence (Hoteit et al., 2014; Al-Mahroos et al., 2013; Khalesi et al., 2012; Khuri-Bulos et al., 2013; Fouda et al., 2017; Mohamed and Al-Shehri, 2013; Djennane et al., 2014; Shady et al., 2015; Ebrahimi et al., 2014; Kelishadi et al., 2014; Nikooyeh et al., 2017; Nichols et al., 2012; Al-Daghri et al., 2015; Al-Daghri et al., 2016; Al Shaikh et al., 2016; Kaddam et al., 2017; Sulimani et al., 2016; Korchia et al., 2013; Bezrati et al., 2016; Narchi et al., 2015; Rajah et al., 2012; Al-Sahlawi et al., 2014; Botros et al., 2015; Khalessi et al., 2015; Naseh et al., 2017; Al-Ajlan et al., 2015; Golbahar et al., 2013; Ardeshir Larijani et al., 2014; Esteghamati et al., 2014; Jaddou et al., 2012; Alfawaz et al., 2014; Ardawi et al., 2012; Tuffaha et al., 2015; Zhang et al., 2016; El Maghraoui et al., 2012; Saliba et al., 2012a; El-Menyar et al., 2012; Sayed-Hassan et al., 2014; Sridhar et al., 2016; Hosseini et al., 2014; Saliba et al., 2012b). Added to that is smoking status in adults and maternal weight at delivery (Appendix II) (Hoteit et al., 2014).
4. Vitamin D assay variation: a pressing need for standardization
The exponential rise in vitamin D publications is paralleled by a similar increase in the number and use of 25(OH)D assays available. Such high test volume necessitated a gradual shift from the kit based radio-immunoassays to the rapid, automated, platform assays, in most clinical and many research laboratories. The Vitamin D External Quality Assessment Scheme (DEQAS), recently reported 26 different assay methods being used in over 1100 laboratories, from 53 countries worldwide, with automated platform assays taking the lion's share (Carter et al., 2017). However, such shift has come at a large price, considering the variability in intra- and interassay performance, and differences in accuracy between methods, making comparison across studies, such as performed in this review, most challenging. Thus the pressing need for clinical and research laboratories to all engage in continuous calibration, recalibration and standardization (Carter et al., 2017; Barake et al., 2012; Binkley and Carter, 2017). The most reliable and accurate method is LCMS (Carter et al., 2017), when performed, but such technology is costly, and seldom used in commercial assays, and even research laboratories in the MENA region. Indeed, based on information obtained from a major vitamin D quality assurance programs DEQAS (DEQAS Vitamin D External Quality Assessment Scheme, n.d.), 22 laboratories in the MENA region currently partake in DEQAS: 14 from Iran, 14 from occupied Palestine/Israel, 2 each from Lebanon, KSA, and UAE (personal communication Graham Carter for DEQAS and Dr Hubert Vesper for VDCSP). Only one laboratory in Qatar is using the LCMS/MS methodology, and only one laboratory, based at our institution, partakes in the CDC VDSP (Laboratory Quality Assurance and Standardization Program, n.d.; Vitamin D CDC Certified Laboratories, 2017).
Our laboratory has been a participant in DEQAS for almost 2 decades, and consistently received certification. DEQAS uses few pooled sera and assesses measurements performed at one point in time. The CDC VDSCP is striving to improve the analytical performance of vitamin D assays and ultimately improve clinical and public health practice. It uses 40 unmodified, single donor samples measured over the course of a year to evaluate assay performance. Because of the more rigorous protocol and stringent evaluation criteria, obtaining certification by CDC VDSCP is more challenging. Between August 2016 and May 2017, our laboratory received a total 160 unknown samples from the VDSP (some duplicates to also assess precision), that were run for 25(OH)D levels using a platform automated assay (Elecsys Cobas) and the laboratory reported values that were compared against a reference value assigned for each sample, as obtained from measurements performed at the Centers for Disease Control and Prevention (Stepman et al., 2011; Mineva et al., 2015). Although the mean precision was quite tight, 4.7(2.7%, 95 CI [1.3–9.1%], the mean bias of 13.7% (SD = 36.8%), 95 CI [1.8–25.6%] exceeded the mean desirable bias range set by the VDSCP of ±5%., with individual values falling much farther off. Indeed, only 24% of the samples fell within this recommended bias range (Fig. 3). Thus, even though our laboratory passes DEQAS challenges it was not able to obtain VDSCP certification, which may well to be reflect of the different purposes of these programs. The goal of clinical laboratories is to provide accurate and reliable vitamin D measurements to help physicians and public health officials in clinical and public health decision making. Factors affecting the accuracy and reliability of vitamin D measurements are related to the extraction methods to extract the vitamin D metabolite from its binding protein, the detection of 25(OH)D2 metabolite, cross-reactivity with 3-epi-25OHD3 and other vitamin D metabolites, and matrix interferences. These factors can result in a percent bias ranging between −25% to +25%, based on means group of laboratories using the same assay (DEQAS Vitamin D External Quality Assessment Scheme, n.d.). In our study, we did observe a high variability in bias among individual samples provided by the CDC VDSCP (Fig. 3). Since the samples provided by CDC are from individual donors, one can assume that similar variability in accuracy exists when measuring patient samples. Programs such as DEQAS combine sera to conduct their program, and this could result in samples showing a high bias being diluted with samples showing a low bias making it difficult to detect high variability in bias among different patients and could explain why certification through DEQAS can be achieved while certification through CDC VDSCP is more difficult to achieve. Clinical practice is based on single measurements performed on individual patients. The high variability in accuracy of measurements in patients is a pressing issue, the resolution of which is crucial to allow the successful pooling of 25(OH)D results across studies, the implementation of meta-analyses, and the development of evidence based vitamin D guidelines (Barake et al., 2012; Binkley and Carter, 2017; Binkley and Sempos, 2014).
Fig. 3.
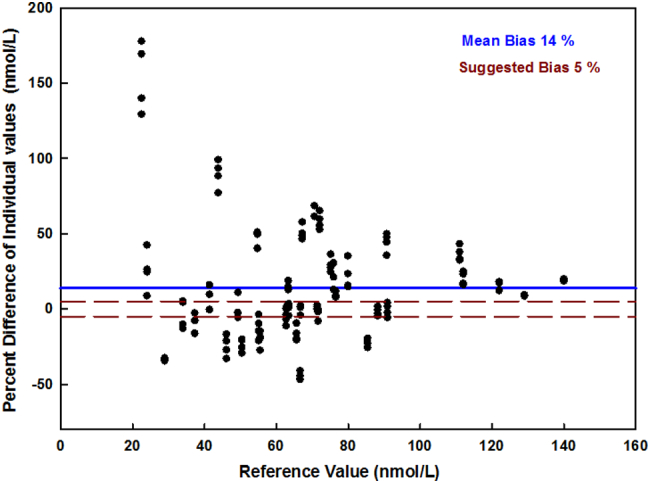
Percent bias in 160 samples run as part of Center of Diseases C (CDC) Vitamin D Standardization Program (VDSP). The percent bias of the individual reported values is shown for all 160 samples run at our institution over 4 quarters in 2016–2017 using the platform automated Roche Elecsys assay compared with reference values defined by the CDC VDSP reference. Percent bias formula: [(reported value − reference value) / reference value] × 100. Although the mean % bias in our samples was 14%, many individual values fell outside that recommended bias range of −5% to +5% (range framed by dotted lines).
5. Randomized trials of vitamin D supplementation in different age categories
We had previously conducted a systematic review on vitamin D randomized trials across all age groups, in the region (Chakhtoura et al., 2017a, Chakhtoura et al., 2017b).
The 4 trials in children and adolescents (El-Hajj Fuleihan et al., 2006; Ghazi et al., 2010; Mayan et al., 2015; Neyestani et al., 2014), mean age of 9.8–16.6 years, baseline 25(OH)D level 7.9–25 ng/ml, showed that a vitamin D dose of 1400–2000 IU/d would be needed to maintain a desirable 25(OH)D level ≥ 20 ng/ml in the majority of the population (Ross et al., 2011; Chakhtoura et al., 2017b). One study demonstrated substantial improvements in body lean mass and total hip bone mineral content (BMC), in girls only, both in the low dose (400 IU/d) and the high dose (2000 IU/d) arms, compared to placebo, and in total hip area, in the high dose group only (El-Hajj Fuleihan et al., 2006). Interestingly, the latter effect persisted at 1 year after the discontinuation of the intervention (Ghazal et al., 2016). The risk of bias in these trials varied widely (Chakhtoura et al., 2017b).
Similarly, we had identified 19 RCTs in adults and elderly (Chakhtoura et al., 2017a; Hosseini et al., 2014; Ahmadi et al., 2013; Al-Sofiani et al., 2015; Al-Zahrani et al., 2014; Begay and Danshiyour, 2012; Breslavsky et al., 2013; El-Hajj Fuleihan et al., 2016; Firouzabadi et al., 2012; Gendelman et al., 2015; Ghavamzadeh et al., 2014; Golan et al., 2013; Moghassemi and Marjani, 2014; Nasri et al., 2014; Rashidi et al., 2009; Sharifi et al., 2014; Salehpour et al., 2012; Taheri et al., 2014; Tehrani et al., 2014). The risk of bias in the trials was unclear to high, except for few (Chakhtoura et al., 2017a). The response of 25(OH)D levels to vitamin D supplementation was blunted at high doses (Chakhtoura et al., 2017a), similarly to findings in Western studies (Ross et al., 2011; Cashman et al., 2011). Starting at a baseline 25(OH)D level of 8.6–15.5 ng/ml, a vitamin D dose ≥2000 IU/d allowed for the majority of subjects to increase their 25(OH)D level to a desirable level of 20 ng/ml (Chakhtoura et al., 2017a). In the meta-regression, baseline 25(OH)D level and vitamin D dose were significant predictors of the achieved 25(OH)D level post-intervention (Chakhtoura et al., 2017a).
We had also identified 10 trials in pregnant women (Chakhtoura et al., 2017b; Sabet et al., 2012; Dawodu et al., 2013; Shakiba and Iranmanesh, 2013; Soheilykhah et al., 2013; Etemadifar and Janghorbani, 2015; Karamali et al., 2015; Samimi et al., 2016a, Samimi et al., 2016b; Shahgheibi et al., 2016; Vaziri et al., 2016). Nine studies were from Iran and one from the UAE (Chakhtoura et al., 2017b). The risk of bias was unclear to high, with the exception of 2 studies (Chakhtoura et al., 2017b). Starting at a mean baseline 25(OH)D level of 7–11 ng/ml, a daily vitamin D dose of 1800–3600 IU was needed to allow for the majority of the subjects to reach a 25(OH)D level of 20 ng/ml (Chakhtoura et al., 2017b).
To update the above systematic review (Chakhtoura et al., 2017a; Chakhtoura et al., 2017b), we conducted a comprehensive search spanning from 2015 to 2017, and only included trials with at least 50 participants per arm (Appendix I) (Arabi et al., 2010; Bassil et al., 2013; MENA Countries Definition World Bank, n.d.; Chakhtoura et al., 2017a; Chakhtoura et al., 2017b; Green, 2011; Weighted Mean Calculation, n.d.; Pooled Standard Deviation Calculation, n.d.). The search yielded 1280 citations after removal of duplicates. We screened the full text of 242 papers, and identified 14 relevant trials (Shahgheibi et al., 2016; Vaziri et al., 2016; Saad et al., 2018; Talaat et al., 2016; Taheri et al., 2015; Tepper et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015; Mohammad-Alizadeh-Charandabi et al., 2015; Shehata, 2016; Rahme et al., 2017; Mojibian et al., 2015; Behjat Sasan et al., 2017).
5.1. Children and adolescents
The current update identified 2 trials in children, with a variable risk of bias from low to high. The study from KSA, randomized children, mean age 8.2 (3.5) years, to 400 IU/d (group 1), 45,000 IU weekly over 2 months then 400 IU/d (group 2), or 2000 IU/d for 3 months then 1000 IU/d for a total of 12 months (group 3) (Talaat et al., 2016). The mean (SD) 25(OH)D level increased from 12.9–14.3(5.4) ng/ml at baseline, to reach 47.7(21.4) ng/ml and 36.2 (7.0) ng/ml at 4 months, in group 2 and 3, respectively, and then decreased to 23.1(12.7) ng/ml in group 2, while it was maintained at 37.7(5.3) ng/ml in group 3 (Talaat et al., 2016). In contrast, it dropped from a high baseline of 21.7(9.5) to 17.0(9.4) ng/ml at 4 months, and 10.2(8.3) ng/ml at 12 months in the low dose group 1 (Talaat et al., 2016). Such findings confirm our previous report that a vitamin D dose of 1000–2000 IU/d may be necessary to maintain a 25(OH)D level ≥ 20 ng/ml, in children and adolescents from this region (Chakhtoura et al., 2017b). A study from Egypt demonstrated that 300 IU/kg/day of vitamin D increased serum 25(OH)D level from 26.3 (12.7) ng/ml to 45.9(17.2) ng/ml, and resulted in a significant 6 unit drop in the Childhood Autism Rating Scale (CARS), in children (mean age 5.4 years) with vitamin D insufficiency at baseline, compared to placebo (Saad et al., 2018). Given the scarcity of vitamin D trials in children with autistic disorders (Mazahery et al., 2016), further trials are needed to confirm the protective effect of vitamin D supplementation in this specific population.
5.2. Pregnant women
In the current update, we identified 4 studies all from Iran, with an unclear (Shahgheibi et al., 2016; Vaziri et al., 2016; Behjat Sasan et al., 2017) or high risk of bias (Mojibian et al., 2015). Two of these studies were already described in our previous systematic review, and described here for completion, as they were published during the period 2015–2017 (Shahgheibi et al., 2016; Vaziri et al., 2016).
5.2.1. Vitamin D supplementation and 25(OH)D level
Two studies only reported on the change in 25(OH)D level following supplementation (Vaziri et al., 2016; Mojibian et al., 2015). The first compared an equivalent daily dose of around 3570 IU/d to 400 IU/d, and reported increments in the mean 25(OH)D level, from a mean baseline of 15 ng/ml, to 27 ng/ml in the low dose group, and to 38 ng/ml in the high dose group (Mojibian et al., 2015). The second reported increments, from a baseline 25(OH)D level of 12 ng/ml, of 6.4 ng/ml using a vitamin D dose of 2000 IU/d, while no change was detected in the placebo group (Vaziri et al., 2016). The increment in 25(OH)D level were 3 ng/ml/100 IU/d vitamin D for the lowest dose, and 0.3–0.6 ng/ml/100 IU for the highest doses (Table 1) (Vaziri et al., 2016; Mojibian et al., 2015). Such findings are comparable to those from our previous review (Chakhtoura et al., 2017b).
Table 1.
Summary of 25(OH)D levels before and after supplementation in trials published during the Period 2015–2017,a with at least 50 participants per arm.
| Author year/country | Dose | Mean age (years) | Gender (% female) | Duration | Baseline 25(OH)D (ng/ml) | Achieved 25(OH)D (ng/ml) | Δ 25(OH)D (ng/ml) | Δ 25(OH)D/100 IU/d (ng/ml) | 25(OH)D level assay |
|---|---|---|---|---|---|---|---|---|---|
| Infants and children | |||||||||
|
Saad et al., 2018 Egypt |
300 IU/kg/d | 5 | 22 | 4 mo | 26 | 46 | 20 | NA | ELISA |
| PBO | 6 | 27 | 28 | 1 | |||||
|
Talaat et al., 2016 KSA |
400 | 8 | 51 | 12 mo | 22 | 10 | −12 | NA | CLIA |
| 45,000 IU/w (2 mo) than 400 IU/d | 9 | 14 | 23 | 9 | |||||
| 2000 IU/d (3 mo) then 1000 IU/d | 8 | 13 | 38 | 25 | |||||
| Pregnant women | |||||||||
|
Mojibian et al., 2015 Iran |
400 IU/d | 27 | 100 | 12 w GA till delivery | 15 | 27 | 12 | 3 | ELISA |
| 50,000 IU/2 w (3570 IU/d) | 28 | 15 | 38 | 23 | 0.6 | ||||
|
Vaziri et al., 2016 Iran |
2000 | 32 | 100 | 26–28 w GA until delivery | 12 | 18 | 6 | 0.3 | CLIA |
| PBO | 26 | 13 | 12 | −1 | NA | ||||
| Adults | |||||||||
|
Taheri et al., 2015 Iran |
2000 | 30 | 100 | 3.7 mo | 9 | 28 | 19 | 0.9 | EIA |
| C | 29 | 9 | 9 | 0 | NA | ||||
|
El-Hajj Fuleihan et al., 2016 Lebanon |
600 | 71 | 57 | 12 mo | 20 | 26 | 6 | 0.9 | LCMS |
| 3750 | 71 | 54 | 21 | 36 | 15 | 0.4 | |||
|
Tepper et al., 2016 Occupied Palestine-Israel |
100,000 IU bimonthly (6666 IU/d) | 48 | 0 | 12 mo | 16 | 26 | 10 | 0.1 | NR |
| PBO | 15 | 21 | 6 | NA | |||||
|
Zarrin et al., 2017b Iran |
1000 | 48 | 53 | 3 mo | 19 | – | 11 | 1.1 | ELISA |
| PBO | 48 | 51 | 15 | – | −2 | NA | |||
|
Kamelian et al., 2017b Iran |
7142 | 39 | 69 | 9 mo | 8 | – | 32 | 0.4 | CLIA |
| PBO | 41 | 63 | 8 | – | 9 | NA | |||
Abbreviations: C: control; CLIA: Chemiluminescence Immunoassay; EIA: Enzymatic Immune Assay; ELISA: Enzyme Linked Immunosorbent Assay; GA: gestational age; LCMS: Liquid Chromatography Mass; mo: months; Spectrometry; NA: not applicable; NR: not reported; PBO: Placebo; w: weeks.
This is an update of a previous systematic review on vitamin D randomized controlled trials conducted in the MENA region. For further details, refer to the main text and to previous publications (Chakhtoura et al., 2017a, Chakhtoura et al., 2017b).
Achieved 25(OH)D level not reported but the change in 25(OH)D level reported.
5.2.2. Vitamin D supplementation and other skeletal and non-skeletal parameters
Two studies (mean baseline 25(OH)D level 13.5–17.4 ng/ml), administered high doses of vitamin D, ranging from 3570 to 5000 IU/d, and compared that to placebo or a low dose (400 IU/d), started in the first and continued until the third trimester or delivery. Both studies reported a protective effect of a high vitamin D dose, compared to a low dose or placebo, on the incidence of Gestational Diabetes Mellitus (GDM), with a calculated relative risk (RR) of 0.3–0.5 (Shahgheibi et al., 2016; Mojibian et al., 2015). These results are consistent with findings from our previous review (Chakhtoura et al., 2017b), and another recent one that was based mostly on data from Iran (Akbari et al., 2017). During pregnancy, vitamin D supplementation (at an equivalent daily dose of 1000–7142 IU) improved glycemic indices, when it was compared to no supplementation (Akbari et al., 2017), or when comparing a high (>2000 IU/d) to a low dose (<800 IU/d) (Chakhtoura et al., 2017a). However, these results were all derived from trials conducted in the same country (Iran) and the majority was conducted by the same research group (Chakhtoura et al., 2017b; Akbari et al., 2017).
One study demonstrated a protective effect of a high vitamin D dose, 50,000 IU every 2 weeks, started early in the second trimester, on the rate of pre-eclampsia, with a RR 1.94 (1.02–3.71), in the control arm compared to the high dose arm (Behjat Sasan et al., 2017). This is consistent with findings from previous reviews on the topic from studies conducted in Western countries (De-Regil et al., 2016; Hypponen et al., 2013).
The effect of vitamin D supplementation on neonatal and infant anthropometrics was assessed in one trial, that administered vitamin D (2000 IU/d), starting at 26–28 weeks gestation, or placebo (Vaziri et al., 2016). It revealed no significant difference between groups (Vaziri et al., 2016). There was also no significant difference in BMD in a sub-group of the population who underwent this measurement, both in the mothers and their neonates (Vaziri et al., 2016). To-date, the effect of vitamin D supplementation during pregnancy on neonatal anthropometric and bone parameters is still a matter of debate. While the neutral results described here-in replicate findings of a study from Brazil, that administered 200 IU of vitamin D with 600 mg of calcium daily, or placebo (Diogenes et al., 2015), other trials from the UK showed a potential beneficial effect with a vitamin D dose of 1000 IU/d, with a trend for a higher neonatal bone mineral content (BMC) at forearm (Congdon et al., 1983), or a higher whole body BMC, in neonates born in the winter season (Cooper et al., 2016). Indeed, baseline 25(OH)D level vitamin D dose administered, and the timing at which supplementation was started, seem to be important modulators of response, and this needs to be taken into consideration.
5.3. Adults and elderly
The current update identified 8 trials: 5 from Iran (Taheri et al., 2015; Zarrin et al., 2017; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015; Mohammad-Alizadeh-Charandabi et al., 2015), 1 from Egypt (Shehata, 2016), one from occupied Palestine/Israel (Tepper et al., 2016), and one from Lebanon (El-Hajj Fuleihan et al., 2016; Rahme et al., 2017). The latter was included in our previous review (Chakhtoura et al., 2017a), since we had access to primary data, but it was not published then, and is described herein (Table 1) (El-Hajj Fuleihan et al., 2016; Taheri et al., 2015; Tepper et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017). The risk of bias in the included trials was unclear (Taheri et al., 2015; Tepper et al., 2016; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015) to high (Zarrin et al., 2017; Mohammad-Alizadeh-Charandabi et al., 2015), with the exception of 2 RCTs considered at low risk of bias (El-Hajj Fuleihan et al., 2016; Shehata, 2016). Three studies were conducted exclusively in women (Taheri et al., 2015; Mohammad-Alizadeh-Charandabi et al., 2015; Shehata, 2016), one study exclusively in men (Tepper et al., 2016) and the others included both genders (El-Hajj Fuleihan et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015). The mean BMI was ≥25 kg/m2 in all studies (El-Hajj Fuleihan et al., 2016; Taheri et al., 2015; Tepper et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017; Shehata, 2016). Several trials were conducted in individuals with diseases not expected to affect vitamin D metabolism, such as pre-diabetes (Zarrin et al., 2017), asymptomatic bacterial vaginosis (Taheri et al., 2015), coronary artery disease (Jozanikohan and Kazemi Saleh, 2015). All the studies compared vitamin D supplementation (low <1000 IU/d (Mohammad-Alizadeh-Charandabi et al., 2015; Shehata, 2016), intermediate dose 1000–2000 IU/d (Taheri et al., 2015; Zarrin et al., 2017), or high dose >2000 IU/d (Tepper et al., 2016; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015) to placebo, except one study that compared a low (600 IU/d) to a high (3750 IU/d) vitamin D dose (El-Hajj Fuleihan et al., 2016).
5.3.1. Serum 25(OH)D levels before and after vitamin D supplementation
Data are derived from 6 studies (El-Hajj Fuleihan et al., 2016; Taheri et al., 2015; Tepper et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017; Jozanikohan and Kazemi Saleh, 2015). Mean baseline 25(OH)D level was <10 ng/ml in 2 studies (Taheri et al., 2015; Kamelian et al., 2017), 10–20 ng/ml in one (Tepper et al., 2016), and between 20 and 30 ng/ml in 2 other studies (El-Hajj Fuleihan et al., 2016; Zarrin et al., 2017). One study did not report on baseline 25(OH)D levels, but specified that all the participants had a level <30 ng/ml (Jozanikohan and Kazemi Saleh, 2015). The increment in 25(OH)D level per 100 IU/d was inversely proportional to the administered dose, similar to our previous findings (Chakhtoura et al., 2017a), ranging between 0.9 and 1.1 ng/ml per 100 IU/d for doses ≤2000 IU/d, and between 0.1 and 0.4 ng/ml per 100 IU/d for doses ≥3000 IU/d (Table 1) (El-Hajj Fuleihan et al., 2016; Taheri et al., 2015; Tepper et al., 2016; Zarrin et al., 2017; Kamelian et al., 2017).
5.3.2. Vitamin D supplementation and other skeletal parameters
Vitamin D at a dose of 50,000 IU/week for 9 months in 119 Iranian women and men resulted in a significant increase in FGF23 level, compared to placebo (Kamelian et al., 2017). An adequately powered study of 221 elderly overweight Lebanese individuals, with a baseline femoral T-score of −1.7 (0.8), demonstrated that vitamin D3 at daily equivalent doses of 600 IU or 3570 IU yielded similar increments in BMD at one year, despite a significant difference in the achieved 25(OH)D level, 36 (9.7) ng/ml in the high dose arm and 26 (7) ng/ml in the low dose arm (Rahme et al., 2017).
5.3.3. Vitamin D supplementation and extra-skeletal parameters
Three studies assessed the effect of vitamin D supplementation on metabolic and glycemic indices, as primary outcomes (El-Hajj Fuleihan et al., 2016; Tepper et al., 2016; Zarrin et al., 2017). Significant improvement in the fasting blood glucose, insulin and HOMA-IR levels in pre-diabetic Iranian patients were noted in subjects receiving vitamin D3 1000 IU/d over 3 months, compared to placebo (Zarrin et al., 2017). Another study conducted on a similar population from occupied Palestine/Israel, administered a higher vitamin D dose, 100,000 IU bi-monthly, and did not show any significant difference in glycemic indices between arms (Tepper et al., 2016). Finally, the above described study in Lebanese overweight elderly did not detect any significant difference in indices of insulin resistance, including HOMA-IR, in the high compared to the low vitamin D dose groups (El-Hajj Fuleihan et al., 2016). In trials conducted in women of reproductive age, vitamin D supplementation allowed an improvement in premenstrual syndrome (Shehata, 2016), a higher rate of cure of asymptomatic bacterial vaginosis (Taheri et al., 2015), and a lower rate of oral contraception discontinuation (Mohammad-Alizadeh-Charandabi et al., 2015).
Clinical trials of vitamin D supplementation and its impact on skeletal and extra-skeletal parameters, and surrogate outcomes, from the MENA, are still scarce. There are no studies on fall or fracture. Findings on metabolic and glycemic parameters are conflicting (El-Hajj Fuleihan et al., 2016; Tepper et al., 2016; Zarrin et al., 2017). While our previous systematic review showed a significant improvement in HOMA-IR with a high vitamin D dose, in studies extending over ≤4 months (Chakhtoura et al., 2017a), results of the current studies were conflicting, possibly related to differences in the risk of bias, race (Persian versus Arab) and study duration; the protective effect of vitamin D supplementation was only detected in short term studies, and not in those extending over 12 months (El-Hajj Fuleihan et al., 2016; Tepper et al., 2016). Indeed, this controversy is not specific to the MENA region, but constitutes a challenge worldwide (Mirhosseini et al., 2017; Lee et al., 2017). The recent scientific statement of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) on vitamin D supplementation for the prevention and management of chronic non-skeletal diseases concluded that “no evidence exists, so far, that administering vitamin D could reduce type 2 diabetes or obesity in the general population” (Cianferotti et al., 2017). Such statement is consistent with the Institute of Medicine 2009 report regarding the effect of vitamin D supplementation on various outcomes including cancer/neoplasms, cardiovascular disease and hypertension, diabetes and metabolic syndrome, falls and physical performance, immune functioning and autoimmune disorders, infections, neuropsychological functioning, and preeclampsia (Ross et al., 2011).
6. Available vitamin D replacement guidelines for MENA - who to screen and replacement doses
The search strategy identified 70 citations for Medline, 60 for Pubmed, and 171 for Embase. After screening titles and abstracts, we considered 5 citations as eligible, targeting the general population (Table 2) (Raef et al., 2011; Al-Daghri et al., 2017; Haq et al., 2018; Lebanese Society of Osteoporosis and Metabolic Bone Disorders, 2012; Maalouf et al., 2007). Two guidelines included recommendations on vitamin D replacement, as part of osteoporosis guidelines (Raef et al., 2011; Maalouf et al., 2007), and recommended a vitamin D dose ranging between 400 and 800 IU/d, depending on the age category. The Lebanese Society for Osteoporosis and Metabolic Bone disorders (OSTEOS) recommends a vitamin D dose of 600–1000 IU/d for children, adolescents and low risk adults (<50 years), and a dose of 1000–2000 IU/d for older and high risk adults (Lebanese Society of Osteoporosis and Metabolic Bone Disorders, 2012). Two other guidelines, from KSA and UAE, were exclusively dedicated to vitamin D supplementation, and recommended 800–2000 IU/d, depending on age category and reproductive status (Al-Daghri et al., 2017; Haq et al., 2018). None of these guidelines were based on a rigorous systematic review on vitamin D dose response in the MENA region, but rather, they were mostly based on expert opinion and review of the evidence from Western studies, regarding target 25(OH)D level, and recommended doses (Table 2) (Raef et al., 2011; Al-Daghri et al., 2017; Haq et al., 2018; Lebanese Society of Osteoporosis and Metabolic Bone Disorders, 2012; Maalouf et al., 2007).
Table 2.
Summary of vitamin D replacement guidelines in the general population, identified in the MENA region.
| Author year/journal | Country | Guidelines developing groupa | Desirable 25(OH) D level | Recommendations |
|---|---|---|---|---|
| A vitamin D targeted guidelines | ||||
|
Raef et al., 2011 Ann Saudi Med |
KSA | Osteoporosis Working Group of King Faisal Specialist Hospital and Research Centre | NA |
|
|
Al-Daghri et al., 2017 Arch Osteoporos |
KSA | Prince Mutaib Chair for Biomarkers of Osteoporosis: 12 local and 2 expert advisers from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) |
|
|
|
Haq et al., 2018 J Steroid Biochem Mol Biol |
UAE | Not defined explicitly, but authors included experts in endocrinology and metabolism, biochemistry and public health | –30 ng/ml as per the Endocrine Society |
|
| B osteoporosis guidelines | ||||
| Lebanese Society for Osteoporosis and Metabolic Bone disorders (OSTEOS), 2012 | Lebanon |
Guidelines developed by: OSTEOS Guidelines endorsed by: Lebanese Society of Endocrinology; Lebanese Society of Obstetrics and Gynecology; Lebanese Association of Orthopedic Surgeons; Lebanese Society of Radiology; Lebanese Society of Rheumatology; Lebanese Society of Family Medicine; Lebanese Society of Internal Medicine; Lebanese Society of General Practitioners |
30–60 ng/ml |
|
|
Maalouf et al., 2007 J Musculo-skelet Neuronal Interact |
MENA | Not defined explicitly, but authors included experts in osteoporosis, endocrinology and metabolism | No clear statement |
|
Abbreviations: 25(OH) D: 25‑hydroxyvitamin D; ESCEO: European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; KSA: Kingdom of Saudi Arabia; MENA: Middle East and North Africa; NA: not available; OSTEOS: Lebanese Society for Osteoporosis and Metabolic Bone disorders; UAE: United Arab Emirates.
None of the documents describe the methodology used in the guideline development process.
7. Ongoing trials
Our search on ClinicalTrials.Gov identified 7 ongoing studies conducted in adults (3 from KSA, one from Iran, one from Qatar and one from occupied Palestine/Israel, Appendix III). Two studies are conducted on patients with multiple sclerosis (n = 240 and 200), and assess the effect of vitamin D supplementation (50,000 IU/week) over one year, on disease relapse rate, and the extended disability state scale score. One study is conducted in diabetic patients (n = 180) and evaluates the effect of vitamin D, given as a monthly loading dose of 120,000 IU over 6 months, compared to placebo, on the change in HbA1c, and other metabolic parameters. Two other studies target patients at high risk of diabetes (n = 200) or those with impaired glucose tolerance (n = 500), and administer a high vitamin D dose (4000–5000 IU/d), or placebo. Their primary outcome is insulin resistance and the incidence of diabetes, respectively. The remaining 2 studies (n = 1080 and 432) compare the effect of different doses of vitamin D on 25(OH)D level and other mineral and muscle parameters.
We identified 2 ongoing studies in pregnant women. One of them from Lebanon (n = 280), administers 2 vitamin D doses, a low (daily equivalent dose of 660 IU) versus a high dose (daily equivalent dose of 2850 IU), started early in the second trimester and continued until delivery. The latter study is powered for 2 primary outcomes: the proportion of women reaching the IOM desirable level of 20 ng/ml and neonatal bone mineral content, assessed by DXA at 4 weeks (±2 weeks) after birth. The other study from occupied Palestine/Israel compares 2 lower vitamin D doses (400 IU/d versus 2000 IU/d), and assesses the impact of supplementation on tibial and radial bone density, using quantitative US.
Although two of the described studies (one in adults and one in pregnant women) had an estimated completion date in 2012 and 2014, we could not identify any results on clinicaltrials.gov, nor in PubMed, Medline, and Embase.
8. Discussion
Nutritional rickets is still seen in our region, due to peculiar lifestyle factors, and genetic rickets is more likely to occur than in other regions, due to high consanguinity rates. There are no population based studies in the MENA region to derive specific statistics.
Our systematic review confirms the high prevalence of silent hypovitaminosis D in the MENA region, and that vitamin D at doses higher than those recommended by the IOM would be necessary to raise serum 25(OH)D levels to the desirable range of 20 ng/ml defined, in western populations (Arabi et al., 2010; Bassil et al., 2013; Chakhtoura et al., 2017a, Chakhtoura et al., 2017b).
However, the clinical significance of 25(OH)D levels in the teens to below 20 ng/ml, such as commonly seen and illustrated in case 3, is totally unclear. Similarly, unknown is the desirable 25(OH)D range due to the scarcity of vitamin D RCTs investigating the impact of its replacement on major health outcomes in this region.
The predictors of low 25(OH)D levels highlighted in this report are well described and include extremes of age, female gender (some studies reported higher levels in women due to the higher likelihood of using vitamin D supplements), pregnancy, latitude, UVB/sun exposure, pollution, concealed clothing style, high BMI, lower socioeconomic status, skin pigmentation, race, and ethnicity (Holick, 2007; Bassil et al., 2013). Seasonal variations of vitamin D are well described, with lower serum 25(OH)D levels in the winter, except for countries from the Gulf states, where they are lower in the summer due to longer in-door dwelling to avoid the scorching heat. In addition, genetic factors, namely polymorphisms in key genes of the vitamin D pathway, modulate vitamin D status in western populations and possibly the Middle East (Wang et al., 2010; Ahn et al., 2010; Arabi et al., 2017). The recognition of these consistent predictors justifies vitamin D supplementation strategies to prevent progression to symptomatic osteomalacia or rickets, which although becoming increasingly rare, is certainly not extinct in the region.
We recognize several limitations of our systematic review, mostly stemming from the original studies available. None of the observational studies or trials was truly population-based, as detailed in Appendix II (Hoteit et al., 2014; Al-Mahroos et al., 2013; Khalesi et al., 2012; Khuri-Bulos et al., 2013; Fouda et al., 2017; Mohamed and Al-Shehri, 2013; Djennane et al., 2014; Shady et al., 2015; Ebrahimi et al., 2014; Kelishadi et al., 2014; Nikooyeh et al., 2017; Nichols et al., 2012; Al-Daghri et al., 2015; Al-Daghri et al., 2016; Al Shaikh et al., 2016; Kaddam et al., 2017; Sulimani et al., 2016; Korchia et al., 2013; Bezrati et al., 2016; Narchi et al., 2015; Rajah et al., 2012; Al-Sahlawi et al., 2014; Botros et al., 2015; Khalessi et al., 2015; Naseh et al., 2017; Al-Ajlan et al., 2015; Golbahar et al., 2013; Ardeshir Larijani et al., 2014; Esteghamati et al., 2014; Jaddou et al., 2012; Alfawaz et al., 2014; Ardawi et al., 2012; Tuffaha et al., 2015; Zhang et al., 2016; El Maghraoui et al., 2012; Saliba et al., 2012a; El-Menyar et al., 2012; Sayed-Hassan et al., 2014; Sridhar et al., 2016; Hosseini et al., 2014; Saliba et al., 2012b). Although we only selected relatively large studies (trials with ≥50 participants per arm, and observational studies with ≥100 participants), sample size calculations and power analyses were missing from several reports. There was a large heterogeneity in 25(OH)D assays used, and only few studies reported 25(OH)D results obtained with the superior method of LCMS/MS. This is an important limitation considering the high variability in 25(OH)D assays and for many limited accuracy. Indeed, none of the reports mentioned participation in an external vitamin D quality assurance program, and the number of participating laboratories, with the exception of a couple of countries, in DEQAS and VDSP was quite low. The risk of bias of RCTs was high, with the exception of a few (El-Hajj Fuleihan et al., 2016; Saad et al., 2018; Shehata, 2016), and although it was not independently assessed by 2 co-authors, this causes serious concerns regarding the internal validity of the trials reported. In studies where mean 25(OH)D levels were not reported, we assumed that the mean and median are the same. Reporting of patients' compliance and adverse events was scarce, and data on the safety of moderate to high vitamin D doses is therefore still lacking.
In summary, most RCTs identified the impact of vitamin D supplementation on serum 25(OH)D levels as the main outcome, and no study has evaluated its effect of supplementation on fractures or falls. Furthermore, the few guidelines available are mostly based on expert opinion. Given the large variability in fracture risk worldwide (Ballane et al., 2014; Kanis et al., 2012), and the high prevalence of hypovitaminosis D in the MENA region, the significance of which is unclear, large randomized controlled trials from the MENA region are needed to confirm the applicability of findings from Western countries, and to enable the rigorous development of region-specific vitamin D guidelines.
The following are the supplementary data related to this article.
Methodology.
Observational studies on prevalence of hypovitaminosis D in middle eastern countries (2012‐2017).
Overview of ongoing vitamin D trials in MENA listed in clinicaltrials.gov by age group and countries.
Conflicts of interest statement
Nothing to disclose.
Transparency document
Transparency document.
Acknowledgements
We would like to thank Miss Aida Farha, Medical Information Specialist, Saab Medical Library at the American University of Beirut - Lebanon, for her advice and assistance in designing comprehensive and complex searches of the various medical literature resources and for the provision of articles, Drs Sami Sanjad and Lamyah Atweh for their sharing of the rickets case and radiographs, and Mr Ali Hamoudi for his help with the manuscript figures. We are grateful for the expert input of Dr Hubert Vesper (VDSP, CDC, USA), Dr Chris Sempos (NIH, Office of Dietary Supplements, USA), and Mr Graham Carter (DEQAS, UK) on the vitamin D assay section. Research reported in this publication was supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health under Award Number D43 TW009118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Ahmadi N., Mortazavi M., Iraj B., Askari G. Whether vitamin D3 is effective in reducing proteinuria in type 2 diabetic patients? J. Res. Med. Sci. 2013;18(5):374–377. [PMC free article] [PubMed] [Google Scholar]
- Ahn J., Yu K., Stolzenberg-Solomon R. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M., Mosazadeh M., Lankarani K.B. The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res. 2017;49(9):647–653. doi: 10.1055/s-0043-115225. [DOI] [PubMed] [Google Scholar]
- Al Shaikh A.M., Abaalkhail B., Soliman A. Prevalence of vitamin D deficiency and calcium homeostasis in Saudi children. J. Clin. Res. Pediatr. Endocrinol. 2016;8(4):461–467. doi: 10.4274/jcrpe.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ajlan A., Krishnaswamy S., Alokail M.S. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Dermatol. 2015;15:314. doi: 10.1186/s12884-015-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ali H., El-Hajj Fuleihan G. Nutritional osteomalacia: substantial clinical improvement and gain in bone density posttherapy. J. Clin. Densitom. 2000;3(1):97–101. doi: 10.1385/jcd:3:1:097. [DOI] [PubMed] [Google Scholar]
- Al-Daghri N.M., Al-Saleh Y., Aljohani N. Vitamin D deficiency and cardiometabolic risks: a juxtaposition of Arab adolescents and adults. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daghri N.M., Al-Saleh Y., Khan N. Sun exposure, skin color and vitamin D status in Arab children and adults. J. Steroid Biochem. Mol. Biol. 2016;164:235–238. doi: 10.1016/j.jsbmb.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Al-Daghri N.M., Al-Saleh Y., Aljohani N. Vitamin D status correction in Saudi Arabia: an experts' consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO) Arch. Osteoporos. 2017;12(1):1–8. doi: 10.1007/s11657-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfawaz H., Tamim H., Alharbi S., Aljaser S., Tamimi W. Vitamin D status among patients visiting a tertiary care center in Riyadh, Saudi Arabia: a retrospective review of 3475 cases. BMC Dermatol. 2014;14:159. doi: 10.1186/1471-2458-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mahroos F., Nagalla D.S., Sandhu A.K. Prevalence and risk factors for vitamin D deficiency among mothers in labor and their newborns. Bahrain Med. Bull. 2013;35(2):60–65. [Google Scholar]
- Al-Sahlawi H.S., Al-Haddad F.A., Al-Mahroos F., Al-Amer E. The impact of dietary intake and sun exposure on vitamin D deficiency among couples. Bahrain Med. Bull. 2014;36(1):33–37. [Google Scholar]
- Al-Sofiani M.E., Jammah A., Racz M. Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: a double blind, randomized clinical trial. Int. J. Endocrinol. Metab. 2015;13(1) doi: 10.5812/ijem.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zahrani M.K., Elnasieh A.M., Alenezi F.M. A 3-month oral vitamin D supplementation marginally improves diastolic blood pressure in Saudi patients with type 2 diabetes mellitus. Int. J. Clin. Exp. Med. 2014;7(12):5421–5428. [PMC free article] [PubMed] [Google Scholar]
- Arabi A., El Rassi R., El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat. Rev. Endocrinol. 2010;6(10):550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- Arabi A., Khoueiry-Zgheib N., Awada Z. CYP2R1 polymorphisms are important modulators of circulating 25‑hydroxyvitamin D levels in elderly females with vitamin insufficiency, but not of the response to vitamin D supplementation. Osteoporos. Int. 2017;28(1):279–290. doi: 10.1007/s00198-016-3713-5. [DOI] [PubMed] [Google Scholar]
- Ardawi M.S., Sibiany A.M., Bakhsh T.M., Qari M.H., Maimani A.A. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos. Int. 2012;23(2):675–686. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- Ardeshir Larijani F., Kalantar Motamedi S.M., Keshtkar A.A. The relation between serum vitamin D levels and blood pressure: a population-based study. Acta Med. Iran. 2014;52(4):290–297. [PubMed] [Google Scholar]
- Avenell A., Mak J.C., O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014;4 doi: 10.1002/14651858.CD000227.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballane G., Cauley J.A., Luckey M.M., Fuleihan Gel H. Secular trends in hip fractures worldwide: opposing trends east versus west. J. Bone Miner. Res. 2014;29(8):1745–1755. doi: 10.1002/jbmr.2218. [DOI] [PubMed] [Google Scholar]
- Barake M., Daher R.T., Salti I. 25‑Hydroxyvitamin D assay variations and impact on clinical decision making. J. Clin. Endocrinol. Metab. 2012;97(3):835–843. doi: 10.1210/jc.2011-2584. [DOI] [PubMed] [Google Scholar]
- Bassil D., Rahme M., Hoteit M., El-Hajj Fuleihan G. Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors and impact on outcomes. Dermato-Endocrinol. 2013;5(2):274–298. doi: 10.4161/derm.25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begay R.A.Z.A., Danshiyour N. The effect of calcium—vitamin D on the success of ovulation induction in infertile women with polycystic ovary syndrome. IJOGI. 2012;15(14):7–13. [Google Scholar]
- Behjat Sasan S., Zandvakili F., Soufizadeh N., Baybordi E. The effects of vitamin D supplement on prevention of recurrence of preeclampsia in pregnant women with a history of preeclampsia. Obstet. Gynecol. Int. 2017;2017:8249–8264. doi: 10.1155/2017/8249264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezrati I., Ben Fradj M.K., Ouerghi N., Feki M., Chaouachi A., Kaabachi N. Vitamin D inadequacy is widespread in Tunisian active boys and is related to diet but not to adiposity or insulin resistance. Libyan J. Med. 2016;11 doi: 10.3402/ljm.v11.31258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley N., Carter G.D. Toward clarity in clinical vitamin D status assessment. Endocrinol. Metab. Clin. 2017;46(4):885–899. doi: 10.1016/j.ecl.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Binkley N., Sempos C.T. Standardizing vitamin D assays: the way forward. J. Bone Miner. Res. 2014;29(8):1709–1714. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G., Gluud L.L., Nikolova D. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 2014;(6) doi: 10.1002/14651858.CD007469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland M.J., Grey A., Gamble G.D., Reid I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–320. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- Botros R.M., Sabry I.M., Abdelbaky R.S., Eid Y.M., Nasr M.S., Hendawy L.M. Vitamin D deficiency among healthy Egyptian females. Endocrinol. Nutr. 2015;62(7):314–321. doi: 10.1016/j.endonu.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Breslavsky A., Frand J., Matas Z., Boaz M., Barnea Z., Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin. Nutr. 2013;32(6):970–975. doi: 10.1016/j.clnu.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Carter G.D., Berry J., Durazo-Arvizu R. Hydroxyvitamin D assays: an historical perspective from DEQAS. J. Steroid Biochem. Mol. Biol. 2017 doi: 10.1016/j.jsbmb.2017.07.018. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Cashman K.D., Fitzgerald A.P., Kiely M., Seamans K.M. A systematic review and meta-regression analysis of the vitamin D intake-serum 25‑hydroxyvitamin D relationship to inform European recommendations. Br. J. Nutr. 2011;106(11):1638–1648. doi: 10.1017/S0007114511005058. [DOI] [PubMed] [Google Scholar]
- Chakhtoura M., Akl E.A., El Ghandour S. Impact of vitamin D replacement in adults and elderly in the Middle East and North Africa: a systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2017;28(1):35–46. doi: 10.1007/s00198-016-3837-7. [DOI] [PubMed] [Google Scholar]
- Chakhtoura M., El Ghandour S., Shawwa K. Vitamin D replacement in children, adolescents and pregnant women in the Middle East and North Africa: a systematic review and meta-analysis of randomized controlled trials. Metabolism. 2017;70:160–176. doi: 10.1016/j.metabol.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Kunutsor S., Vitezova A. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348 doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L., Bertoldo F., Bischoff-Ferrari H.A. Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: research for evidence and a scientific statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Endocrine. 2017;56(2):245–261. doi: 10.1007/s12020-017-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon P., Horsman A., Kirby P.A., Dibble J., Bashir T. Mineral content of the forearms of babies born to Asian and white mothers. Br. Med. J. 1983;286(6373):1233–1235. doi: 10.1136/bmj.286.6373.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Harvey N.C., Bishop N.J. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(5):393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawodu A., Saadi H.F., Bekdache G., Javed Y., Altaye M., Hollis B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J. Clin. Endocrinol. Metab. 2013;98(6):2337–2346. doi: 10.1210/jc.2013-1154. [DOI] [PubMed] [Google Scholar]
- DEQAS Vitamin D External Quality Assessment Scheme. http://www.deqas.org/ available from. (Last Accessed January, 2017)
- De-Regil L.M., Palacios C., Lombardo L.K., Pena-Rosas J.P. Vitamin D supplementation for women during pregnancy. Sao Paulo Med. J. 2016;134(3):274–275. doi: 10.1590/1516-3180.20161343T2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes M.E., Bezerra F.F., Rezende E.P., Donangelo C.M. Calcium plus vitamin D supplementation during the third trimester of pregnancy in adolescents accustomed to low calcium diets does not affect infant bone mass at early lactation in a randomized controlled trial. J. Nutr. 2015;145(7):1515–1523. doi: 10.3945/jn.114.208140. [DOI] [PubMed] [Google Scholar]
- Djennane M., Lebbah S., Roux C., Djoudi H., Cavalier E., Souberbielle J.C. Vitamin D status of schoolchildren in northern Algeria, seasonal variations and determinants of vitamin D deficiency. Osteoporos. Int. 2014;25(5):1493–1502. doi: 10.1007/s00198-014-2623-7. [DOI] [PubMed] [Google Scholar]
- Ebrahimi M., Khashayar P., Keshtkar A. Prevalence of vitamin D deficiency among Iranian adolescents. J. Pediatr. Endocrinol. Metab. 2014;27(7–8):595–602. doi: 10.1515/jpem-2013-0428. [DOI] [PubMed] [Google Scholar]
- El Maghraoui A., Ouzzif Z., Mounach A. Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Dermatol. 2012;12:11. doi: 10.1186/1472-6874-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G., Nabulsi M., Tamim H. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J. Clin. Endocrinol. Metab. 2006;91(2):405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G., Bouillon R., Clarke B. Serum 25‑hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J. Bone Miner. Res. 2015;30(7):1119–1133. doi: 10.1002/jbmr.2536. [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G., Baddoura R., Habib R.H. Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am. J. Clin. Nutr. 2016;104(2):315–323. doi: 10.3945/ajcn.116.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Menyar A., Rahil A., Dousa K. Low vitamin D and cardiovascular risk factors in males and females from a sunny, rich country. Open Cardiovasc. Med. J. 2012;6:76–80. doi: 10.2174/1874192401206010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rassi R., Baliki G., El-Hajj Fuleihan G. American University of Beirut Medical Center, Department of Internal Medicine; Beirut, Lebanon: 2012. Vitamin D Status in Middle East and Africa. available from www/of bone heart org/site/default/files, pdf/Vitamin-D-M East-Africa pdf. (Last Accessed December 29, 2017) [Google Scholar]
- Esteghamati A., Aryan Z., Esteghamati A., Nakhjavani M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabete Metab. 2014;40(5):347–355. doi: 10.1016/j.diabet.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Etemadifar M., Janghorbani M. Efficacy of high-dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: preliminary findings of a randomized-controlled trial. Iran. J. Neurol. 2015;14(2):67–73. [PMC free article] [PubMed] [Google Scholar]
- Firouzabadi R., Aflatoonian A., Modarresi S., Sekhavat L., MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement. Ther. Clin. Pract. 2012;18(2):85–88. doi: 10.1016/j.ctcp.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Ford J.A., MacLennan G.S., Avenell A., Bolland M., Grey A., Witham M. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutr. 2014;100(3):746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- Fouda M.A., Turkestani I.Z., Almusharraf S. Extremely high prevalence of maternal and neonatal vitamin D deficiency in the Arab population. Neonatology. 2017;112(3):225–230. doi: 10.1159/000475704. [DOI] [PubMed] [Google Scholar]
- Gendelman O., Itzhaki D., Makarov S., Bennun M., Amital H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus. 2015;24(4–5):483–489. doi: 10.1177/0961203314558676. [DOI] [PubMed] [Google Scholar]
- Ghavamzadeh S., Mobasseri M., Mahdavi R. The effect of vitamin D supplementation on adiposity, blood glycated hemoglobin, serum leptin and tumor necrosis factor-alpha in type 2 diabetic patients. Int. J. Prev. Med. 2014;5(9):1091–1098. [PMC free article] [PubMed] [Google Scholar]
- Ghazal N., Al-Shaar L., Maalouf J. Persistent effect of vitamin D supplementation on musculoskeletal parameters in adolescents one year after trial completion. J. Bone Miner. Res. 2016;31(7):1473–1480. doi: 10.1002/jbmr.2802. [DOI] [PubMed] [Google Scholar]
- Ghazi A.A., Hosseinpanah F., MA E., Ghazi S., Hedayati M., Azizi F. Effects of different doses of oral cholecalciferol on serum 25(OH)D, PTH, calcium and bone markers during fall and winter in schoolchildren. Eur. J. Clin. Nutr. 2010;64(12):1415–1422. doi: 10.1038/ejcn.2010.169. [DOI] [PubMed] [Google Scholar]
- Golan D., Staun-Ram E., Glass-Marmor L. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav. Immun. 2013;32:180–185. doi: 10.1016/j.bbi.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Golbahar J., Al-Saffar N., Diab D.A., Al-Othman S., Darwish A. Vitamin D status in adults: a cross sectional study. Bahrain Med. Bull. 2013;35(1):17–22. [Google Scholar]
- Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]http://handbook-5-1.cochrane.org/ available from. (Last Accessed January 2, 2018) [Google Scholar]
- Haq A., Wimalawansa S.J., Pludowski P., Anouti F.A. Clinical practice guidelines for vitamin D in the United Arab Emirates. J. Steroid Biochem. Mol. Biol. 2018;175:4–11. doi: 10.1016/j.jsbmb.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Hilger J., Friedel A., Herr R. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Hosseini S.R., Cumming R.G., Kheirkhah F. Cohort profile: the Amirkola Health and Ageing Project (AHAP) Int. J. Epidemiol. 2014;43(5):1393–1400. doi: 10.1093/ije/dyt089. [DOI] [PubMed] [Google Scholar]
- Hoteit M., Al-Shaar L., Yazbeck C., Bou Sleiman M., Ghalayini T., El-Hajj Fuleihan G. Hypovitaminosis D in a sunny country: time trends, predictors, and implications for practice guidelines. Metabolism. 2014;63(7):968–978. doi: 10.1016/j.metabol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Hypponen E., Cavadino A., Williams D. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann. Nutr. Metab. 2013;63(4):331–340. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- Jaddou H.Y., Batieha A.M., Khader Y.S., Kanaan S.H., El-Khateeb M.S., Ajlouni K.M. Depression is associated with low levels of 25‑hydroxyvitamin D among Jordanian adults: results from a national population survey. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262(4):321–327. doi: 10.1007/s00406-011-0265-8. [DOI] [PubMed] [Google Scholar]
- Jozanikohan Z., Kazemi Saleh D.A. Semi-experimental study to assess whether the current recommended protocol for treating vitamin D deficiency is enough? Iran Red Crescent Med J. 2015;17(7) doi: 10.5812/ircmj.22779v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddam I.M., Al-Shaikh A.M., Abaalkhail B.A. Prevalence of vitamin D deficiency and its associated factors in three regions of Saudi Arabia. Saudi Med. J. 2017;38(4):381–390. doi: 10.15537/smj.2017.4.18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamelian T., Saki F., Jeddi M., Dabbaghmanesh M.H., Omrani G.H.R. Effect of cholecalciferol therapy on serum FGF23 in vitamin D deficient patients: a randomized clinical trial. J. Endocrinol. Investig. 2017 doi: 10.1007/s40618-017-0739-2. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Oden A., McCloskey E.V., Johansson H., Wahl D.A., Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012;23(9):2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamali M., Beihaghi E., Mohammadi A.A., Asemi Z. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm. Metab. Res. 2015;47(12):867–872. doi: 10.1055/s-0035-1548835. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Ardalan G., Motlagh M.E. National report on the association of serum vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): the CASPIAN-III Study. Nutrition. 2014;30(1):33–38. doi: 10.1016/j.nut.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Keum N., Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br. J. Cancer. 2014;111(5):976–980. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi N., Bahaeddini S.M., Shariat M. Prevalence of maternal vitamin D deficiency in neonates with delayed hypocalcaemia. Acta Med. Iran. 2012;50(11):740–745. [PubMed] [Google Scholar]
- Khalessi N., Kalani M., Araghi M., Farahani Z. The relationship between maternal vitamin D deficiency and low birth weight neonates. J Family Reprod Health. 2015;9(3):113–117. [PMC free article] [PubMed] [Google Scholar]
- Khuri-Bulos N., Lang R.D., Blevins M. Vitamin D deficiency among newborns in Amman, Jordan. Glob. J. Health Sci. 2013;6(1):162–171. doi: 10.5539/gjhs.v6n1p162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchia G., Amitai Y., Moshe G. Vitamin D deficiency in children in Jerusalem: the need for updating the recommendation for supplementation. Isr. Med. Assoc. J. 2013;15(7):333–338. [PubMed] [Google Scholar]
- Laboratory Quality Assurance and Standardization Program. https://www.cdc.gov/labstandards/hs.html available from. (Last Accessed January, 2017)
- Lebanese Society of Osteoporosis and Metabolic Bone Disorders, available from http://website.aub.edu.lb/fm/cmop/downloads/vitD_g.pdf. (Last Accessed Januray 2018) (2012).
- Lee C.J., Iyer G., Liu Y. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J. Diabetes Complicat. 2017;31(7):1115–1126. doi: 10.1016/j.jdiacomp.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 2010;121(1–2):297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Maalouf G., Gannage-Yared M.H., Ezzedine J. Middle East and North Africa consensus on osteoporosis. J. Musculoskelet. Neuronal Interact. 2007;7(2):131–143. [PubMed] [Google Scholar]
- Malloy P.J., Feldman D. Genetic disorders and defects in vitamin D action. Endocrinol. Metab. Clin. N. Am. 2010;39(2):333–346. doi: 10.1016/j.ecl.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P.J., Zhang C., Tang L. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int. J. Cardiol. 2013;169(2):106–111. doi: 10.1016/j.ijcard.2013.08.055. [DOI] [PubMed] [Google Scholar]
- Mayan I., Somech R., Lev A., Cohen A.H., Constantini N.W., Dubnov-Raz G. Thymus activity, vitamin D, and respiratory infections in adolescent swimmers. Isr. Med. Assoc. J. 2015;17(9):571–575. [PubMed] [Google Scholar]
- Mazahery H., Camargo C.A., Jr., Conlon C., Beck K.L., Kruger M.C., von Hurst P.R. Vitamin D and autism spectrum disorder: a literature review. Nutrients. 2016;8(4):236. doi: 10.3390/nu8040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENA Countries Definition World Bank http://www.worldbank.org/en/region/mena available from. (Last Accessed Januray 03 2018)
- Mineva E.M., Schleicher R.L., Chaudhary-Webb M. A candidate reference measurement procedure for quantifying serum concentrations of 25‑hydroxyvitamin D(3) and 25‑hydroxyvitamin D(2) using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015;407(19):5615–5624. doi: 10.1007/s00216-015-8733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhosseini N., Vatanparast H., Mazidi M., Kimball S.M. The effect of improved serum 25‑hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J. Clin. Endocrinol. Metab. 2017;102(9):3097–3110. doi: 10.1210/jc.2017-01024. [DOI] [PubMed] [Google Scholar]
- Moghassemi S., Marjani A. The effect of short-term vitamin D supplementation on lipid profile and blood pressure in post-menopausal women: a randomized controlled trial. Iran. J. Nurs. Midwifery Res. 2014;19(5):517. [PMC free article] [PubMed] [Google Scholar]
- Mohamed W.A., Al-Shehri M.A. Cord blood 25‑hydroxyvitamin D levels and the risk of acute lower respiratory tract infection in early childhood. J. Trop. Pediatr. 2013;59(1):29–35. doi: 10.1093/tropej/fms042. [DOI] [PubMed] [Google Scholar]
- Mohammad-Alizadeh-Charandabi S., Mirghafourvand M., Froghy L., Javadzadeh Y., Razmaraii N. The effect of multivitamin supplements on continuation rate and side effects of combined oral contraceptives: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2015;20(5):361–371. doi: 10.3109/13625187.2015.1010115. [DOI] [PubMed] [Google Scholar]
- Mojibian M., Soheilykhah S., Zadeh M.A.F., Moghadam M.J. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iran. J. Reprod. Med. 2015;13(11):687–696. [PMC free article] [PubMed] [Google Scholar]
- Narchi H., Kochiyil J., Al Hamad S., Yasin J., Laleye L., Al Dhaheri A. Hypovitaminosis D in adolescent females-an analytical cohort study in the United Arab Emirates. Paediatr. Int. Child Health. 2015;35(1):36–43. doi: 10.1179/2046905514Y.0000000144. [DOI] [PubMed] [Google Scholar]
- Naseh A., Ashrafzadeh S., Rassi S. Prevalence of vitamin D deficiency in pregnant mothers in Tehran and investigating its association with serum glucose and insulin. J. Matern. Fetal Neonatal Med. 2017:1–7. doi: 10.1080/14767058.2017.1342796. [DOI] [PubMed] [Google Scholar]
- Nasri H., Behradmanesh S., Maghsoudi A.R., Ahmadi A., Nasri P., Rafieian-Kopaei M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J. Renal Inj. Prev. 2014;3(1):31–34. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyestani T.R., Hajifaraji M., Omidvar N. Calcium-vitamin D-fortified milk is as effective on circulating bone biomarkers as fortified juice and supplement but has less acceptance: a randomised controlled school-based trial. J. Hum. Nutr. Diet. 2014;27(6):606–616. doi: 10.1111/jhn.12191. [DOI] [PubMed] [Google Scholar]
- Nichols E.K., Khatib I.M., Aburto N.J. Vitamin D status and determinants of deficiency among non-pregnant Jordanian women of reproductive age. Eur. J. Clin. Nutr. 2012;66(6):751–756. doi: 10.1038/ejcn.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikooyeh B., Abdollahi Z., Hajifaraji M. Vitamin D status, latitude and their associations with some health parameters in children: national food and nutrition surveillance. J. Trop. Pediatr. 2017;63(1):57–64. doi: 10.1093/tropej/fmw057. [DOI] [PubMed] [Google Scholar]
- Pooled Standard Deviation Calculation. http://wps.aw.com/wps/media/objects/15/15512/formulas.pdf available from. (Last Accessed in January 2018)
- Raef H., Al-Bugami M., Balharith S. Updated recommendations for the diagnosis and management of osteoporosis: a local perspective. Ann. Saudi Med. 2011;31(2):111–128. doi: 10.4103/0256-4947.77502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme M., Sharara S.L., Baddoura R. Impact of calcium and two doses of vitamin D on bone metabolism in the elderly: a randomized controlled trial. J. Bone Miner. Res. 2017;32(7):1486–1495. doi: 10.1002/jbmr.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah J., Haq A., Pettifor J.M. Vitamin D and calcium status in urban children attending an ambulatory clinic service in the United Arab Emirates. Dermato-Endocrinol. 2012;4(1):39–43. doi: 10.4161/derm.18250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi B., Haghollahi F., Shariat M., Zayerii F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan. J. Obstet. Gynecol. 2009;48(2):142–147. doi: 10.1016/S1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- Ross A.C., Manson J.E., Abrams S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad K., Abdel-Rahman A.A., Elserogy Y.M. Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. J. Child Psychol. Psychiatry. 2018;59(1):20–29. doi: 10.1111/jcpp.12652. [DOI] [PubMed] [Google Scholar]
- Sabet Z., Ghazi A., Tohidi M., Oladi B. Vitamin d supplementation in pregnant iranian women: effects on maternal and neonatal vitamin D and parathyroid hormone status. Acta Endocrinol. 2012;8(1) [Google Scholar]
- Salehpour A., Hosseinpanah F., Shidfar F. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutr. J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba W., Barnett O., Stein N., Kershenbaum A., Rennert G. The longitudinal variability of serum 25(OH)D levels. Eur. J. Intern. Med. 2012;23(4):e106–11. doi: 10.1016/j.ejim.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Saliba W., Rennert H.S., Kershenbaum A., Rennert G. Serum 25(OH)D concentrations in sunny Israel. Osteoporos. Int. 2012;23(2):687–694. doi: 10.1007/s00198-011-1597-y. [DOI] [PubMed] [Google Scholar]
- Samimi M., Kashi M., Foroozanfard F. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016;29(4):505–515. doi: 10.1111/jhn.12339. [DOI] [PubMed] [Google Scholar]
- Samimi M., Foroozanfard F., Amini F., Sehat M. Effect of vitamin D supplementation on unexplained recurrent spontaneous abortion: a double-blind randomized controlled trial. Glob. J. Health Sci. 2016;9(3):95–102. [Google Scholar]
- Sayed-Hassan R., Abazid N., Alourfi Z. Relationship between 25‑hydroxyvitamin D concentrations, serum calcium, and parathyroid hormone in apparently healthy Syrian people. Arch. Osteoporos. 2014;9:176. doi: 10.1007/s11657-014-0176-1. [DOI] [PubMed] [Google Scholar]
- Sedrani S.H., Elidrissy A.W., El Arabi K.M. Sunlight and vitamin D status in normal Saudi subjects. Am. J. Clin. Nutr. 1983;38(1):129–132. doi: 10.1093/ajcn/38.1.129. [DOI] [PubMed] [Google Scholar]
- Shady M.M., Youssef M.M., Shehata M.A., El-Din E.M., ElMalt H.A. Association of serum 25‑hydroxyvitamin D with life style and dietary factors in Egyptian prepubescent children. Macedonian J. Med. Sci. 2015;3(1):80–84. doi: 10.3889/oamjms.2015.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahgheibi S., Farhadifar F., Pouya B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: results from a randomized placebo-controlled trial. J. Res. Med. Sci. 2016;21 doi: 10.4103/1735-1995.175148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba M., Iranmanesh M.R. Vitamin D requirement in pregnancy to prevent deficiency in neonates: a randomised trial. Singap. Med. J. 2013;54(5):285–288. doi: 10.11622/smedj.2013110. [DOI] [PubMed] [Google Scholar]
- Sharifi N., Amani R., Hajiani E., Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- Shehata N.A. Calcium versus oral contraceptive pills containing drospirenone for the treatment of mild to moderate premenstrual syndrome: a double blind randomized placebo controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;198:100–104. doi: 10.1016/j.ejogrb.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Soheilykhah S., Mojibian M., Moghadam M.J., Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol. Endocrinol. 2013;29(4):396–399. doi: 10.3109/09513590.2012.752456. [DOI] [PubMed] [Google Scholar]
- Sperati F., Vici P., Maugeri-Sacca M. Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S.B., Rao P.G., Multani S.K., Jain M. Assessment of prevalence of hypovitaminosis D in multiethnic population of the United Arab Emirates. J. Adv. Pharm. Technol. Res. 2016;7(2):48–53. doi: 10.4103/2231-4040.177202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepman H.C., Vanderroost A., Van Uytfanghe K., Thienpont L.M. Candidate reference measurement procedures for serum 25‑hydroxyvitamin D3 and 25‑hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin. Chem. 2011;57(3):441–448. doi: 10.1373/clinchem.2010.152553. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Brefka S., Denkinger M.D. What works to prevent falls in community-dwelling older adults? Umbrella review of meta-analyses of randomized controlled trials. Phys. Ther. 2015;95(8):1095–1110. doi: 10.2522/ptj.20140461. [DOI] [PubMed] [Google Scholar]
- Sulimani R.A., Mohammed A.G., Alfadda A.A. Vitamin D deficiency and biochemical variations among urban Saudi adolescent girls according to season. Saudi Med. J. 2016;37(9):1002–1008. doi: 10.15537/smj.2016.9.15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri M., Baheiraei A., Rahimi Foroushani A., Modarres M., Resolving Vitamin D. Deficiency in the preconception period among high-risk reproductive women: a randomized controlled trial. Iran Red Crescent Med J. 2014;16(1) doi: 10.5812/ircmj.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri M., Baheiraei A., Foroushani A.R., Nikmanesh B., Modarres M. Treatment of vitamin D deficiency is an effective method in the elimination of asymptomatic bacterial vaginosis: a placebo-controlled randomized clinical trial. Indian J. Med. Res. 2015;141(6):799–806. doi: 10.4103/0971-5916.160707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat I.M., Kamal N.M., Alghamdi H.A., Alharthi A.A., Alshahrani M.A. A randomized clinical trial comparing 3 different replacement regimens of vitamin D in clinically asymptomatic pediatrics and adolescents with vitamin D insufficiency. Ital. J. Pediatr. 2016;42(1):106–114. doi: 10.1186/s13052-016-0314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavera-Mendoza L.E., White J.H. Cell defenses and the sunshine vitamin. Sci. Am. 2007;297(5) doi: 10.1038/scientificamerican1107-62. (62-5, 8-70, 2) [DOI] [PubMed] [Google Scholar]
- Tehrani H.G., Mostajeran F., Shahsavari S. The effect of calcium and vitamin D supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with poly cystic ovarian syndrome. J. Res. Med. Sci. 2014;19(9):875–880. [PMC free article] [PubMed] [Google Scholar]
- Tepper S., Shahar D.R., Geva D., Ish-Shalom S. Differences in homeostatic model assessment (HOMA) values and insulin levels after vitamin D supplementation in healthy men: a double-blind randomized controlled trial. Diabetes Obes. Metab. 2016;18(6):633–637. doi: 10.1111/dom.12650. [DOI] [PubMed] [Google Scholar]
- Tuffaha M., El Bcheraoui C., Daoud F. Deficiencies under plenty of sun: vitamin D status among adults in the Kingdom of Saudi Arabia, 2013. N. Am. J. Med. Sci. 2015;7(10):467–475. doi: 10.4103/1947-2714.168675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schoor N., Lips P. Global overview of vitamin D status. Endocrinol. Metab. Clin. N. Am. 2017;46(4):845–870. doi: 10.1016/j.ecl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Vaziri F., Dabbaghmanesh M.H., Samsami A., Nasiri S., Shirazi P.T. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: a randomized placebo clinical trial. Early Hum. Dev. 2016;103:61–68. doi: 10.1016/j.earlhumdev.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Vitamin D CDC Certified Laboratories 2017. https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures.pdf available from. (Last Accessed January, 2018)
- Wang T.J., Zhang F., Richards J.B. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.M., Alexander D.D., Boushey C.J. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016;27(1):367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weighted Mean Calculation. http://www.statisticshowto.com/weighted-mean/ available from. (Last Accessed in January 2018)
- Zarrin R., Ayremlou P., Ghassemi F. The effect of vitamin D supplementation on the glycemic status and the percentage of body fat mass in adults with prediabetes: a randomized clinical trial. Iran Red Crescent Med J. 2017;19(3) [Google Scholar]
- Zhang F.F., Al Hooti S., Al Zenki S. Vitamin D deficiency is associated with high prevalence of diabetes in Kuwaiti adults: results from a national survey. BMC Dermatol. 2016;16:100. doi: 10.1186/s12889-016-2758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Zhu J., Zhou M., Cui L., Yao W., Liu Y. Meta-analysis of long-term vitamin D supplementation on overall mortality. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methodology.
Observational studies on prevalence of hypovitaminosis D in middle eastern countries (2012‐2017).
Overview of ongoing vitamin D trials in MENA listed in clinicaltrials.gov by age group and countries.
Transparency document.