Abstract
With the widespread use of measurement of bone mineral density to detect, diagnose, and monitor therapy in the management of osteoporosis, bone histomorphometry has largely been relegated to research settings and academic pursuits. However, bone density measurement cannot distinguish between osteoporosis and other metabolic bone disorders such as different types of osteomalacia, osteitis fibrosa, renal osteodystrophy, hypophosphatasia, and Paget’s disease of bone. Furthermore, bone density test cannot tell us anything about microarchitecture of bone, tissue level dynamics, bone cellular activity, bone mineralization and bone remodeling, understanding of which is essential to make a specific diagnosis of a suspected metabolic bone disease, to evaluate beneficial (or adverse) effects of various therapies, treatment (medical or surgical) decisions in hyperparathyroid states. As a research tool, bone histomorphometry contributed immensely to our understanding of bone biology, revolutionized the study of the mechanism of actions of various therapies, and provided crucial understanding of the adverse effects of drugs.
Keywords: Osteomalacia, Osteoid, Mineralization defect, Bone remodeling, Vitamin D deficiency, Tumor induced osteomalacia, Hereditary hypophosphatemic osteomalacia
Highlights
-
•
Nutritional osteomalacia is rare in the developed countries where routine food fortification is practiced. It frequently escapes recognition, especially in its early stages, because of vague symptoms, non-specofic bone pain, and muscle weakness.
-
•
Abnormal imaging studies may raise the possibility of malignancy as was in the case vignette. However, elevated serum PTH with low normal serum calcium is unusual in patients with metastatic bone disease.
-
•
Bone histomorphometry plays a crucial role in arriving at precise diagnosis and help in therapeutic approach such as high-dose vitamin D therapy.
-
•
Despite impressive and rapid improvements in symptoms, and normalization of mineralization defect on bone biopsy, continued secondary hyperparathyroidism persists for years as in the case vignette.
-
•
Early recognition in the right clinical context will prevent unnecessary testing and irreversible cortical bone loss with increased life-long fracture risk, persistent hyperparathyroidism, and prevent development of tertiary hyperparathyroidism.
With the widespread availability and use of dual energy x-ray absorptiometry (DEXA) to detect, diagnose, and monitor therapy in the management of osteoporosis, bone histomorphometry has largely been relegated to research settings and academic pursuits. However, DEXA cannot distinguish between osteoporosis and several other metabolic bone disorders such as various types of osteomalacia, osteitis fibrosa, uremic osteodystrophy, hypophosphatasia, Paget's disease of bone, etc. Furthermore, DEXA cannot tell us anything about microarchitecture of bone, tissue level dynamics, bone cellular activity, bone mineralization and bone remodeling. Understanding the latter is essential to make a specific diagnosis of a suspected bone disease, to evaluate beneficial (or adverse) effects of various therapies, treatment (medical or surgical) decisions in hyperparathyroid states. As a research tool, bone histomorphometry contributed immensely to our understanding of bone biology (Dempster, 1997; Khosla, 2013; Kulak and Dempster, 2010), revolutionized the study of the mechanism of actions of various therapies (Dempster et al., 2016a; Dempster et al., 2016b), and has been crucial in understanding the rare adverse effects of drugs to be sure (Odvina et al., 2005). To put bone histomorphometry in the proper context, we begin with a case vignette to illustrate the crucial role bone histomorphometry in arriving at precise diagnosis. The clinical presentation is in bold font followed by clinician's assessment during evaluation of the patient in regular font.
1. The case vignette
A 59-year-old white woman was seen in the Bone and Mineral Clinic for evaluation of diffuse bone pain. She reported progressive worsening of rib cage tenderness and lower extremity pain over the past year, which made it difficult for her to ambulate or climb a flight of stairs. She also described generalized muscle weakness and unsteady gait, necessitating the use of a cane.
Her medical history revealed a diagnosis of chronic obstructive pulmonary disease from chronic smoking, requiring intermittent oral steroid therapy. She had undergone a left hip replacement several years prior for aseptic necrosis of the femoral head, presumably due to long-term steroid therapy. A few years later, she sustained a low trauma fracture of the right femoral neck. She received bisphosphonate therapy for presumed diagnosis of “osteoporosis” for at least 3 years, but did not receive any calcium or vitamin D supplementation during the 3 years of bisphosphonate therapy. She gave a history of some weight loss, but no past history of malignancy.
Before referral to the Bone and Mineral Clinic, the patient had several visits to urgent care and emergency rooms for evaluation of bone pain. X-rays at that time showed diffuse osteopenia and suspicious lytic lesions, which prompted a whole body bone scan revealing several “hot spots” throughout the skeleton and symmetrical bilateral uptake of the nucleotide in several ribs. Because of abnormal x-rays and bone scan, increasing diffuse bone pain, and an elevated serum alkaline phosphatase (411 IU/l; reference range 40–140 IU/l), she was referred to oncology for evaluation of an underlying malignancy. She underwent whole body CT, MRI, and a bone marrow biopsy, none of which revealed malignancy. Initial laboratory evaluation by the oncologist showed a serum calcium of 8.5 mg/dl (reference range 8.2–10.2 mg/dl), serum phosphate 3.2 mg/dl (reference range 2.5–4.5 mg/dl), normal renal function (serum creatinine 0.8 mg/dl; reference range < 1.03 mg/dl), and elevated serum bone specific alkaline phosphatase of 187 IU/l (reference range < 15 IU/l). Because of persistently elevated serum total and bone specific alkaline phosphatase and abnormal bone scan, serum PTH was measured and showed a value of 974 pg/ml (reference range 15–65 pg/ml) prompting referral to a bone and mineral specialist for evaluation of possible metabolic bone disease.
The history is highly suggestive of osteomalacia due to vitamin D deficiency considering the poor intake of calcium and vitamin D, findings on physical examination, and the classic appearance on bone scan (bilateral symmetrical uptake in the ribs, an unusual finding in metastatic bone disease). Osteomalacia frequently escapes recognition, especially in its early stages, because of vague symptoms, non-specific bone pain, and muscle weakness. Abnormal imaging studies with elevated serum alkaline phosphatase in a known smoker raises the possibility of malignancy as was suspected in this patient. However, elevated serum PTH, especially an extremely high level, with low normal serum calcium is unusual in patients with metastatic bone disease.
Physical examination in the Bone and Mineral Clinic revealed a woman who looked chronically ill and appeared older than her stated age. She could not arise unaided from the chair and used the arm rest to stand up from a sitting position. She used her cane for ambulation and had a waddling gait characteristic of osteomalacia. There was tenderness to palpation of her upper arms, rib cage, on lateral pressing of the pelvic brim, and on light tapping over the tibial shins. A detailed food and supplement inquiry revealed that her total calcium intake was <300 mg/day and no vitamin D supplementation. Being housebound because of progressive debility, she had minimal or no exposure to sunlight.
Repeat laboratory evaluation in the Bone and Mineral Clinic, one month after the initial testing by the oncologist, confirmed elevated serum total and bone specific alkaline phosphatase (397 and 187 IU/l, respectively) and PTH (758 pg/ml). Serum 25-hydroxyvitamin D was very low at 7 ng/ml, with a remote value of 6 ng/ml 5 years previously.
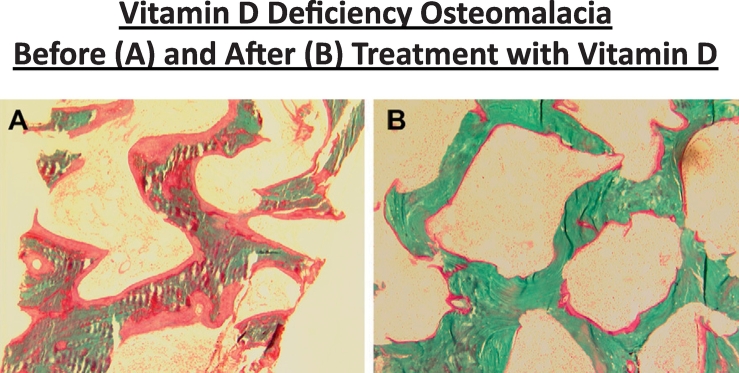
Although the biochemical findings are suggestive of severe vitamin D deficiency and secondary hyperparathyroidism, no specific cause for vitamin D deficiency was found. Therefore, a trans-iliac bone biopsy following double tetracycline labeling was performed to confirm the presence of nutritional osteomalacia, a rare occurrence in this Western part of the world, and to better guide therapeutic approach. Bone histomorphometry confirmed severe osteomalacia with wide osteoid seams and no tetracycline uptake indicating complete cessation of mineralization, conforming to the traditional description of osteomalacia (Fig. 1A).
Fig. 1.
Bone histomorphometry in the Case Vignette. Note wide and extensive osteoid before treatment (A) and almost complete disappearance of osteoid after treatment (B).
A diagnosis was made of severe nutritional vitamin D deficiency osteomalacia due to poor calcium and vitamin D intake and the lack of sunlight exposure because of progressive debility. Detailed evaluation for malabsorption including celiac disease was non-revealing. The patient was treated with weekly doses of ergocalciferol, 50,000 units, and daily calcium supplements for a few months. The dose of vitamin D was progressively reduced as her serum alkaline phosphatase and PTH levels returned towards normal and serum 25-hydroxyvitamin D levels increased. Within 2–3 months, her symptoms improved dramatically and she was able to ambulate without a cane.
One year later, a follow-up bone biopsy showed complete healing of osteomalacia (Fig. 1B). At the time of repeat bone biopsy, serum calcium was 9.8 mg/dl. Both total and bone specific alkaline phosphatase levels returned to within the reference ranges, PTH fell to 99 pg/ml, and serum 25-hydroxyvitamin D rose to 81 ng/ml, as a result of high-dose vitamin D therapy to suppress PTH secretion. However, despite continued high-dose vitamin D therapy, serum PTH levels remained high (80–100 pg/ml) even after 6 years, implying some autonomy of PTH secretion. She never developed hypercalcemia and renal function remained normal throughout vitamin D therapy.
Clinical manifestations of osteomalacia are related to poorly-understood underlying pathogenic mechanisms. The clinical hallmarks of the presentation are vague and diffuse bone pain, progressive muscle weakness mainly of the proximal lower extremities, and difficulty walking with waddling gait. Since the onset of symptoms is almost always insidious, most patients escape early diagnosis with their symptoms initially attributed to a wide variety of illnesses such as arthritis, rheumatism, fibromyalgia, myopathy, and even cancer, as in our patient.
The case highlights several important clinical points of relevance. First, nutritional osteomalacia is commonly assumed to be rare in the Western world, but can occur with some regularity in susceptible individuals (Gloth 3rd and Greenough 3rd., 2004; Gloth 3rd et al., 1991; Prabhala et al., 1999). Second, because of the “similarity” of findings on bone scintigraphy and vague symptoms especially with weight loss, malignancy is often suspected, and the patient is subjected to unnecessary diagnostic procedures resulting in delay in diagnosis. Third, the diagnostic value of bone histomorphometry lies in establishing the precise diagnosis. Fourth, if vitamin D deficiency osteomalacia is not detected early, there is irreversible cortical bone loss (Parfitt et al., 1985a) and increased life-time fracture risk. In the following sections, we review the conceptual pathophysiologic basis and histologic evolution of osteomalacia (Rao et al., 1983; Rao, 1990), and the value of bone histomorphometry (Parfitt et al., 1979) in evaluating different types of osteomalacia.
2. Introduction
Worldwide, nutritional vitamin D deficiency remains the most common cause of osteomalacia, numerically the most frequent, and occurs almost exclusively in parts of the world where vitamin D deficiency is endemic (Bhan et al., 2010; Basha et al., 2000a). Because of its rarity in developed countries, the diagnosis of nutritional osteomalacia is often missed or delayed or both (Basha et al., 2000b), as exemplified by the case vignette. The two principle mechanisms by which osteomalacia develops are: vitamin D deficiency (nutritional or malabsorption), as adequate vitamin D is required for mineralization of newly laid down matrix, and deficiency of phosphate or calcium, the two most abundant mineral components of bone. Hypophosphatemia, however caused, is the most common cause of osteomalacia in the West; its two most common causes are: hereditary hypophosphatemic syndromes (Drezner, 2000) and FGF-23 secreting tumors (Florenzano et al., 2017). Other rare causes of hypophosphatemic osteomalacia include: antacid induced (Boutsen et al., 1996; Kassem et al., 1991), poor nutrition due to critical illness (Honasoge and Rao, 1995), and various acquired and genetic renal tubular defects (Clarke et al., 1995). Even rarer, but histologically distinct from vitamin D and phosphate deficiency osteomalacia, are the atypical and focal osteomalacia (AOM and FOM respectively) due to toxic effects of the drugs used to treat osteoporosis such as sodium fluoride (Lundy et al., 1995; Kiely et al., 1999) and non‑nitrogen containing bisphosphonate, etidronate (Thomas et al., 1995), aluminum containing phosphate binders in patients on dialysis (Ott et al., 1982; Parfitt et al., 1986), and cadmium (Takebayashi et al., 2000). Interestingly, although calcium deficiency rickets (and presumably osteomalacia) has been reported in children (Thacher et al., 1999), osteomalacia solely due to calcium deficiency has not been reported in adults.
3. Osteomalacia: A historical perspective
The term was originally intended for and restricted to the generalized softening of bones and crippling deformities. The histologic differentiation of osteomalacia from osteoporosis and osteitis fibrosa was first made by the German pathologist Pommer in the 19th century based on examination of cadaveric bones, and later by Fuller Albright. Parfitt's restatement based on the current concepts of bone remodeling is probably the most appropriate description (Parfitt, 1998). In the normal course of bone remodeling, a moiety of resorbed old bone is replaced by the same volume of normal lamellar bone in young adults, by a lesser amount of normal lamellar bone in age related osteoporosis, by a complex mixture of woven bone and fibrous tissue in osteitis fibrosa, and by unmineralized bone matrix (or osteoid tissue) in osteomalacia (Parfitt, 1998). Thus, Parfitt's definition explicitly separates osteomalacia from all other types of bone disorders including those that may mimic osteomalacia by clinical, radiologic, or histological features. Harold Frost from the Henry Ford Hospital was probably the first to describe the detailed tetracycline-based bone histomorphometry in rib biopsy specimens from 6 patients with osteomalacia (Ramser et al., 1966).
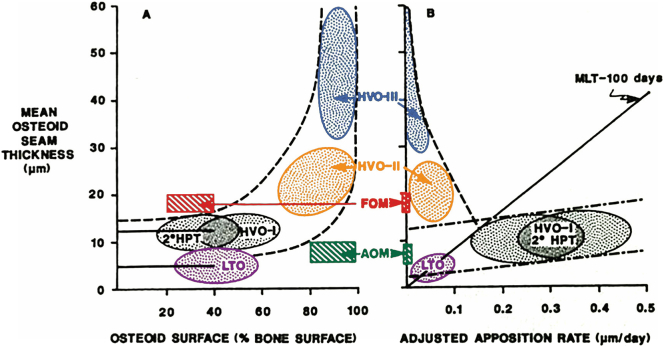
After the discovery of vitamin D, it became obvious that almost all “bone softening” conditions were due to vitamin D deficiency, and by implication, osteomalacia became synonymous with any condition that could be cured by vitamin D but not necessarily caused by its deficiency. Bone disorders that did not respond to “usual doses of vitamin D” were designated as “vitamin D dependent or resistant” (Parfitt, 1972) rickets and osteomalacia, and are now known due to abnormalities in vitamin D action or its metabolism (see other chapters in this issue). Thus, osteomalacia viewed principally as a mineralization defect can manifest in several types, but differ from one another by the characteristic relationships of osteoid thickness with osteoid surface (Fig. 2A) and with adjusted mineral apposition rate (Fig. 2B) (Rao et al., 1983; Rao, 1990); strictly speaking, an increase in only osteoid surface or osteoid thickness is really not osteomalacia. Accordingly, expanding the use of the term “osteomalacia” to include conditions that do not conform to this unique relationship (AOM & FOM in Fig. 2A & B, for instance) obfuscates the original descriptions of the condition with its characteristic clinical, radiographic, scintigraphic, and histologic features. Furthermore, excess osteoid accumulation (surface or volume) can occur in several conditions: states of high bone turnover (primary or secondary hyperparathyroidism and hyperthyroidism) (Silverberg et al., 1989; Rao et al., 1993; Mosekilde and Melsen, 1978; Moore et al., 2009), enzyme defects (hypophosphatasia) (Whyte, 2016; Belkhouribchia et al., 2016), matrix disorders (fibrogenesis imperfecta ossium (Ralphs et al., 1989), Paget's disease of bone (Singer, 2016), and axial osteomalacia (Demiaux-Domenech et al., 1996). In fact, such assumptions have led to the use of large doses of vitamin D to treat some of these disorders in the past! (Kolb, 1959; Bhadada et al., 2017).
Fig. 2.
A & B: Diagrammatic depiction showing the relationship of osteoid thickness with fractional osteoid surface on the left (A) and with adjusted appositional rate on the right (B). There is no relationship between osteoid thickness and surface in normal subjects or in patients with 2°HPT, HVO-i and LTO until the osteoid surface is >70% (straight horizontal lines), after which the relationship is hyperbolic (interrupted curvilinear lines). On the right (B), there is a positive relationship between osteoid thickness and adjusted mineral apposition rate (straight interrupted lines), in normal subjects, and in patients with 2°HPT, HVO-i and LTO. The oblique interrupted line indicates the reversal of this relationship in patients with more severe osteomalacia (HVO-ii and iii), a cardinal feature of osteomalacia unlike all other conditions. The solid straight line represents a mineralization lag time of 100 days that separates patients with and without osteomalacia. Location of each category of osteomalacia is diagrammatically shown for clarity and simplicity. Note significant overlap of 2°HPT, HVO-i and LTO.
2°HPT: secondary hyperparathyroidism; HVO-i,ii,iii: hypovitaminosis D osteopathy stages; FOM: focal osteomalacia; AOM: atypical osteomalacia; LTO: low turnover osteoporosis.
Modified from Rao et al (Rao, 1990).
4. Histologic evolution and definition of vitamin D deficiency osteomalacia
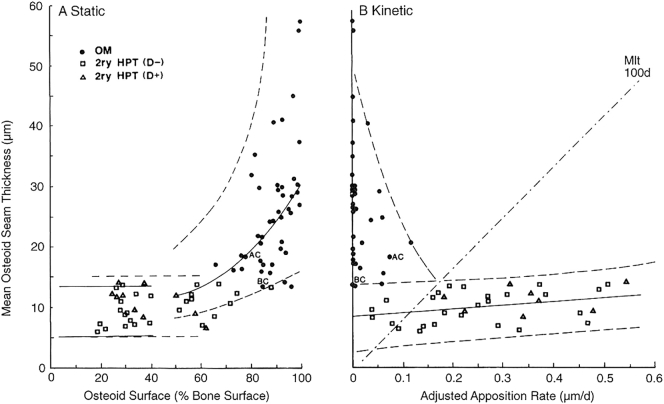
In the development of classical nutritional vitamin D deficiency osteomalacia, the earliest bone histomorphometric finding is an increased bone turnover due to secondary hyperparathyroidism (Parfitt et al., 1985a; Rao et al., 1983), an inevitable consequence of vitamin D depletion. This stage, which we refer to as hypovitaminosis D osteopathy stage-i (HVO-i or pre-osteomalacia), is characterized by an increase mainly in osteoid surface with a slight increase in osteoid thickness (usually <12.5 μm) (Rao et al., 1983; Rao, 1990). Both in normal subjects and in patients with osteoporosis, there is no relationship between osteoid surface and osteoid thickness (Figs. 2A & 3A, Table 1). By contrast, in patients with vitamin D deficiency there is a hyperbolic relationship between these two variables indicating that osteoid surface increases first, and osteoid thicknesses increases only when osteoid surface exceeds >70% of the total bone surface. Thereafter, any further increase in osteoid surface is accompanied by a substantial increase in osteoid thickness (Figs. 2A & 3A) and osteoid volume. However, the relationship between osteoid thickness and adjusted mineral apposition rate as determined by tetracycline labeling, is more complex (Figs. 2B & 3B). When the adjusted mineral apposition rate is >0.15 μm/day, there is a positive (direct, albeit weak) relationship with osteoid thickness (Figs. 2B & 3B, Table 1), but the relationship is inversed when the adjusted apposition rate falls <0.15 μm/day such that osteoid thickness increases in patients with vitamin D deficiency, but decreases in all other conditions (Fig. 2B). A mineralization lag time, an index of the time interval between matrix apposition and its subsequent mineralization, of >100 days separates patients with and without osteomalacia (Figs. 2B & 3B). Accordingly, histologic osteomalacia is defined by a combination of osteoid thickness > 12.5 μm and a mineralization lag time of >100 days (Table 2) (Rao et al., 1983; Rao, 1990). Although a decreased bone mineral density (by x-rays or DEXA) is seen in all patients with nutritional vitamin D deficiency, the latter techniques cannot distinguish the effects of vitamin D deficiency from age-related bone loss. Unless osteomalacia is far advanced with pseudo-fractures on x-rays, bone histomorphometry is required in arriving at the correct diagnosis in the appropriate patient (see the case vignette above). As the degree and duration of vitamin D deficiency progresses, there is progressively more osteoid accumulation with preservation of tetracycline uptake (designated as HVO-ii) indicating some mineral apposition is occurring, and finally mineral apposition ceases without any tetracycline labeling (designated as HVO-iii; Table 2; Fig. 2, Fig. 3) (Rao et al., 1983; Rao, 1990; Parfitt, 1998; Ramser et al., 1966). An improved definition that incorporates several mineralization related indices by bone histomorphometry into a single composite number, the “mineralization index (MI),” has a high sensitivity and specificity (Parfitt et al., 2004) for the diagnosis of osteomalacia (Rao et al., 1983; Rao, 1990) and may even contribute to management strategies (Parfitt et al., 2004).
Fig. 3.
A & B: The description of the figure layout is same as in Fig. 2 with individual patient points with various types of mineralization defects. Open Squares: HVO-i; Open Triangles: 2°HPT without vitamin D deficiency (mainly due to calcium malabsorption); Closed Circles: patients with HVO-ii & iii. AC and BC represent one patient each with osteomalacia due to anticonvulsant therapy and biliary cirrhosis respectively. Modified from (Parfitt, 1998).
Table 1.
Fundamental differences in bone histomorphometry between osteoporosis and osteomalacia.
| Histomorphometric feature | Osteoporosis | Osteomalacia |
|---|---|---|
| Mineralization lag time (Mlt) | <100 days or shorter | >100 days or infinity |
| Osteoid maturation time | Normal | Prolonged |
| Osteoid thickness (O·Th) | Normal/low | Always high (>12.5 μm) |
| O·Th correlation with OS/BS | None | Positive & hyperbolic |
| O·Th correlation with Aj.AR | Weakly positive | Negative & hyperbolic |
| Osteoblast defect | Matrix | Mineral |
Aj.AR: adjusted mineral apposition rate; OS/BS: osteoid and total bone surfaces respectively; see also Fig. 1, Fig. 2). Modified from reference (Parfitt, 1998).
Table 2.
Bone histomorphometric criteria for different morphological forms of osteomalacia and for different stages in the evolution of hypovitaminosis D osteopathy (HVO).
| Type of osteomalacia | Stage | OS/BS (%) | O·Th (μm) | Mlt (days) | OV/BV (%) |
|---|---|---|---|---|---|
| Pre-osteomalacia | HVO-i | 30–70 | <12.5 | <100 | >5a |
| Generalized osteomalacia | HVO-ii | >70 | >12.5 | >100 | >10b |
| Generalized osteomalacia | HVO-iii | >70 | >12.5 | ∞ | >10b |
| Focal osteomalacia | – | <30 | >12.5 | >100 | <5c |
| Atypical osteomalacia | – | >70 | <12.5 | >50 | >5d |
OS/BS: osteoid and total bone surfaces respectively; O·Th: osteoid thickness; Mlt: mineralization lag time; OV/BV: osteoid and bone volume respectively; HVO-i, ii, iii: Hypovitaminosis D Osteopathy stages.
Modified from reference (Rao et al., 1983; Parfitt, 1998).
Note the differences in Mlt in different types of osteomalacia.
OV/BV is increased mainly by an increase in OS with only slightly increase in O·Th.
OV/BV is increased because of an increase both in O·Th and OS.
OV/BV is increased mainly by an increase in O·Th.
OV/BV is increased mainly by an increase OS.
Osteomalacia as defined above, conforms to all of the characteristic clinical, biochemical, and radiologic features traditionally known to be associated with vitamin D deficiency, and can be collectively considered as the “osteomalacia syndrome” (Parfitt, 1998). In addition, the definition incorporates the current concepts of bone remodeling and the mechanisms underlying osteoid accumulation with increasing severity of vitamin D depletion (Parfitt, 1998; Parfitt et al., 1985b). Osteomalacia in its early stage (i.e., HVO-i or pre-osteomalacia) can be diagnosed only by bone histomorphometry before any irreversible cortical bone loss (Parfitt et al., 1985a) and skeletal deformities have occurred by the time osteomalacia is detected by conventional clinical, biochemical, or imaging methods. The use of the term “biochemical osteomalacia” based on a constellation of biochemical findings (low serum calcium and/or phosphate, low serum 25-hydroxyvitamin D, or serum high alkaline phosphatase and/or PTH), or equating “hyperosteoidosis” in a bone biopsy specimen with osteomalacia is not warranted.
Nutritional vitamin D deficiency osteomalacia, as defined, occurs only when the serum 25-hydroxyvitamin D level falls below 10 ng/ml, and almost all patients will have elevated serum levels of alkaline phosphatase and PTH (Bhan et al., 2010; Basha et al., 2000a). However, not everyone with serum 25-hydroxyvitamin D level < 10 ng/ml will have osteomalacia. Although histologic osteomalacia in its late stages can be completely cured, the patient's symptoms resolved, and pseudo-fractures healed with vitamin D therapy, the deformed skeleton (vertebral and pelvic deformities, protrusio-acetabuli) and cortical thinning in long bones are irreversible; hence recognition of osteomalacia in its early stages (i.e., HVO-i) is of paramount importance (Parfitt et al., 1985c).
5. Osteomalacia due to phosphate deficiency
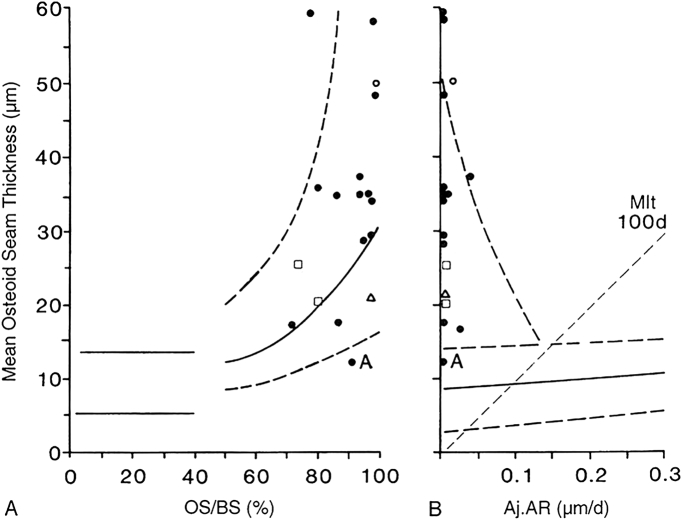
The essential similarities and differences between vitamin D deficiency and phosphate deficiency osteomalacia are contrasted in Table 3, and can be readily explained by the bone remodeling concepts with one exception: we lack bone histomorphometric data at an earlier stage in patients with hypophosphatemic osteomalacia that is analogous to HVO-i. In all varieties of hypophosphatemic osteomalacia, the relationship of osteoid thickness both with osteoid surface and adjusted mineral appositional rate is similar to that seen in HVO-ii & iii, although osteoid thickness is greater, and a higher proportion of patients are in HVO-iii than in HVO-ii (see Fig. 4A & B, Fig. 5, and Table 4). Absence of focal osteomalacia (FOM; Fig. 2) implies that the evolution of osteoid accumulation is similar to that seen in HVO; i.e., osteoid surface increases before an increase in osteoid thickness, but without increased remodeling, as occurs in HVO-i. Osteoid surface can, therefore, increase only as a result of prolongation of the formation, a well-known characteristic feature of hypophosphatemic osteomalacia. Accordingly, it is inferred that all patients with impaired mineralization due to hypophosphatemia evolve through a stage of atypical osteomalacia (AOM; Fig. 2, Fig. 3), in which a reduction in the rate of mineral apposition is accompanied by a parallel reduction in the rate of matrix apposition. However, data supporting this hypothesis are lacking, since many patients are not symptomatic during the development of hypophosphatemia and thus unlikely to have had a bone biopsy.
Table 3.
Contrasting biochemical and bone histomorphometric features of vitamin D and phosphate deficiency osteomalacia.
| Measurement | Vitamin D deficiency | Phosphate deficiency |
|---|---|---|
| Serum calcium | Normal or low | Almost always normalb |
| Serum phosphate | Normal or lowa | By definition <2.5 mg/dl |
| Serum PTH | ↑ or ↑↑ | Normalb |
| Serum alkaline phosphatase | Almost always elevated | Almost always elevated |
| Osteoclast surface | ↑↑ | Normalb |
| Marrow fibrosis | Frequent | Almost neverb |
| Cortical thickness | ↓↓ | Normal or ↑ or ↓c |
| Cancellous bone volume | Normal or↓ | Normal or ↑ or ↓c |
Occasionally high due to severe hypocalcemia causing renal resistance to PTH action (Rao et al., 1985).
Except in patients with tertiary hyperparathyroidism due to long term oral phosphate therapy (Bhadada et al., 2013);
Decreased only in acquired forms of hypophosphatemia most likely due to associated deficiency of vitamin D or calcium or both. Modified from reference (Parfitt, 1998).
Fig. 4.
A & B: The description of the figure layout is same as in Fig. 2. The relationship between osteoid thickness and osteoid surface is shown on the left (A), and between osteoid thickness and adjusted appositional rate is shown on the right (B). Individual patient points with various types of hypophosphatemic osteomalacia (hereditary, tumor-induced, acquired renal tubular defects) are shown. Note all cases are shifted to the right in A and to the left in B indicating a more severe mineralization defect, similar to that seen in HVO-ii & iii (compare to Fig, 3 A & B). There is no data on “earlier stage” of hypophosphatemic osteomalacia that corresponds to HVO-i. Modified from (Parfitt, 1998).
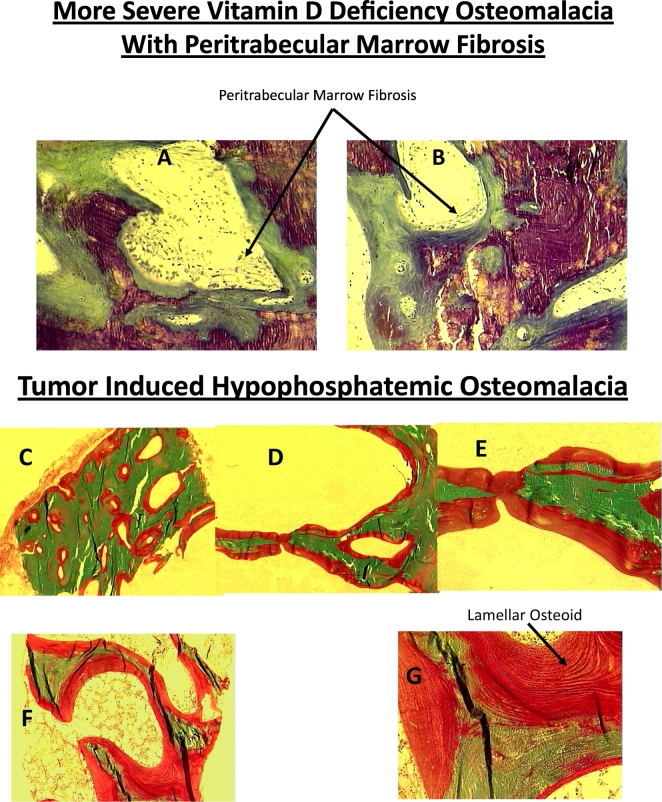
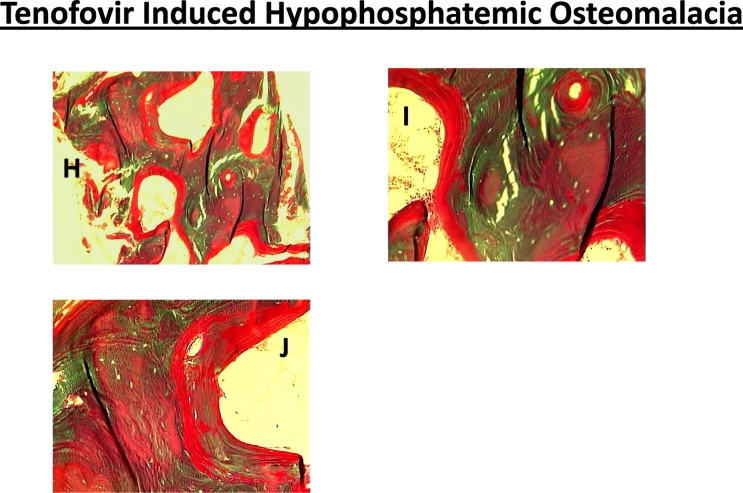
Fig. 5.
Representative bone biopsy photomicrographs in different types of osteomalacia. Increased osteoid thickness and surface (A & B) with marrow fibrosis (arrows) in a patient with severe vitamin D deficiency osteomalacia (Al-Shoha et al., 2009). In contrast, marrow fibrosis is not seen in tumor (C-G) or tenofovir induced (H-J) hypophosphatemic osteomalacia. Also, the osteoid thickness and surface (red color) is more extensive in both types of hypophosphatemic osteomalacia (C-J) compared to vitamin D deficiency osteomalacia (A & B). Finally, the osteoid is of lamellar type (arrow) rather than woven type as seen in fracture repair, Paget's disease of bone, osteitis fibrosa, all of which may also show increased osteoid. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Representative values for bone histomorphometry in vitamin D and phosphate deficiency osteomalacia.
| Measurement | Vitamin D deficiency | Phosphate deficiency | Reference range |
|---|---|---|---|
| OS/BS (%) | 61.3 ± 18.0 | 70.1 ± 15.9 | 21 ± 11 |
| O·Th (μm) | 29.7 ± 10.5 | 38.0 ± 12.2 | <12.5 |
| OV/BV (%) | 21.7 ± 11.5 | 33.6 ± 19.1 | 2.6 ± 1.4 |
| ES/NOS (%) | 5.27 ± 3.59 | 1.99 ± 1.53 | 4 ± 2 |
| TBV/TV (% TV) | 24.4 ± 9.97 | 26.4 ± 16.1 | 20 ± 6 |
| CBV/TV (%) | 84.1 ± 23.4 | 92.5 ± 45.7 | 94.5 ± 2.5 |
| C·Th (cm) | 0.51 ± 0.35 | 0.94 ± 0.71 | 1.27 ± 0.37 |
OS: Osteoid surface; BS: bone surface; O·Th: Osteoid thickness; OV: Osteoid volume; ES: Eroded surface; NOS: Non-osteoid surface; BV: Trabecular bone volume; TV: Total tissue volume; CBV: Cortical bone volume; C·Th: Cortical thickness.
Note higher mean values for OS, O·Th, OV, TBV, and CBV in phosphate deficiency osteomalacia, and lower mean value for C·Th in vitamin D deficiency osteomalacia, a characteristic feature due to associated secondary hyperparathyroidism.
Differences in bone volumes and C·Th are not significant since the phosphate deficiency osteomalacia group is a mixture of both hereditary and acquired (tumor induced and tenofovir treated) hypophosphatemic osteomalacia. Bone volumes C.Th. are high in hereditary forms, but are low in the acquired forms. (Rao, SD, unpublished data).
The key histomorphometric findings in various types of hypophosphatemic osteomalacia are depicted in Fig. 4A & B and representative bone biopsy photomicrographs are shown in Fig. 5. As seen, almost all cases of hypophosphatemic osteomalacia are concentrated towards right in Fig. 4A and towards left in Fig. 4B. In contrast, all cases of vitamin D deficiency osteomalacia are distributed along the regression intervals of the relationships (Fig. 3A & B). This does not necessarily imply that an earlier stage of hypophosphatemic osteomalacia does not exist; we just simply do not know of its evolution as well as we do about vitamin D deficiency osteomalacia. The contrasting biochemical and bone histologic features of vitamin D and phosphate deficiency osteomalacia are summarized in Table 3 and the representative bone histomorphometric values are shown in Table 4. Serum calcium, 25-hydroxyvitamin D, and PTH are always normal in untreated hypophosphatemic osteomalacia. Consequently, cortical thinning and marrow fibrosis, the specific skeletal effects of excess PTH are almost never seen in phosphate deficiency osteomalacia (Table 3). However, serum alkaline phosphatase is elevated and is the most common abnormality in all forms of osteomalacia regardless of its cause, although occasional cases of osteomalacia have been reported with normal alkaline phosphatase.
Several points deserve worth noting: First, a distinct feature of hypophosphatemic osteomalacia is the degree of osteoid accumulation (Table 4 & Fig. 5); both osteoid surface and thickness are much greater than in vitamin D-related osteomalacia at any stage. Second, osteoid indices usually return to normal after successful therapy in vitamin D deficiency osteomalacia, but not in hypophosphatemic osteomalacia; a substantial osteoid is still present in hypophosphatemic osteomalacia despite improvement in biochemical indices and patients' symptoms. Therefore, continued therapy with phosphate and calcitriol in the absence of symptoms may not be necessary. Third, cortical thinning and bone marrow fibrosis (Table 3; Fig. 5), a characteristic feature of vitamin D related osteomalacia, is not seen in hereditary hypophosphatemic osteomalacia. However, hypophosphatemic osteomalacia due to FGF-23 secreting tumors and genetic or acquired renal tubular defects is often associated with cortical thinning, perhaps related to some other reason (secondary hyperparathyroidism due to co-existent vitamin D deficiency, age related osteoporosis), or direct effects of the drug as in patients with adefovir and tenofovir-induced renal tubular defects (Mateo et al., 2016).
6. Osteomalacia due to toxic effects of drugs
In contrast to the generalized osteomalacia caused by adefovir and tenofovir, focal and atypical osteomalacia (FOM and AOM in Figures) are distinct histologic entities seen in patients with an underlying metabolic bone disease for which the drugs are given (Lundy et al., 1995; Kiely et al., 1999; Thomas et al., 1995; Takebayashi et al., 2000; Demiaux-Domenech et al., 1996; Hoppe et al., 2012). Aluminum-related osteomalacia is also generalized, like vitamin D and phosphate deficiency osteomalcia, and is associated with deposition of aluminum at the osteoid-mineralized bone interface (Parfitt et al., 1986).
7. Diagnostic value of bone histomorphometry
Microscopic examination of tissues and tissue fluids is routinely used in clinical practice to arrive at a precise diagnosis; similarly bone biopsy to diagnose metabolic bone disease should be no exception (Kulak and Dempster, 2010; Parfitt et al., 1979; Recker, 1994). Despite significant advancements in non-invasive methods to diagnose osteoporosis (DEXA, QCT, pQCT, micro-MRI, etc.), none of the imaging or quantitative measures can distinguish between osteoporosis and osteomalacia. Future refinements might allow the realization of a “virtual bone biopsy”, but are unlikely to be useful in assessing tissue level dynamics, cellular activity, and rate of mineralization without in vivo double tetracycline labeled detailed bone histomorphometry (Recker, 2008). Regrettably, except those involved in clinical research, bone histomorphometry is rarely performed even in major academic medical centers, often when it is necessary, and is bypassed by noninvasive techniques, which may result in a delay in diagnosis or inappropriate diagnosis and treatment or both (Kulak and Dempster, 2010; Parfitt et al., 1979; Parfitt, 1998; Recker, 1994). As a research tool, bone histomorphometry of an un-decalcified trans-iliac bone biopsy specimen has contributed immensely to the understanding of the mechanisms of age-related bone loss, pathogenesis of osteoporosis and other metabolic and genetic disorders of bone, to study the effects of treatment, and better characterization of newer forms of bone diseases. A detailed review of the procedure and the standardized nomenclature used for bone histomorphometry have been published (Dempster et al., 2013; Rao, 1983).
The reasons to choose a diagnostic procedure in clinical practice are: that it confirms a diagnosis under consideration, helps plan management and treatment strategy, and, most importantly, it gives potential information on the prognosis of the condition as well as response to specific treatment. Bone biopsy is no exception and bone histomorphometry has been essential in evaluating the effects of various drugs (both for their efficacy and potential adverse effects) in osteoporosis and various metabolic and genetic disorders of bone. Because of its invasive nature, risks, discomfort, time required to obtain histomorphometric data, and expense should be weighed against potential benefit beyond that which could be obtained by noninvasive technique such as bone mineral density, biochemical measurements of bone and mineral metabolism, or markers of bone turnover. In some clinical situations, as in the case vignette, bone histomorphometry is necessary to establish the correct diagnosis to render appropriate treatment. One limitation to the use of bone histomorphometry is that it is not widely available and remains within the purview of specialized laboratories in academic centers. A select few institutions provide detailed bone histomorphometric analysis, including our own laboratory, but must be collaborated before the bone biopsy is performed to insure proper in vivo tetracycline labeling, specimen handling and storage, and transportation to the laboratory. A list of specialized laboratories is given at the end of the chapter and the reader is strongly urged to contact and discuss the possibility of performing bone histomorphometry before the procedure is performed. Also, patient should be informed that results will not available for 4–8 weeks or more because of the time required for preparing un-decalcified sections. Although there are no precise indications for obtaining a detailed bone histomorphometry, in the senior author's (DSR) opinion the following list is offered as potential indications where a precise diagnosis cannot be established by currently available non-invasive methods:
-
•
Renal osteodystrophy
-
•
Suspected osteomalacia
-
•
Bone disease associated with gastrointestinal disease, resection, or bypass
-
•
Anticonvulsant-induced osteoporosis
-
•
Selected cases of primary hyperparathyroidism
-
•
Selected cases of postmenopausal osteoporosis
-
•
Selected cases of idiopathic osteoporosis (both in men and premenopausal women).
8. Bone biopsy procedure
The trans-iliac approach is preferred over the vertical approach since the latter provides only trabecular bone with tiny amount of cortical bone of irregular morphology. The trans-iliac approach provides both cortical and trabecular bone with adequate amount and good quality intervening trabecular bone between the cortices. The suggested location is approximately 2 cm below and behind the anterior and superior iliac spine, the Bordier Triangle (Rao, 1983). A 7 mm diameter trephine is preferred although a vertical or trans-iliac approach with a smaller diameter (3–5 mm) with a Jamshidi needle could be used in cases of osteomalacia where qualitative assessment is more important than quantitative assessment. Since dynamic measures are not as crucial to make a diagnosis of osteomalacia, prior in vivo tetracycline labeling is not mandatory. In fact it will not delay the diagnosis by 21 days required for in vivo tetracycline labeling. Such method can also be used in selected cases of renal osteodystrophy where qualitative assessment of PTH effect on bone (fibrosis), degree of marrow fibrosis, and absence of excess osteoid accumulation are all that is needed. In addition it predicts the possibility of hungry bone syndrome in cases where parathyroidectomy is contemplated. Both the vertical and trans-iliac bone biopsy can be performed with minimal discomfort and complications (Rao et al., 1980); the senior author (DSR) has performed >2000 bone biopsy procedures both for clinical and research purposes over the past 40 years without any long term complications. Currently, we use conscious sedation for a 7 mm trans-iliac approach and local anesthesia for the smaller diameter Jamshidi needle biopsy in selected cases. At present, bone biopsy is the only available method to unambiguously establish the diagnosis of osteomalacia. Bone histomorphometry consists of several measurements; a full description is beyond the scope of current discussion, but is summarized in the ASBMR taskforce report (Dempster et al., 2013).
Apart from the problems resulting from use of incorrect histological criteria to diagnose osteomalacia, several misconceptions exist. The use of phrase such as “biochemical osteomalacia”, and “hyperosteoidosis” may falsely imply some defective mineralization; however, these abnormalities can occur without osteomalacia as we have defined and without vitamin D or phosphate deficiency. Others have claimed that osteomalacia can be present even when non-invasive diagnostic tests are normal, but in the senior author's (DSR) experience this is a rare occurrence. Unsuspected osteomalacia has been reported in 10% of patients with apparent age-related osteoporosis and compression fractures, most likely due to the referral bias that inevitably occurs at academic and specialized centers of tertiary care. No case of osteomalacia was found in an unselected bone histomorphometric study of healthy pre- and post-menopausal women despite some with serum 25-hydroxyvitamin D levels of <10 ng/ml (Han et al., 1997). Furthermore, the prevalence of osteomalacia in unselected series of patients with postmenopausal osteoporosis in Detroit is <1%. Nevertheless, it is important to recognize that patients with a low bone mineral density due to osteomalacia usually remain for many years having diagnosed as only osteoporosis.
9. Conclusions
Osteomalacia is a histologic diagnosis and bone histomorphometry is essential to establish a definitive diagnosis. Distinction between different types of osteomalacia is important since not all varieties respond to vitamin D therapy. Ideally, the therapeutic decisions should be based on the severity of mineralization defect and its response to therapy rather than simply relying on biochemical findings. Patients with atypical osteomalacia due to aluminum or focal osteomalacia due to drug toxicity do not respond to vitamin D therapy. All patients with vitamin D deficiency osteomalacia have serum 25-hydroxyvitamin D levels <10 ng/ml, but not all with low 25-hydroxyvitamin D levels have osteomalacia. The most consistent biochemical abnormality in osteomalacia is raised serum alkaline phosphatase regardless of it cause, but this has a low specificity. Because of these inconsistent biochemical features, it is essential to perform bone histomorphometry to establish the diagnosis of osteomalacia in susceptible ot suspected cases as illustrated in the case vignette. Otherwise unnecessary and sometimes invasive procedures are undertaken in search of a non-existent malignancy and delay in diagnosis!
Conflict of interest
All authors declare that they have no conflicts of interest.
Transparency document
Transparency document.
Acknowledgements
The authors thank Ms. Stephanie Stebens, MLIS, Sladen Library for literature search and citation help, Sarah Whitehouse, Senior Medical Writer for editing and proof reading, and Wendy Gill for the preparation of all illustrations. The article is dedicated to the memory of late A. Michael Parfitt, who along with the senior author (DSR) first described the irreversible cortical bone loss due to secondary hyperparathyroidism as result of vitamin D deficiency. One of Dr. Parfitt's last desires was to report on the histological evolution of osteomalacia and its response to 25-hydroxyvitamin D therapy, but never materialized.
Footnotes
Partly supported by Indian Society for Bone Mineral Research (ISBMR) & NIH grant AR062103.
The Transparency document associated with this article can be found, in online version.
References
- Al-Shoha A., Qiu S., Palnitkar S., Rao D.S. Osteomalacia with bone marrow fibrosis due to severe vitamin D deficiency after a gastrointestinal bypass operation for severe obesity. Endocr. Pract. 2009;15(6):528–533. doi: 10.4158/EP09050.ORR. [DOI] [PubMed] [Google Scholar]
- Basha B., Rao D.S., Han Z.H., Parfitt A.M. Osteomalacia due to vitamin D depletion in the US. Am. J. Med. 2000;108:296–300. doi: 10.1016/s0002-9343(99)00460-x. [DOI] [PubMed] [Google Scholar]
- Basha B., Rao D.S., Han Z.H., Parfitt A.M. Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am. J. Med. 2000;108(4):296–300. doi: 10.1016/s0002-9343(99)00460-x. [DOI] [PubMed] [Google Scholar]
- Belkhouribchia J., Bravenboer B., Meuwissen M., Velkeniers B. Osteomalacia with low alkaline phosphatase: a not so rare condition with important consequences. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-212827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadada S.K., Palnitkar S., Qiu S., Parikh N., Talpos G.B., Rao S.D. Deliberate total parathyroidectomy: a potentially novel therapy for tumor-induced hypophosphatemic osteomalacia. J. Clin. Endocrinol. Metab. 2013;98(11):4273–4278. doi: 10.1210/jc.2013-2705. [DOI] [PubMed] [Google Scholar]
- Bhadada S.K., Dhiman V., Mukherjee S., Aggarwal S., Bal A., Sukumar S.P. Fibrogenesis imperfecta ossium and response to human growth hormone: a potential therapy. J. Clin. Endocrinol. Metab. 2017;102(5):1750–1756. doi: 10.1210/jc.2016-3055. [DOI] [PubMed] [Google Scholar]
- Bhan A., Rao A.D., Rao D.S. Osteomalacia as a result of vitamin D deficiency. Endocrinol. Metab. Clin. N. Am. 2010;39(2):321–331. doi: 10.1016/j.ecl.2010.02.001. [Review] [19 refs] [DOI] [PubMed] [Google Scholar]
- Boutsen Y., Devogelaer J.P., Malghem J., Noel H., Nagant de Deuxchaisnes C. Antacid-induced osteomalacia. Clin. Rheumatol. 1996;15:75–80. doi: 10.1007/BF02231691. [DOI] [PubMed] [Google Scholar]
- Clarke B.L., Wynne A.G., Wilson D.M., Fitzpatrick L.A. Osteomalacia associated with adult Fanconi's syndrome: clinical and diagnostic features. Clin. Endocrinol. 1995;43(4):479–490. doi: 10.1111/j.1365-2265.1995.tb02621.x. [DOI] [PubMed] [Google Scholar]
- Demiaux-Domenech B., Bonjour J.P., Rizzoli R. Axial osteomalacia: report of a new case with selective increase in axial bone mineral density. Bone. 1996;18(6):633–637. doi: 10.1016/8756-3282(96)00087-7. [DOI] [PubMed] [Google Scholar]
- Dempster D.W. Exploiting and bypassing the bone remodelling cycle to optimize the treatment of osteoporosis. J. Bone Miner. Res. 1997;12:1152–1154. doi: 10.1359/jbmr.1997.12.8.1152. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry nomenclature committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster D.W., Zhou H., Recker R.R., Brown J.P., Recknor C.P., Lewiecki E.M. Differential effects of teriparatide and denosumab on intact PTH and bone formation indices: AVA osteoporosis study. J. Clin. Endocrinol. Metab. 2016;101(4):1353–1363. doi: 10.1210/jc.2015-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster D.W., Roschger P., Misof B.M., Zhou H., Paschalis E.P., Alam J. Differential effects of teriparatide and zoledronic acid on bone mineralization density distribution at 6 and 24 months in the SHOTZ study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016;31(8):1527–1535. doi: 10.1002/jbmr.2825. [DOI] [PubMed] [Google Scholar]
- Drezner M.K. PHEX gene and hypophosphatemia. Kidney Int. 2000;57(1):9–18. doi: 10.1046/j.1523-1755.2000.00807.x. [DOI] [PubMed] [Google Scholar]
- Florenzano P., Gafni R.I., Collins M.T. Tumor-induced osteomalacia. Bone Rep. 2017;7:90–97. doi: 10.1016/j.bonr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloth F.M., 3rd, Greenough W.B., 3rd. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin. Proc. 2004;79(5):696–699. doi: 10.1016/S0025-6196(11)62303-3. (author reply 9) [DOI] [PubMed] [Google Scholar]
- Gloth F.M., 3rd, Lindsay J.M., Zelesnick L.B., Greenough W.B., 3rd Can vitamin D deficiency produce an unusual pain syndrome? Arch. Intern. Med. 1991;151(8):1662–1664. [PubMed] [Google Scholar]
- Han Z.H., Palnitkar S., Rao D.S., Nelson D., Parfitt A.M. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J. Bone Miner. Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- Honasoge M., Rao D.S. Metabolic bone disease in gastrointestinal, hepatobiliary, and pancreatic disorders and total parenteral nutrition. Curr. Op. Rheumatol. 1995;7:249–254. doi: 10.1097/00002281-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Hoppe E., Masson C., Laffitte A., Chappard D., Audran M. Osteomalacia in a patient with Paget's bone disease treated with long-term etidronate. Morphologie. 2012;96(313):40–43. doi: 10.1016/j.morpho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kassem M., Eriksen E.F., Melsen F., Mosekilde L. Antacid-induced osteomalacia: a case report with a histomorphometric analysis. J. Intern. Med. 1991;229(3):275–279. doi: 10.1111/j.1365-2796.1991.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Khosla S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(10):1226–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely P.D., Chow J., Eastwood J.B., Bourke B.E. Fluorosis and osteomalacia. Arthritis Rheum. 1999;42(9):2012–2013. doi: 10.1002/1529-0131(199909)42:9<2012::AID-ANR31>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kolb F.O. Paget's disease; changes occurring following treatment with newer hormonal agents. Calif. Med. 1959;91:245–250. [PMC free article] [PubMed] [Google Scholar]
- Kulak C.A., Dempster D.W. Bone histomorphometry: a concise review for endocrinologists and clinicians. Arq. Bras. Endocrinol. Metabol. 2010;54(2):87–98. doi: 10.1590/s0004-27302010000200002. [DOI] [PubMed] [Google Scholar]
- Lundy M.W., Stauffer M., Wergedal J.E., Baylink D.J., Featherstone J.D., Hodgson S.F. Histomorphometric analysis of iliac crest bone biopsies in placebo-treated versus fluoride-treated subjects. Osteoporos. Int. 1995;5(2):115–129. doi: 10.1007/BF01623313. a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. [DOI] [PubMed] [Google Scholar]
- Mateo L., Holgado S., Marinoso M.L., Perez-Andres R., Bonjoch A., Romeu J. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin. Rheumatol. 2016;35(5):1271–1279. doi: 10.1007/s10067-014-2627-x. (Epub 2014 May 3) [DOI] [PubMed] [Google Scholar]
- Moore C., Yee J., Malluche H., Rao D.S., Monier-Faugere M.C., Adams E. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009;4(9):1484–1493. doi: 10.2215/CJN.01770408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosekilde L., Melsen F. A tetracycline-based histomorphometric evaluation of bone resorption and bone turnover in hyperthyroidism and hyperparathyroidism. Acta Med. Scand. 1978;204:97–102. doi: 10.1111/j.0954-6820.1978.tb08406.x. [DOI] [PubMed] [Google Scholar]
- Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N., Gottschalk F.A., Pak C.Y. Severely suppressed bone turnover: a potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Ott S.M., Maloney N.A., Coburn J.W., Alfrey A.C., Sherrard D.J. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N. Engl. J. Med. 1982;307:709–713. doi: 10.1056/NEJM198209163071202. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M. Hypophosphatemic vitamin D refractory rickets and osteomalacia. Orthop. Clin. N. Am. 1972;3:653–680. [PubMed] [Google Scholar]
- Parfitt A.M. Osteomalacia and related disorders. In: Avioli L.V., Krane S.M., editors. Metabolic Bone Disease and Clinically Related Disorders. 3rd ed. Academic Press; New York: 1998. pp. 327–386. [Google Scholar]
- Parfitt A.M., Oliver I., Villanueva A.R. Bone histology in metabolic bone disease: the diagnostic value of bone biopsy. Orthop. Clin. N. Am. 1979;10(2):329–345. [PubMed] [Google Scholar]
- Parfitt A.M., Rao D.S., Stanciu J., Villanueva A.R., Kleerekoper M., Frame B. Irreversible bone loss in osteomalacia: comparison of radial photon absorptiometry with iliac bone histomorphometry during treatment. J. Clin. Investig. 1985;76:2403–2412. doi: 10.1172/JCI112253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A.M., Podenphant J., Villanueva A.R., Frame B. Metabolic bone disease with and without osteomalacia after intestinal bypass surgery: a bone histomorphometric study. Bone. 1985;6(4):211–220. doi: 10.1016/8756-3282(85)90003-1. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Rao D.S., Stanciu J., Villanueva A.R., Kleerekoper M., Frame B. Irreversible bone loss in osteomalacia. Comparison of radial photon absorptiometry with iliac bone histomorphometry during treatment. J. Clin. Invest. 1985;76(6):2403–2412. doi: 10.1172/JCI112253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A.M., Rao D.S., Stanciu J., Villanueva A.R. Comparison of aluminum related with vitamin D related osteomalacia by tetracycline based bone histomorphometry. Adv. Exp. Med. Biol. 1986;208:283–287. doi: 10.1007/978-1-4684-5206-8_35. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Qiu S., Rao D.S. The mineralization index—a new approach to the histomorphometric appraisal of osteomalacia. Bone. 2004;35:320–325. doi: 10.1016/j.bone.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Prabhala A., Garg R., Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch. Intern. Med. 1999;160:1199–1203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- Ralphs J.R., Stamp T.C., Dopping-Hepenstal P.J., Ali S.Y. Ultrastructural features of the osteoid of patients with fibrogenesis imperfecta ossium. Bone. 1989;10(4):243–249. doi: 10.1016/8756-3282(89)90060-4. [DOI] [PubMed] [Google Scholar]
- Ramser J.R., Frost H.M., Frame B., Arnstein A.R., Smith R. Tetracycline-based studies of bone dynamics in rib of 6 cases of osteomalacia. Clin. Orthop. Relat. Res. 1966;46:219–236. [PubMed] [Google Scholar]
- Rao D.S. Practical approach to bone biopsy. In: Recker R., editor. Bone Histomorphometry: Techniques and Interpretations. 1st. CRC Press; Boca Raton, FL: 1983. pp. 3–11. [Google Scholar]
- Rao D.S. Metabolic bone disease in gastrointestinal and biliary disorders. In: Favus M.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 1st ed. Americal Society for Bone and Mineral Research; Kelseyville, CA: 1990. pp. 175–178. [Google Scholar]
- Rao D.S., Matkovic V., Duncan H. Transiliac bone biopsy: complications and diagnostic value. Henry Ford Hosp. Med. J. 1980;28:112–115. [PubMed] [Google Scholar]
- Rao D.S., Villanueva A.R., Mathews M. Histologic evolution of vitamin D depletion in patients with intestinal malabsorption or dietary deficiency. In: Frame B., JTJ Potts, editors. Clinical Disorders of Bone and Mineral Metabolism. Excerpta Medica; Amsterdam: 1983. pp. 224–226. [Google Scholar]
- Rao D.S., Parfitt A.M., Kleerekoper M., Pumo B.S., Frame B. Dissociation between the effects of endogenous parathyroid hormone on adenosine 3′,5′-monophosphate generation and phosphate reabsorption in hypocalcemia due to vitamin D depletion: an acquired disorder resembling pseudohypoparathyroidism type II. J. Clin. Endocrinol. Metab. 1985;61(2):285–290. doi: 10.1210/jcem-61-2-285. [DOI] [PubMed] [Google Scholar]
- Rao D.S., Shih M.S., Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N. Engl. J. Med. 1993;328:171–175. doi: 10.1056/NEJM199301213280304. [DOI] [PubMed] [Google Scholar]
- Recker R.R. Bone biopsy and histomorphometry in clinical practice. Rheum. Dis. Clin. N. Am. 1994;20(3):609–627. [PubMed] [Google Scholar]
- Recker R. Bone biopsy and histomorphometry in clinical practice. In: Rosen C.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolis. 7th. American Society for Bone and Mineral Research; Washington, DC, USA: 2008. pp. 180–186. [Google Scholar]
- Silverberg S.J., Shane E., De La Cruz L., Dempster D.W., Feldman F., Seldin D. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- Singer F.R. Bone quality in Paget's disease of bone. Curr. Osteoporos. Rep. 2016;14(2):39–42. doi: 10.1007/s11914-016-0303-6. [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Jimi S., Segawa M., Kiyoshi Y. Cadmium induces osteomalacia mediated by proximal tubular atrophy and disturbances of phosphate reabsorption. A study of 11 autopsies. Pathol. Res. Pract. 2000;196(9):653–663. doi: 10.1016/S0344-0338(00)80010-2. [DOI] [PubMed] [Google Scholar]
- Thacher T.D., Fischer P.R., Pettifor J.M., Lawson J.O., Isichei C.O., Reading J.C. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N. Engl. J. Med. 1999;341(8):563. doi: 10.1056/NEJM199908193410803. [DOI] [PubMed] [Google Scholar]
- Thomas T., Lafage M.H., Alexandre C. Atypical osteomalacia after 2 year etidronate intermittent cyclic administration in osteoporosis. J. Rheumatol. 1995;22(11):2183–2185. [PubMed] [Google Scholar]
- Whyte M.P. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2016;12(4):233–246. doi: 10.1038/nrendo.2016.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.