Abstract
Objective
To evaluate whether extended-release gabapentin is more effective than placebo among women with vulvodynia.
Methods
In a multicenter double-blind, placebo-controlled randomized crossover trial, gabapentin (1200 – 3000 mg/day) was compared to placebo. The primary outcome was mean pain intensity (0, no pain at all to 10, worse pain ever)on the tampon test (a standardized tampon insertion and removal test used as a surrogate marker for dyspareunia) during the last 7 days of the maintenance phase. Secondary outcomes included sexual intercourse pain and daily pain. A sample size of 53 provided 90% power to detect a one-point reduction on the tampon test (.05 level, two-sided) between the two treatment phases.
Results
From August 2012 to January 2016, 230 women were screened at three academic institutions and 89 (mean age: 37; 66% black) were randomized: 45 to gabapentin first and then placebo; and 44 to placebo first and then gabapentin. Tampon pain with gabapentin was not different compared to placebo (adjusted mean: 4.0 [95% confidence interval, CI, 3.0–4.9] vs. 4.3 [95% CI, 3.4–5.2], difference: -0.3 [95% CI, -0.7–0.0]; P=.07). Gabapentin also did not improve pain over placebo for sexual intercourse pain (adjusted mean: 3.9 [95% CI, 2.4–5.3] vs. 4.0 [95% CI, 2.5–5.4], difference: -0.1 [95% CI, -0.9–0.6]; P=.76) and daily pain (adjusted mean: 2.7 [95% CI, 1.8–3.6] vs. 2.9 [95% CI, 2.0–3.8], difference: -0.2 [95% CI, -0.5– -0.2]; P=.36). Subset analyses found that longer pain duration and oral contraceptive nonuse were associated with minimal improvement in tampon testpain with gabapentin.
Conclusion
In this cohort, extended-release gabapentin, as compared with placebo did not reduce tampon pain. These data do not support the recommendation of gabapentin alone as treatment for vulvodynia.
Optimal pain management has become a concern across many medical conditions, especially when addictive pharmacologic and/or mood-altering interventions are prescribed for extended periods of time. Vulvodynia, characterized by burning and stinging pain of the vulva, is a chronic pain syndrome with a 7-16% lifetime prevalence.1 To date, vulvodynia randomized clinical trials (RCT's) have been limited in number, geographic region of sampling, and demographic diversity. The few published vulvodynia RCT's find no difference in pain or other quality of life measures between pharmacologic intervention andplacebo.2-6
Despite lack of evidence-based data, gabapentin iscommonly prescribed for vulvodynia and is recommended in several vulvodynia treatment guidelines.7,8 Gabapentin has been found effective in RCT's for neuropathic conditions.9 Women with vulvodynia often present with certain neuropathic characteristics, such as dynamic allodynia (pain in response to light touch). Although some women report symptom relief with gabapentin use in clinical practice, evidence supporting its benefit is limited, based on descriptive/observational reports that have methodological flaws, including reliance on subjective outcome measures, sampling bias, and small sample sizes.10-16 No placebo controlled RCT's have been conducted.17
We evaluated the efficacy of gabapentin, in an extended release formulation, in women diagnosed with localized provoked vulvodynia, defined as superficial vulvar vestibular pain that is provoked by touch, in a demographically diverse sample.
Materials and Methods
This was an 18-week, multicenter, placebo-controlled, double-blinded RCT with a two-treatment, two-period crossover design that studied the efficacy of extended release gabapentin (Gralise™) (1200–3000 mg/day) for localized provoked vulvodynia (previously known as vulvar vestibulitis). The study consisted of 8 phases:1) a 2-week screening phase, 2) randomization, 3) 4-week dose titration, 4) a 2-week maintenance phase, 5) 2-week dose-taper, 6) 4-week dose titration after crossover, 7) a 2-week post-crossover maintenance phase, and 8) a 2-week dose-taper phase with scheduled visits at 6, 8, 14, and 16 weeks. Clinical response was assessed by the pain intensity from vaginal insertion by the participant of a tampon (tampon test)18 during the last 7 days of the initial maintenance phase and post-crossover maintenance phase.
The study was conducted between August 8, 2012 and January 19, 2016 and received IRB approval at the University of Tennessee Health Science Center (#10-00985-FB), Rutgers Robert Wood Johnson Medical School (#0220110309), and the University of Rochester (#31720) (see Acknowledgments in Appendix 1, available online at http://links.lww.com/xxx). All participants provided written informed consent. The GABA RCT was registered in ClinicalTrials.gov (NCT1301001).
Inclusion criteria included women between the ages of 18-50 years (later extended to women aged 50 years and above since the reported upper age for vulvodynia is well above 50 years of age),19 reporting greater than 3 continuous months of insertional dyspareunia, pain to vulvar touch or vulvar pain with tampon insertion or both and during pelvic examination. Participants were allowed to use oral contraceptives, hormone therapy or selective serotonergic reuptake inhibitors if they were prescribed prior to randomization and were on a stable dose.
Following informed consent, study candidates completed questionnaires on medical and gynecological history and underwent a physical and pelvic examination. Candidates fulfilled Friedrich's criteria,20 by their reporting tenderness of the vulvar vestibule with intercourse or touch, as with tampon insertion, using modified diagnostic criteria of Bergeron et al.21 A mean score equal to or greater than 4 out of 10 on a numeric rating scale of pain intensity was required, with the score in the vulvar vestibule greater than the score on the peripheral vulva area or inside the vagina.
Block randomized drug assignments were determined using a computer-generated random numbers technique and a concealed allocation schedule generated by the Pharmaceuticals Department at the University of Tennessee Health Science Center. Identical-appearing tablets were packaged and distributed by the department. Investigators, research staff and participants were blinded after assignment to interventions.
The two-period crossover design consisted of 8 weeks per treatment period with weekly telephone contact. Dosing incrementally increased one tablet per week, for 4 weeks and plateaued at 5 tablets daily for study weeks 5 and 6. Participants were asked to take the oral medication at bedtime during week 1 and in divided doses at week 2 through 6 and to incrementally increase dose regardless of point of response (pain relief). In the event of non-serious side effects, participants were asked to decrease tablet dose by one and to remain at that dose for the remainder of the clinical trial. In the event of further non-serious side effects, the reduction by one tablet was repeated.
Participants were analyzed on an intention-to-treat, last observation carried forward basis. When deemed medically necessary, an unblinding protocol was followed. Pain “rescue medication” such as acetaminophen, aspirin, or nonsteroidal anti-inflammatory drugs was permitted and documented, if used. The use of opioid analgesics and topical medications were protocol violations.
Data collection utilized a proprietary database platform Slim-Prim, (Scientific Laboratory & Patient-care Research Information management system, University of Tennessee),22 a web-accessible, modular data based system mounted on an Oracle server acted as the Central Data Repository. Data was entered electronically by participants (or by research staff if diaries were completed by hand) and the accuracy of the database was validated by a research staff member. The primary trial outcome was measured by the tampon test, a surrogate marker for vaginal penetration pain, performed once weekly, according to a previously validatedmethod.18 The outcome measure uses an 11-point numeric rating scale (0 = no pain at all; 10 = worse pain ever), and has been shown to have reliability, construct validity, and responsiveness.18 It is often used as the primary outcome in vulvodynia research because pain with sexual intercourse may be so severe that women with vulvodynia may abstain, limiting the number of data points for vaginal penetration pain analysis; and pain with elicited tampon insertion is included within the definition of vulvodynia.20
Secondary outcomes included daily pain and pain with sexual intercourse. Participants were asked to record intensity of general pain experienced over the past 24 hours; and sexual intercourse, if it occurred within the evaluation period, using an 11-point (0–10) numeric rating scale.
During the pre-randomization visit, women were instructed and demonstrated proficiency on performance of the tampon test, and completion of electronic or handwritten diary including daily 24-hour pain, and intercourse pain. All information was reviewed and approved during weekly telephone calls by the team and later confirmed by data-entry validation. A baseline level of pain (average 4 out of 10 or greater on the 11-point tampon test [0 = no pain at all; 10 = worse pain ever])was required to proceed to randomization.
Participants also were instructed to record intensity of general pain experienced over the past 24 hours on a 11-point (0–10)numeric rating scale and to record any side effects experienced with study medication. Side effects were listed individually and included a severity estimate (mild, moderate, or severe). They also were instructed to refrain from using lubricants or moisturizers throughout the trial. If lubricants were used, the participant was to report this.
Exploratory analyses included determining the effect of age, race, duration, oral contraceptive use, onset (pain always present with sexual intercourse or if there was a pain-free period), and sexual abuse history (single item on questionnaire) on treatment outcome, since more recent literature suggests that demographic characteristics may play a role.23
During scheduled study visits 1 through 5, participants were evaluated bypelvic examination, cotton swab test, pelvic muscle tenderness testing (pelvic muscle algometry) and assessment of vaginal milieu; the cotton swab test was performed at the last study visit. Cotton swab test was performed on pre-defined points of the labia majora, minora, and lower vagina, as described.21 We performed pelvic muscle algometry by random staircase method and 11-point (0–10) numeric rating scale direct scaling of pain response to a load cell-mediated, digitally applied for cestimulus to selected pelvic floor muscle groups including levator ani, obturator internus, and piriformis muscles.
Vaginal milieu assessment included microscopic wet mount vaginal smears, Rakoff stain24 for vaginal maturation index, Affirm™ (VPIII Microbial Identification System, Becton, Dickinson and Company, Sparks, MD) test to assess for vaginitis, a phenazine test tape for vaginal pH, and urine pregnancy test. Those with vulvovaginal atrophy assessed by maturation index, were treated with hormone therapy for at least 6 weeks prior to randomization. Those with vaginitis were treated and re-screened for eligibility and treated, when necessary, throughout the trial.
The primary outcome was participant reported pain intensity by an 11-point (0-10) numeric rating scale of the last participant inserted tampon during the last 7 days of the maintenance phase. Sexual intercourse and daily pain, secondary outcome measures, also were measured by the 11-point numeric rating scale during the same 7-day period. The study was an intent-to-treat design, last observation carried forward,25 which meant once a participant was randomized, if she was lost to follow up, all future values were assumed to be equal to her last observed value.
All analyses were done using the Mixed Procedure in SAS 9.4 (SAS Institute Inc., Cary, North Carolina) according to the crossover design. The mixed model included sequence, period, and treatment. If the center effect was significant, it would be included in the model. Efficacy parameters are reported as least squares means (95% confidence interval, CI). Statistical significance was defined as a P value less than 0.05 using two-tailed tests.
The exploratory analyses were conducted in two stages. In the first stage, univariate analysis was used to test for associations of the demographic variables with tampon test pain, according to the crossover design. The model also included interaction terms with treatment. The P value was set at .15 in the univariate analysis in order to avoid missing any potential variables significantly associated with tampon pain in the first stage toward multivariate analysis. Those found significant were all included in the multivariate analysis in the final stage, in which statistical significance was set at the 1% level.
Demographic characteristics were analyzed with the chi square test and adverse events were analyzed with the McNemar's test; both are reported as percentages. Statistical significance was set at the 5% level.
Power analysis was based on data from neuropathic pain RCTs using gabapentin since there was no clinical trial data available in participants with vulvodynia.26 In order to have a power of 90% and a significance level of 5% to detect a one-point difference between the two phases on a scale of 1 to 10 of the tampon test, a sample size of 53 was needed, assuming the following assumptions were made: no carry-over effect, no interactions between participants, treatments, and periods, no period effect, no center effect, a standard deviation during the gabapentin and placebo cycles of 2.2, and acorrelation between gabapentin and placebo tampon test measure of 0.5. A multiplicity adjustment was not performed for the secondary measures since it was not the intent of the study to assess these measures at the same experimental significance level as established for the primary outcome variable. Assuming a 40% drop-out rate, 89 participants were randomized to complete 53.
Results
Figure 1 summarizes the flow diagram of progression from baseline visit to week 18. Of the 230 women screened, 89 met entry criteria and were randomized and 66 completed the trial. Of the 141 participants who were excluded, 101 did not meet inclusion criteria, 16 decided not to participate in the trial, and 24 did not return for drug randomization. Of the 23 individuals randomly assigned to study drug who did not complete the trial, eight were lost-to-follow up, seven withdrew consent, seven were removed by research staff because of adverse events, and one was removed for a protocol deviation.
Figure 1.
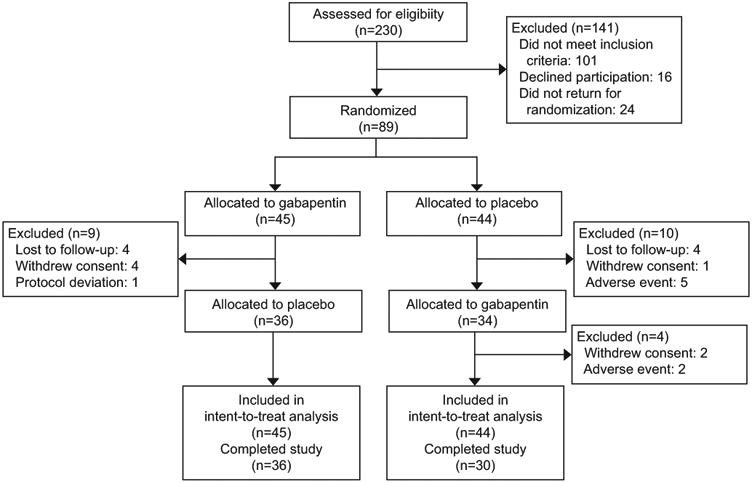
Flow of participants through the randomized clinical trial. Participants were allocated to either gabapentin first and then placebo, or placebo first and then gabapentin. Data were analyzed using true intent-to-treat.
Of the 26% of participants who discontinued treatment, a greater proportion discontinued during the placebo treatment arm compared to the intervention arm (32% vs. 20%). The 74% attrition rate is comparable to other RCTs in vulvodynia.2-6 Analyses were based on the 89 participants who met entry criteria.
Demographic and baseline characteristics at randomization are presented in Table 1. There were no statistically significant differences in distribution of any of the variables between the gabapentin and placebo crossover phases. The average patient was 37years of age, with a large proportion of participants reporting their race as black. Most had attended college, the majority had their vulvodynia pain duration of over 5 years, about 25% had a history of sexual abuse, about 25% were taking oral contraceptives, 7 participants were using hormone therapy, and 2 were taking selective serotonergic reuptake inhibitors. Approximately half of the participants had primary onset and half had secondary onset.
Table 1. Demographic and Clinical Characteristics of Participants.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
|
| |||
| Placebo First (n = 44) |
Gabapentin First (n = 45) |
P Value* | |
| Age | .19 | ||
| Less than 52 years | 41 (93.2) | 38 (84.4) | |
| 52 years or older | 3 (6.8) | 7 (15.6) | |
| Race | .77 | ||
| White | 14 (31.8) | 16 (35.6) | |
| Black | 29 (65.9) | 29 (64.4) | |
| More than one race | 1 (2.3) | 0 (0.0) | |
| Educational status | .56 | ||
| College | 29 (65.9) | 27 (60.0) | |
| No college | 15 (34.1) | 18 (40.0) | |
| Duration of pain, years | .93 | ||
| 5 yrs. or less | 17 (38.6) | 17 (37.8) | |
| Greater than 5 yrs. | 27 (61.4) | 28 (62.2) | |
| History of sexual abuse | .51 | ||
| Yes | 10 (22.7) | 13 (28.9) | |
| No | 34 (77.3) | 32 (71.1) | |
| Onset | .92 | ||
| Primary | 21 (47.7) | 21 (46.7) | |
| Secondary | 22 (50.0) | 23 (51.1) | |
| Not reported | 1 (2.3) | 1 (2.2) | |
Data are reported according to sequence of treatment.
Based on chi square test.
Nearly all participants (88 of 89) were therapeutically naive to gabapentin. Comparison of the 23 individuals who failed to complete the randomized phase found no difference in age, race, years of education, marital status, or duration of disease compared with participants completing the randomized phase.
The primary outcome measure of this study, the tampon test, failed to demonstrate improved pain with gabapentin over placebo (unadjusted mean: 3.9 [95% confidence interval, CI, 3.4–4.5] vs. 4.3 [95% CI, 3.7–4.9], adjusted mean: 4.0 [95% CI, 3.0–4.9] vs. 4.3 [95% CI, 3.4–5.2]; P = .07) (Table 2).Based on the multicenter, crossover design, primary and secondary outcomes were analyzed as group means (unadjusted) and least squares means (adjusted for center, sequence and period).A center effect was found for the primary outcome, tampon test pain (P = .02) and thus was included in all mixed model analyses. No effect was observed on sequence (P = .67) or period (P = .13)
Table 2. Summary of Primary and Secondary Outcome Measures.
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Outcome | No. | Placebo (95% CI) |
Gabapentin (95%) CI) |
Difference (95% CI) |
Placebo (95% CI) |
Gabapentin (95% CI) |
Difference (95% CI) |
| Primary Outcome | |||||||
| Tampon Test | 83† | 4.3 (3.7–4.9) | 3.9 (3.4–4.5) | -0.3 (-0.7–0.1) | 4.3 (3.4–5.2) | 4.0 (3.0–4.9) | -0.3 (-0.7–0.0) |
| Secondary Outcomes | |||||||
| Intercourse | 27‡ | 4.3 (3.5–5.1) | 4.2 (3.3–5.0) | 0.0 (-0.9–0.8) | 4.0 (2.5–5.4) | 3.9 (2.4–5.3) | -0.1 (-0.9–0.6) |
| Daily Pain | 87§ | 3.0 (2.5–3.6) | 2.9 (2.3–3.4) | -0.2 (-0.5–0.2) | 2.9 (2.0–3.8) | 2.7 (1.8–3.6) | -0.2 (-0.5–0.2) |
CI, confidence interval. Rated on an 11-point numeric rating scale, where 0 = no pain at all and 10 = worse pain ever.
Based on univariate analysis, mixed model. Adjusted for center, sequence and period.
Six subjects did not insert a tampon during the last 7 days of the maintenance phase in one or both treatment arms.
Sixty-two subjects did not have sexual intercourse during the last 7 days of the maintenance phase in one or both treatment arms.
Two subjects did not report daily pain during the last 7 days of the maintenance phase in one or both treatment arms.
Similar to the tampon test, gabapentin failed to improve pain over placebo for secondary outcome measures of sexual intercourse pain (unadjusted mean: 4.2 [95% CI, 3.3–5.0] vs. 4.3 [95% CI, 3.5–5.1]; adjusted mean: 3.9 [95% CI, 2.4–5.3] vs. 4.0 [95% CI, 2.5–5.4]; P = .76) and daily pain (unadjusted mean: 2.9 [95% CI, 2.3–3.4] vs. 3.0 [95% CI, 2.5 to 3.6], adjusted mean: 2.7 [95% CI, 1.8–3.6] vs. 2.9 [95% CI, 2.0–3.8]; P = .36), adjusted for center, sequence and period, as described above.
Exploratory analyses of gabapentin effect on selected demographic subsets was also adjusted for center, sequence, and period based on the multicenter, crossover design. Gabapentin reduced tampon test pain in women with vulvar pain for over 5 years (unadjusted difference: -0.6 [95% CI, -1.1–0.0], adjusted difference: -0.6 [95% CI, -1.1– -0.1]; P =.01); in women aged 52 years or older (unadjusted difference: -1.3 [95% CI, -3.1–0.6, adjusted difference: -1.2 [95% CI, -2.3– -0.1]; P = .03); in white women (unadjusted difference: -0.6 [95% CI, -1.3–0.0], adjusted difference: -0.7 [95% CI, -1.3–0.0]; P = .04); in women not taking oral contraceptives (unadjusted difference: -0.4 [95% CI, -0.9–0.0], adjusted difference: -0.5 [95% CI, -0.9– -0.1]; P = .03), and in women with secondary onset vulvodynia (unadjusted difference: -0.4 [95% CI, -0.9–0.1], adjusted difference -0.4 [95% CI, -1.0– -0.1]; P = .10). There was no significant treatment effect difference by sexual abuse history.
We subsequently developed a multivariate mixed model which included age, race, duration of pain, onset, oral contraceptive use, center, sequence, and period. Controlling for other variables in the model, a gabapentin treatment (improvement) difference over placebo was associated with duration of pain (> 5 yrs.) (unadjusted difference: -0.6 [95% CI, -1.1–0.0],adjusted difference: -1.0 [95% CI, -1.8– -0.2]; P = .01); white racial category (unadjusted difference: -0.6 [95 % CI, -1.33– -0.0], adjusted difference: -0.9 [95% CI, -1.6 to -0.2]; P=.02;and oral contraceptive non-use (unadjusted difference: -0.4 [95% CI, -0.9–0.0], adjusted difference: -0.8 [95% CI, -1.4– -0.2]; P = .01). There was no treatment effect difference by age or onset (Table 3). Demographic variables that differed by center included race (P=0.003) and age ≥ 52 years (P< 0.001). No effect was observed on sequence (P = .67) or period (P = .13).
Table 3. Demographic and Clinical Characteristics and Treatment Effect on Tampon Test.
| Characteristic | No. | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Placebo (95% CI) |
Gabapentin (95% CI) |
Difference (95% CI) |
Placebo (95% CI) |
Gabapentin (95% CI) |
Difference (95% CI) |
||
| Duration of pain | |||||||
| > 5 years | 55 | 4.4 (3.8–5.1) | 3.9 (3.2–4.6) | -0.6 (-1.1–0.0) | 4.7 (3.2–6.1) | 3.7 (2.2–5.1) | -1.0 (-1.8– -0.2)† |
| ≤ 5 years | 34 | 4.1 (3.0–5.1) | 4.0 (3.0–5.1) | 0.1 (-0.3–0.5) | 4.5 (3.0–5.9) | 4.4 (2.9–5.9) | -0.1 (-0.9– -.6) |
| Race‡ | |||||||
| White | 30 | 4.5 (3.6–5.4) | 3.9 (3.0–4.8) | -0.6 (-1.3–0.0) | 4.4 (3.1–5.7) | 3.5 (2.2–4.8) | -0.9 (-1.6– -0.2)§ |
| Black | 58 | 4.3 (3.5–5.0) | 4.0 (3.3–4.8) | -0.1 (-0.6–0.3) | 4.8 (3.2–6.5) | 4.6 (2.9–6.2) | -0.3 (-1.1–0.5) |
| Oral Contraception | |||||||
| Yes | 23 | 4.7 (3.5–5.9) | 4.7 (3.5–5.9) | 0.0 (-0.6–0.6) | 4.9 (3.2–6.6) | 4.5 (2.8–6.2) | -0.4 (-1.3–0.6) |
| No | 66 | 4.2 (3.5–4.8) | 3.6 (3.0–4.3) | -0.4 (-0.9–0.0) | 4.3 (3.1–5.6) | 3.6 (2.3–4.8) | -0.8 (-1.4– -0.2)† |
| Onset║ | |||||||
| Primary | 42 | 4.4 (3.6–5.2) | 4.2 (3.3–5.0) | -0.3 (-0.8– -0.3) | 4.8 (3.3–6.2) | 4.4 (2.9–5.8) | -0.4 (-1.1–0.3) |
| Secondary | 45 | 4.2 (3.3–5.0) | 3.7 (2.9–4.5) | -0.4 (-0.9–0.1) | 4.5 (2.9–5.9) | 3.7 (2.2–5.2) | -0.7 (-1.5–0.1) |
| Age | |||||||
| 52 yrs. or older | 10 | 4.4 (1.6–7.2) | 3.2 (1.2–5.2) | -1.3 (-3.1–0.6) | 4.4 (2.3–6.4) | 3.4 (1.3–5.5) | -1.0 (-2.2–0.2) |
| Less than 52 yrs. | 79 | 4.3 (3.7– -4.9 | 4.0 (3.4–4.6) | -0.2 (-0.6–0.2) | 4.8 (3.7–5.9) | 4.7 (3.5–5.8) | -0.2 (-0.6–0.3) |
CI, confidence interval. Yrs., years. Rated on an 11-point numeric rating scale, where 0 = no pain at all and 10 = worse pain ever.
Based on multivariate analysis, mixed model. Adjusted for center, sequence, period, age, race, duration of pain, onset, and oral contraceptive use. Multivariate analysis included all variables with P ≤.15 from the univariate analysis. Those found significant were all included in the multivariate analysis in the final stage, in which statistical significance was set at P = .01.
P≤ .01.
One participant reported more than one race and was not included in the analysis.
P< .05.
Two participants did not report onset and were not included in the analysis.
Figure 2. displays longitudinal changes in the numeric rating scale tampon test score (mean, ± 95% CI) over the Phase 1 and Phase 2 treatment sequences of the study. Phase 1 (Week 0 to Week 6) displays a 28% decline in tampon test pain with placebo: baseline 6.4 [95 % CI, 5.7– -6.1] to end Phase 1: 4.6 [95% CI, 5.4– -3.7], t=5.34; P <0.001 compared to a 37% decline in tampon test pain with gabapentin: baseline 6.3 [95% CI, 5.6– -7.1] to end Phase 1: 3.9 [95% CI, 3.32– -4.7], t=5.89; P< 0.0001. No further decline is evident with placebo (following crossover): end Phase 2: 4.0 [95% CI, 3.3– -4.8], t=0.09; P = ns, compared to a further 15% decline with gabapentin (following crossover) end Phase 2: 3.9 [95% CI, 3.2– -4.7], t=2.49; P < 0.02.
Figure 2.
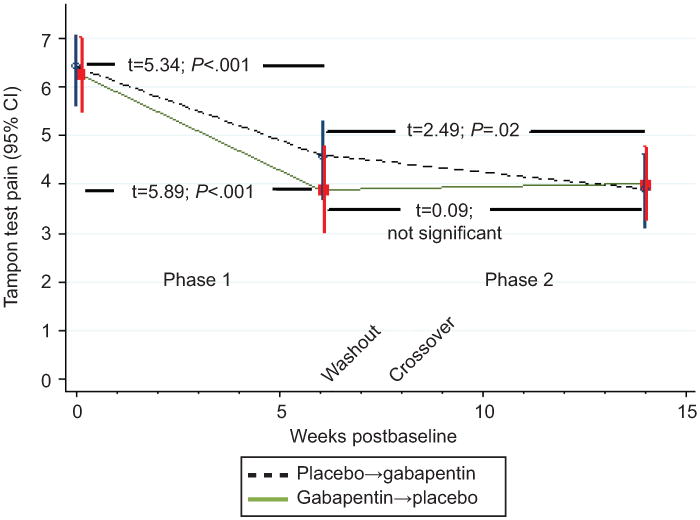
Longitudinal changes in the numeric rating scale tampon test score over the Phase 1 and Phase 2 treatment sequences of the study.
During the last 7 days of the maintenance phase, the average daily dose of gabapentin was 2,476 ±866 mg. Compliance rate, determined by retained container pill count, was 94.7%. The incidence of adverse effects was slightly higher, but not significantly different, with gabapentin compared to placebo: Rhinitis (11.2% vs 4.5%; P =.08), dizziness (10.1% vs. 3.4%; P = .08), nausea (8.9% vs. 3.4%: P = .10), headache (7.9% vs. 5.6%; P =.53), somnolence (7.9% vs 4.5%: P =.32), bacterial vaginosis (7.9% vs. 4.5%; P =.37), and fatigue (5.6% vs. 1.1%: P =.10).No serious adverse events occurred during gabapentin treatment.
During the placebo treatment arm, 5 (6%) of participants withdrew from the study due to adverse events and 2 (3%)withdrew due to adverse events during the gabapentinarm. At study completion, 42% of the participants correctly identified when they were taking gabapentin.
Dicussion
Gabapentin in the treatment of vulvodynia in this cohort was not more effective than placebo on the primary outcome measure, the tampon test (a surrogate marker for dyspareunia), or on secondary outcomes: sexual intercourse pain and daily pain. A lack of gabapentin treatment effect over placebo is consistent with previous controlled studies investigating other pharmacologic treatments for vulvodynia.2-6
We found improvement in tampon scores for both active treatment and placebo during the first treatment arm (Figure 2), suggesting an early placebo effect, which leveled off for Phase 2 placebo participants but continued for Phase 2 gabapentin participants, suggesting a modest treatment effect. It remains difficult to distinguish a placebo effect from the therapeutic benefit of interacting with research staff (Hawthorne effect)26 or from spontaneous improvement (natural history).27 The likelihood for an effect due to natural history is lessened by the lack of a period effect, but only a no-treatment arm would be able to dissect out placebo and placebo-independent effects. As well, it is unlikely that low pain severity was a contributor, since previous trials reporting greater pain intensity also reported negative findings.2-6
Our findings contrast with previous descriptive/observational studies where most women reported improvement with gabapentin.10-16 Whereas the placebo effect and other methodological flaws most likely accounted for the discrepant findings, it is possible that women with more generalized symptoms may have obtained benefit, as one small, retrospective study revealed that 55% (6/11) of those with generalized vulvodynia achieved complete pain relief post treatment with topical gabapentin as compared with 17% (4/17) of those with provoked vulvodynia.10
The strength of this multicenter controlled trialis that it included a demographically and geographically diverse sample. Another strength of this RCT is that participants entered their pain ratings electronically, which may have minimized recall bias.
We investigated the possibility that gabapentin therapy may benefit women with certain demographic characteristics, such as onset, age, pain duration, race, and oral contraceptive use. Because the sample was too small in these subsets of women and the differences found indicated “minimal” change,28no definitive statement can be made based on these findings. Further research is necessary to determine whether symptom presentation or demographic characteristics predict treatment outcome.
Regarding study limitations, this study did not include a no-treatment arm to better define the true placebo effect. Also, power analysis and calculation of sample size was based on a previously reported parallel design RCT of gabapentin on neuropathic pain.29 Since the analysis was not based on a vulvodynia population, it is possible that a Type II error may have occurred. However, crossover designs are statistically efficient, and the multiple outcomes of the trial show little evidence of trends in data that might achieve statistical significance with a larger sample size.
Carryover effects from placebo to active phases and failure to measure gabapentin plasma concentrations during each phase may also have clouded the treatment effect. In prior gabapentin studies, crossover trial period effect and plasma concentrations have not correlated with clinical response.30 The choice of treatment duration may have been too brief to detect a clinical response, as one retrospective report found improvement in patient pain ratings during gabapentin treatment only after 12 months to 18 months of continuous treatment.11 While external validity was enhanced by the multicenter design, it may have been strengthened with a larger sample.
This RCT provides insufficient evidence to support the recommendation of gabapentin alone as first-line treatment of vulvodynia. Since previous RCTs studying pharmacologic management options also have been negative, practitioners may want to consider all therapeutic options, including non-pharmacologic management interventions such as physical therapy and behavioral and sexual counseling.
Supplementary Material
Acknowledgments
Supported by R01 HD065740 (to Dr. Brown) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Office of Women's Health Research and University of Tennessee General Clinical Research Center. Depomed, Inc. provided gabapentin extended release and matching placebo, but was not involved in the concept or design of the clinical trial, did not have access to data for analysis or interpretation of the data, and did not contribute to writing of the manuscript.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal's requirements for authorship.
Presented at the International Society of Vulvovaginal Disease World Congress, September 13-15, 2017, Mendoza, Argentina.
Clinical Trial Registration: ClinicalTrials.gov, NCT01301001.
Contributor Information
Candace S. Brown, Department of Clinical and Translational Science, University of TennesseeHealth Science Center, Memphis, TN.
Gloria A Bachmann, Department of Obstetrics, Gynecology and Reproductive Sciences, Rutgers-Robert Wood Johnson Medical School, New Brunswick, NJ.
Jim Wan, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN.
David C. Foster, Department of Obstetrics and Gynecology, University of Rochester School of Medicine and Dentistry, Rochester, NY.
References
- 1.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Women's Assoc. 2003;58:82–8. [PubMed] [Google Scholar]
- 2.Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583–93. doi: 10.1097/AOG.0b013e3181e9e0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyirjesy P, Sobel JD, Weiz MV, Leaman DJ, Small MJ, Gelone SP. Cromolyn cream for recalcitrant idiopathic vulvar vestibulitis: results of a placebo-controlled study. Sex Transm Infect. 2001;77:53–7. doi: 10.1136/sti.77.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein J. Efficacy study of topical application of nifedipine cream to treat vulvar vestibulitis. J Lower Gen Tract Dis. 2009;13:S1–S28. 33. [Google Scholar]
- 5.Petersen CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A-a novel treatment for provoked vestibulodynia? Results from a randomized, placebo controlled, double blinded study. J Sex Med. 2009;6:2523–37. doi: 10.1111/j.1743-6109.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 6.Farajun Y, Zarfati D, Abranow L, Livoff A, Bornstein J. Enoxaparin Treatment of vulvodynia: a randomized controlled trial. Obstet Gynecol. 2012;120:565–72. doi: 10.1097/AOG.0b013e3182657de6. [DOI] [PubMed] [Google Scholar]
- 7.De Andres J, Sanchis-Lopez N, Asensio-Samper JM, et al. Vulvodynia – An evidenced-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204–36. doi: 10.1111/papr.12274. [DOI] [PubMed] [Google Scholar]
- 8.Stockdale CK, Lawson HW. 2013 Vulvodynia guideline update. Am Soc Colposcopy Cerv Pathol. 2014;18:93–100. doi: 10.1097/LGT.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 9.Mellegers MA, Furlan AD, Mailis A. Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain. 2001;17:284–95. doi: 10.1097/00002508-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet Gynecol. 2008;112:579–85. doi: 10.1097/AOG.0b013e3181827c77. [DOI] [PubMed] [Google Scholar]
- 11.Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia, unprovoked. J Reprod Med. 2007;52:103–6. [PubMed] [Google Scholar]
- 12.Bates CM, Timmons DJ. Vulvodynia – New and more effective approaches to therapy. Int J STD AIDS. 2002;13:210–2. doi: 10.1258/0956462021924802. [DOI] [PubMed] [Google Scholar]
- 13.Reed BD, Haefner HK, Cantor L. Vulvar dysesthesia (vulvodynia): A follow-up study. J Reprod Med. 2003;48:409–16. [PubMed] [Google Scholar]
- 14.Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg. 1999;89:1459–60. doi: 10.1097/00000539-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Jeon Y, Kim Y, Shim B, Yoon H, Park Y, Shim B, et al. A retrospective study of the management of vulvodynia. Korean J Urol. 2013;54:48–52. doi: 10.4111/kju.2013.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventolini G, Barhan S, Duke J. Vulvodynia, a step-wise therapeutic prospective cohort study. J Obstet Gynaecol. 2009;29:648–50. doi: 10.1080/01443610903095882. [DOI] [PubMed] [Google Scholar]
- 17.Leo RJ. A systematic review of the utility of anticonvulsant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10:2000–8. doi: 10.1111/jsm.12200. [DOI] [PubMed] [Google Scholar]
- 18.Foster DC, Kotok MB, Huang LS, et al. The Tampon Test for vulvodynia treatment outcomes research: reliability construct validity, and responsiveness. Obstet Gynecol. 2009;113:825–32. doi: 10.1097/AOG.0b013e31819bda7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, et al. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol. 2012;206:170.e1–9. doi: 10.1016/j.ajog.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich EG. Vulvar vestibulitis syndrome. J Reprod Med. 1987;32:110–4. [PubMed] [Google Scholar]
- 21.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI. Vulvar vestibulitis syndrome: reliability of diagnosis and evaluation of current diagnostic criteria. Obstet Gynecol. 2001;98:45–51. doi: 10.1016/s0029-7844(01)01389-8. [DOI] [PubMed] [Google Scholar]
- 22.Viangteeravat T, Brooks IM, Smith EJ, et al. Slim-Prim: a biomedical informatics database to promote translational research. Perspect Health Inf Manag. 2009;6:6. [PMC free article] [PubMed] [Google Scholar]
- 23.Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet Gynecol. 2016;127:745–751. doi: 10.1097/AOG.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 24.Rakoff AE. The endocrine factors in pelvic tumors, with a discussion of the Papanicolaou smear method of diagnosis. Radiology. 1948;50:190–201. doi: 10.1148/50.2.190. [DOI] [PubMed] [Google Scholar]
- 25.Ware JH. Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348:2136–7. doi: 10.1056/NEJMe030054. [DOI] [PubMed] [Google Scholar]
- 26.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. doi: 10.1136/bmj.h4672. [DOI] [PubMed] [Google Scholar]
- 27.Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112:231–7. doi: 10.1097/AOG.0b013e318180965b. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACTrecommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 30.Gatti G, Ferrari AR, Guerrini R, Bonanni P, Bonomi I, Perucca E. Plasma gabapentin concentrations in children with epilepsy: influence of age, relationship with dosage, and preliminary observations on correlation with clinical response. Ther Drug Monit. 2003;25:54–60. doi: 10.1097/00007691-200302000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


