Abstract
Pancreatic metastases are rare; they account for only 2% of all pancreatic malignancies and usually occur when associated with a disseminated metastatic disease. Solitary pancreatic metastases are even less frequent, and there are few reports regarding surgical resection. We report the case of a 77-year-old female patient diagnosed with a single cephalo-pancreatic metastasis of renal cell carcinoma, 16 years after a total nephrectomy. The patient underwent successful pancreaticoduodenectomy, and the diagnosis was confirmed. A subsequent positron emission tomography (PET) scan showed disease relapse, and tyrosine kinase inhibitor treatment with sunitinib was initiated. After 1 year and 4 months, the PET-computed tomography scan showed a complete radiologic response.
Keywords: Neoplasm Metastasis, Carcinoma, Kidney Neoplasm, Pancreas
CASE REPORT
A 77-year-old female patient sought medical care complaining of vague, mild, and non-specific abdominal pain for the past week. The physical examination and laboratory blood tests were unremarkable. Her medical history included a total left nephrectomy due to a renal cell carcinoma (RCC) diagnosed 16 years ago (pathological stage pT1N0M0), cholelithiasis, controlled arterial hypertension, and an ischemic stroke episode 10 years ago without major physical restraints.
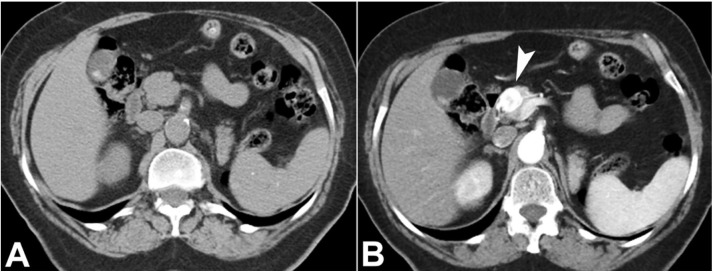
The contrast-enhanced abdominal computed tomography (CT) scan was performed, showing a single cephalo-pancreatic nodule, isodense with the pancreas on the non-contrast-enhanced images, markedly hypervascular on the arterial phase, and slightly hyperdense compared with the pancreas on the portal-venous phase (Figure 1).
Figure 1. Abdominal CT, axial plane. A – A single nodular lesion in the pancreatic head; B – markedly hypervascular on the arterial phase (arrowhead).
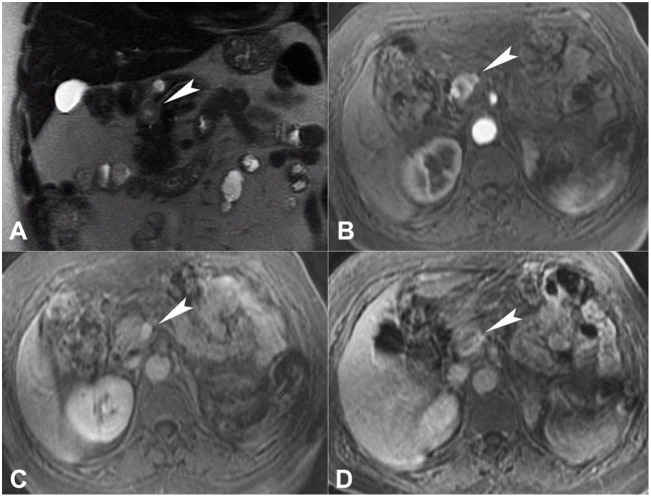
At magnetic resonance imaging (MRI), the nodule was hyperintense on T2-weighed images relative to the normal pancreas, showing a more hyperintense central area, representing cystic/necrotic changes. On T1-weighed fat saturated images, the nodule was slightly hypointense compared to the normal pancreatic parenchyma. On diffusion-weighted images, a mild restriction could be noted. The contrast uptake dynamics were similar to the CT scan; also, on the delayed phase, a thin hyperintense capsule was noted (Figure 2).
Figure 2. Abdominal MRI. A –T2-weighted sequence shows a hyperintense pancreatic nodule (arrowhead); B and C – The contrast uptake dynamics was similar to the CT scan; D – On the delayed phase, note a thin hyperintense capsule.
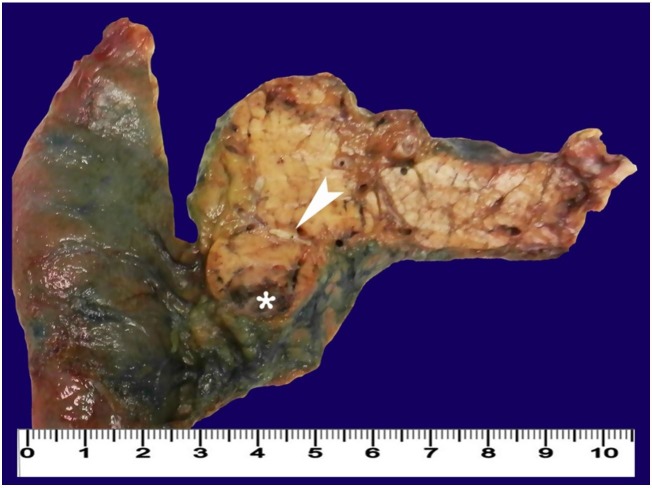
As there wasn’t any other evidence of extrapancreatic disease, the patient was submitted to a pancreaticoduodenectomy 10 months after the initial CT scan. The surgical specimen showed a 15 mm cephalo-pancreatic brownish nodule, with central cystic/necrotic changes and a surrounding thin fibrous capsule (Figures 3 and 4).
Figure 3. Gross view of the surgical specimen of cephalic duodenopancreactectomy showing a pancreatic nodule (arrowhead) with central cystic/necrotic changes (asterisk).
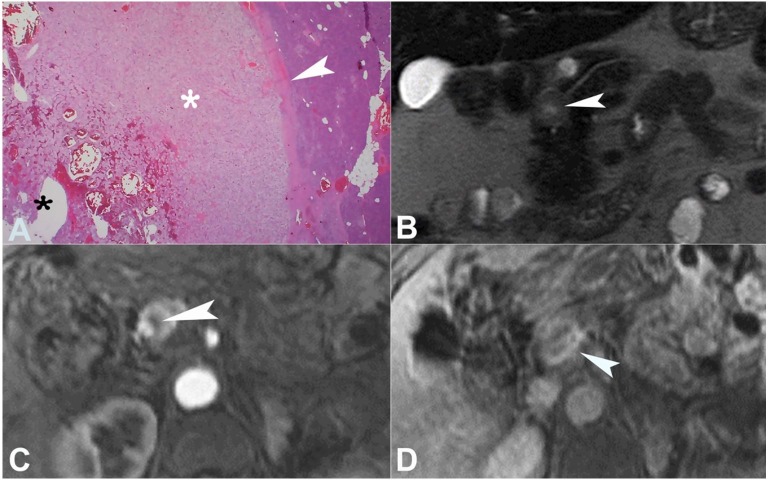
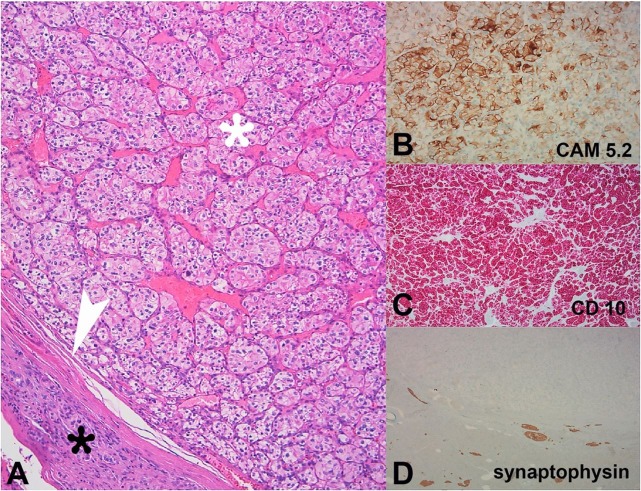
Figure 4. A – Photomicrography of the tumor showing large polygonal cells with clear cytoplasm (white asterisk) with a surrounding fibrous capsule (white arrowhead). Note the cystic/necrotic part of the tumor (black asterisk) (H&E, 20X); B – MRI representation of the cystic/necrotic changes within the nodule, which appear bright on T2-weighted images (arrowhead); C – with decreased contrast uptake (arrowhead); D – The delayed phase shows the late enhancement of the nodule surrounding the fibrous capsule (arrowhead).
The histological study with hematoxylin and eosin (H&E) staining revealed alveolar growth of large polygonal cells with clear cytoplasm, uniform round nuclei, and inconspicuous nucleoli, surrounded by a fibrous capsule (Figure 4).
Immunohistochemical study showed positivity for CAM 5.2 and CD10, and was negative for synaptophysin, Ck7, vimentin, and CD117. These findings were compatible with clear cell renal carcinoma pancreatic metastasis (Figure 5). The Memorial Sloan-Kettering Cancer Center score for metastatic RCC yielded 0 points, classifying the patient in a favorable risk group.
Figure 5. Photomicrography of the tumor. A – Alveolar growth of large polygonal cells with clear cytoplasm, uniform round nuclei, and inconspicuous nucleoli (white asterisk), surrounded by a thin fibrous capsule (arrowhead) and normal pancreatic tissue (black asterisk) (H&E, 100x); B–D – The immunohistochemical study shows positivity for CAM 5.2 and CD10, and negativity for synaptophysin.
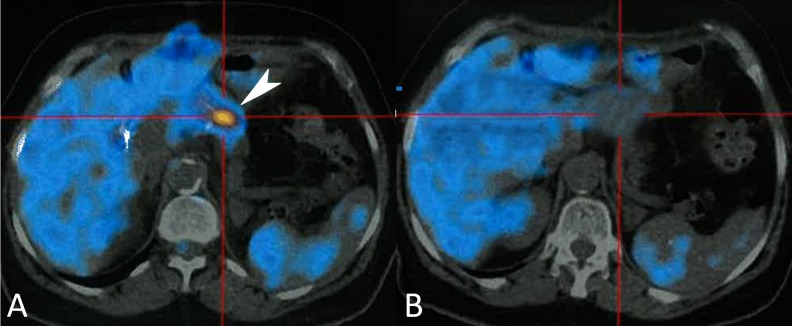
Fludeoxyglucose (FDG) positron emission tomography (PET)-CT scan was performed 1 month and 11 days after surgery to exclude residual disease. It showed several foci with a very high FDG uptake on the remaining pancreatic parenchyma, with an increase in standardized uptake value from the early to the delayed phase. We believe this was consistent with residual metastatic disease, which was not conspicuous on surgery (Figure 6A). Tyrosine kinase inhibitor treatment with sunitinib 50 mg a day was then initiated: 4 weeks on, 2 weeks off. After 1 year and 4 months, the PET-CT scan showed no signs of disease, which was consistent with a complete radiological treatment response (Figure 6B).
Figure 6. PET scan. A – Nodular hyperfixation area of FDG on the remaining parenchyma (white arrow); B – At 1 year and 4 months follow-up, the PET-CT scan showing no signs of disease relapse.
DISCUSSION
Pancreatic metastases are rare, accounting for only 2% of all pancreatic malignancies.1
The most common tumors that metastasize to the pancreas are RCC, melanoma, breast cancer, lung cancer, gastric cancer, and colorectal carcinoma.2,3 The metastatic pancreatic disease is often asymptomatic, and is usually detected during disease follow-up.1 When symptomatic, the manifestations may include abdominal pain, nausea, weight loss, and gastrointestinal bleeding. Pancreatic duct obstruction may also occur, leading to jaundice or acute pancreatitis.3
Pancreatic metastases follow three major patterns: (i) single localized mass in 50%-73% of cases; (ii) diffuse pancreatic enlargement (15%-44%); and (iii) multiple pancreatic nodules (5%-10%). No predominance of a distinct part of the pancreas has been reported to be affected by the metastases, and the lesions’ pattern of enhancement on CT or MRI tend to mimic that of a primary tumor.3-5 Metastases from pulmonary, gastric, and colorectal adenocarcinoma are normally hypovascular, mimicking the pattern of enhancement of pancreatic adenocarcinoma. RCC metastases tend to appear as hypervascular on the arterial phase reflecting a viable tumor, with decreased or absent perfusion centrally, representing necrosis—a feature that is also typical of primary RCC.6
Pancreatic metastases do not usually represent a challenging diagnosis as they normally develop late in the presentation of a known primary malignancy. An exception is RCC, which is notable for metastasizing long after the diagnosis and treatment of the primary tumor, with a mean interval between primary RCC and pancreatic metastasis of 8–10 years.7-9 It is believed that the pancreas is affected by more indolent variants of RCC, representing a favorable subgroup of patients with tumor recurrence.10,11
In the case of our patient with previous RCC and a solitary hypervascular nodule, apart from the diagnosis of metastatic malignancy, the differential diagnosis included a pancreatic metastasis from a synchronous primary RCC in the contralateral kidney, a pancreatic neuroendocrine tumor, vascular lesions (as aneurysms of the splenic artery), or accessory intrapancreatic spleens.12 A synchronous primary RCC was ruled out as the contralateral kidney was normal at CT scan and MRI. The lesion appearance at imaging was not suggestive of an aneurysm, and an accessory intrapancreatic spleen was not very probable as the spleen did not present any significant changes. In this case, the most reasonable diagnosis would be a pancreatic neuroendocrine tumor or metastasis from the previous RCC. When the diagnosis is uncertain, percutaneous or endoscopic ultrasound-guided biopsy may be useful, since its diagnostic accuracy may reach 90%.6,13
Surgery is usually not viable due to the disseminated metastatic disease, and tyrosine kinase inhibitors (TKI), immunotherapy, and palliative care are the most appropriate treatments. First-line therapy usually consists of TKI or an anti-vascular endothelial growth factor antibody, being sunitinib or pazopanib, and bevacizumab plus interferon-alfa2a, which are the drugs most commonly used, respectively. Second-line therapy has been recently changed with nivolumab (anti-programmed death 1 inhibitor) and cabozantinib (TKI) showing very significant improvement in overall survival.14
Experience in managing patients with pancreatic RCC metastases is limited, and decisions should be made on a case-specific basis as there are no definitive recommended guidelines.11 In a meta-analysis published by Tanis et al.9, which included 311 patients surgically treated for pancreatic RCC metastases, the authors concluded that even though there might be a potential selection bias, the pancreatic operation is justified by a remarkably high survival probability with a low postoperative mortality. Overall survival rates were estimated at 80.6% at 2 years and 72.6% at 5 years. However, patients should be informed about the substantial morbidity of pancreatic surgery, although it is mostly of mild clinical significance.10
Due to the indolent behavior of pancreatic metastatic RCC, one could consider managing these patients with observation and best supportive care alone, or by starting systemic therapy. Only a multicentric study comparing resection versus non-resection would provide the best treatment to adopt. Till such a study is conducted, it is believed that surgery—when technically feasible—represents the best therapeutic option, even in the presence of extrapancreatic disease or pancreatic multifocality.10 The combination of surgery with immunotherapy and antiangiogenic agents is also possible, although more trials are needed to formulate clearer evidence-based recommendations.
Footnotes
How to cite: Nogueira M, Dias SC, Silva AC, Pinto J, Machado J. Solitary pancreatic renal cell carcinoma metastasis. Autops Case Rep [Internet].2018;8(2):e2018023. http://dx.doi.org/10.4322/acr.2018.023
The patient signed an informed consent and the manuscript has been approved by the Ethics Committee of Hospital Pedro Hispano, Matosinhos, Porto, Portugal.
Financial support: None
REFERENCES
- 1.Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: the role of surgery. World J Gastrointest Oncol. 2014;6(10):381-92. 10.4251/wjgo.v6.i10.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998;18(2):369-78. 10.1148/radiographics.18.2.9536484. [DOI] [PubMed] [Google Scholar]
- 3.Scatarige JC, Horton KM, Sheth S, Fishman EK. Pancreatic parenchymal metastases: observations on helical CT. AJR Am J Roentgenol. 2001;176(3):695-9. 10.2214/ajr.176.3.1760695. [DOI] [PubMed] [Google Scholar]
- 4.Muranaka T, Teshima K, Honda H, Nanjo T, Hanada K, Oshiumi Y. Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989;30(6):615-9. 10.1177/028418518903000609. [DOI] [PubMed] [Google Scholar]
- 5.Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, Bassi P. Pancreatic metastases: CT assessment. Eur Radiol. 1997;7(2):241-5. 10.1007/s003300050144. [DOI] [PubMed] [Google Scholar]
- 6.Yoshinaga S, Suzuki H, Oda I, Saito Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(Suppl. 1):29-33. 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 7.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611-23. 10.1016/S0022-5347(05)65640-6. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie AW, Chisholm GD. The natural history of renal carcinoma. Semin Oncol. 1983;10(4):390-400. [PubMed] [Google Scholar]
- 9.Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96(6):579-92. 10.1002/bjs.6606. [DOI] [PubMed] [Google Scholar]
- 10.Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008;15(4):1161-8. 10.1245/s10434-007-9782-0. [DOI] [PubMed] [Google Scholar]
- 11.Yagi T, Hashimoto D, Taki K, et al. . Surgery for metastatic tumors of the pancreas. Surg Case Rep. 2017;3(1):31. 10.1186/s40792-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raman SP, Hruban RH, Cameron JL, Wolfgang CL, Fishman EK. Pancreatic imaging mimics: Part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am J Roentgenol. 2012;199(2):309-18. 10.2214/AJR.12.8627. [DOI] [PubMed] [Google Scholar]
- 13.Itoi T, Tsuchiya T, Itokawa F, et al. . Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: A single-center experience. Dig Endosc. 2011;23(Suppl. 1):34-8. 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 14.Escudier B, Porta C, Schmidinger M, et al. . Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl. 5):v58-68. 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]