Abstract
Background
Patients undergoing hematopoietic stem cell transplant (HSCT) can experience gastrointestinal (GI) side effects as a complication of the treatment. Limited research exists describing how the duration and severity of GI side effects influence the consumption of adequate calorie intake in this population. The purpose of this study was to assess differences in GI side effects between patients who consumed adequate calories compared with those who did not.
Methods
The MD Anderson Symptom Inventory–Gastrointestinal (MDASI-GI) tool was used to record daily GI side effects of 72 HSCT patients. Daily calorie intake was determined via calorie counts. Data were collected from day of transplant until engraftment.
Results
Median percentage of caloric needs consumed for all patients was 49.2% (interquartile range, 35.1–66.6). Calorie intake decreased from baseline to transplant day 8 as severity of GI symptoms increased. An inverse relationship between percentage of caloric needs met and MDASI-GI component score, MDASI-GI symptom score, and lack of appetite score was observed. The only significant difference in MDASI-GI symptom scores between those who consumed adequate calories and those who consumed inadequate calories was for diarrhea; subjects who consumed >60% of caloric needs had significantly lower median diarrhea scores.
Conclusion
Most patients consumed <60% of their caloric needs from time of transplant to time of engraftment. More research is needed to provide insight into strategies to increase intake and to describe the implications of prolonged inadequate intake in HSCT patients. (Nutr Clin Pract. 2015;30:305–310)
Keywords: nutritional support, adverse effects, nutrition therapy, enteral nutrition, stem cell transplantation
Background
Patients receiving hematopoietic stem cell transplantation (HSCT) often experience symptoms of treatment that impair their ability to consume adequate calories. In the past, patients who experienced these gastrointestinal (GI) side effects were often administered parenteral nutrition (PN) to help prevent a decline in their nutrition status. However, several studies have linked the use of PN to complications such as increased incidence of hyperglycemia and delayed time to engraftment.1,2 When HSCT patients are unable to consume or absorb an adequate amount of nutrients for extended time frames, PN may be essential, but its discontinuation is recommended once toxicities from treatment resolve.3 Enteral nutrition (EN) has been shown to help minimize some of the risks associated with PN. While EN has been demonstrated to be safe and feasible in some HSCT populations,4,5 routine use in all HSCT patients has been limited. The limited use of EN is most likely due to the lack of evidence for EN’s impact on clinical outcomes, clinicians’ concerns with increased potential for a higher risk of infection and bleeding associated with GI tube access, and effect of EN on GI toxicities. There is also limited research regarding how oral food consumption affects frequency and severity of GI symptom distress in HSCT patients. Many HSCT patients tolerate treatment without nutrition support and continue consuming an oral diet ad libitum. However, oral ingestion of adequate calories is often difficult for HSCT patients due to the many side effects experienced secondary to HSCT treatment. The tolerance and effectiveness of oral and/or EN in HSCT patients and the relationship between adequate energy and frequency, severity, and duration of GI symptoms have not been well reported. The purpose of this study was to assess differences in GI side effects in HSCT patients who consumed adequate energy intake compared with those who consumed inadequate energy intakes during the course of their HSCT treatment.
Materials and Methods
Patients
During the study period, 116 patients were admitted to the bone marrow transplant unit of a tertiary care urban academic medical center. Of those patients, 88 agreed to participate in the study (76%). Those who failed to consent were non–English speaking (n = 8), had previously participated in the study (n = 4), did not want to participate daily (n = 4), were too anxious (n = 2), or declined for various other miscellaneous reasons (n = 10). Of the 88 patients who agreed to participate, 2 dropped out of the study due to personal reasons and 1 patient did not engraft. Thirteen patients received PN at some point during time of transplant to time to engraftment and were excluded in the analyses to avoid bias from type of feeding modality. A total of 72 patients were included in the analysis. Institutional review board approval was obtained for conducting this study, and informed consent was required from all patients. Eligible patients were undergoing HSCT, English speaking, and 18 years or older. Non–English-speaking patients, those who received PN at any time from transplant to engraftment, and nonconsenting patients were excluded.
Dietary Intake
Dietary intake was assessed using calorie counts to determine the amount of calories and protein patients consumed. Patients were visited after every meal, and a calorie count was conducted by a diet technician or a registered dietitian. Patients were given an informational sheet describing why they were placed on a calorie count as well as a worksheet to record any snacks consumed in between meals. Prior to the start of the study, to establish the consistency of dietary intake measures, a random sample of 10 meal trays from patients in the main hospital was assessed for caloric consumption by 11 raters (8 diet technicians, 2 registered dietitians, and 1 dietetic intern). Raters calculated the number of calories consumed from each meal tray after estimating the amount of food consumed by the patient. Results using intraclass correlation (ICC) showed an ICC of 0.95, indicating good interrater agreement. Calorie counts began on the day patients received their transplantation (day 0) and continued until engraftment. After each meal, the percentage of each food item consumed at that meal was recorded and entered into the CBORD Foodservice Suite version 6.8.100 (CBORD, Ithaca, NY), where the total calories and total protein were calculated for each meal. Snacks and nutrition supplements that patients consumed were also included. If a food item was not in CBORD Foodservice Suite, the caloric and protein information for the food item was obtained from the U.S. Department of Agriculture National Nutrient Database for Standard Reference version 1.2.2.6 If there were missing calorie counts, the research team used the electronic medical record to obtain the percentage of meals consumed from the nursing flow sheet. The research team then calculated that percentage of calories and protein from the average daily values of the 14-day menu cycle. If there was nothing recorded in the electronic medical record, the research team took an average of the other 2 meals from that day to determine the calories and protein consumed for the missing meal as is standard practice at the medical center where the study took place. The total amount of calories and protein consumed per day was calculated from meals, snacks, and nutrition supplements. The percentage of caloric needs that were met for the study period was calculated as a proportion of net caloric intake (day of transplant to day of engraftment) divided by estimated caloric needs (30 kcal/kg/d and 1.5 g of protein/kg/d). If the patient’s admission weight was >120% of ideal body weight, adjusted body weight was used when calculating energy needs.7 For the purpose of this study, consuming ≥60% of caloric needs was considered adequate. Patients were divided into 2 groups: those who consumed ≥60% of their calorie needs and those who consumed <60% of their calorie needs.8
GI Symptoms
The MD Anderson Symptom Inventory–Gastrointestinal (MDASI-GI) was used to evaluate patients from the first day of transplant until engraftment. The MDASI-GI tool includes 13 core items, 5 GI items, and 6 interference items and uses a 10 point rating scale for scoring.9,10 The MDASI-GI tool has been validated using a sample of autologous bone marrow transplant (BMT) patients.10 For the purpose of this study, a MDASI-GI component score was calculated using the 13 core items and 5 GI items. A MDASI-GI symptom score was also calculated and included 4 select core items (nausea, lack of appetite, dry mouth, and vomiting) and 4 select GI items (constipation, diarrhea, difficulty swallowing, and change in taste). These subset groupings are endorsed by the developers of the MDASI.10 The daily scores were averaged over the duration of the study (day 0 to engraftment) for each patient and used in the analysis. Ratings for each individual symptom were also summed and averaged for each patient over the duration of the study.
Additional Variables
Additional data were collected to describe the study sample, including age, race, sex, body mass index (BMI, defined as kg/m2), HSCT type, and conditioning regimen.
Data Analysis
Nonparametric statistics were used to accommodate violations in the normality assumptions and differences in sample size for groups. Mann-Whitney U tests were used to determine differences in patient characteristics based on adequacy of oral intake (≥60% of caloric needs vs <60% of caloric needs). Spearman rank order correlation was used to determine the relationship between caloric intake and MDASI-GI component and individual GI symptom scores.
Results
Patient Characteristics
Table 1 summarizes the patient characteristics for all patients included in the analysis (n = 72) and stratified by amount of calories consumed during the study. The study population was 65% white and 64% male with a mean ± SD age of 54.9 ± 11.8 years and median BMI of 28.0 (interquartile range [IQR], 24.4–32.3). Most patients received an autologous (72%) transplant compared with allogeneic (20%) and matched unrelated donor (MUD) (8%) transplants. This is consistent with the population of HSCT patients treated at the medical center where the study was conducted.
Table 1. Characteristics of Patients Receiving HSCT.
| Characteristic | Total (N = 72) |
≥60% of Caloric Needs (n = 22) |
<60% of Caloric Needs (n = 50) |
P Value |
|---|---|---|---|---|
| Sex | .59 | |||
| Male | 46 (63.9) | 14 (63.6) | 32 (64.0) | |
| Female | 26 (36.1) | 8 (36.4) | 18 (36.0) | |
| Race | .44 | |||
| White | 47 (65.3) | 14 (63.6) | 33 (66.0) | |
| Black | 16 (22.2) | 4 (18.2) | 12 (24.0) | |
| Asian | 1 (1.4) | 1 (4.5) | 0 | |
| Hispanic | 8 (11.1) | 3 (13.6) | 5 (10.0) | |
| Age, mean ± SD, y | ||||
| Total | 54.9 ± 11.8 | 56.6 ± 8.7 | 54.1 ± 12.9 | .42 |
| Autologous | 55.7 ± 13.1 | 59.6 ± 10.4 | 54.6 ± 13.7 | .25 |
| Allogeneic | 51.9 ± 7.7 | 53.4 ± 4.5 | 50.0 ± 10.8 | .44 |
| MUDa | 54.7 ± 5.5 | 51.5 ± 2.1 | 56.3 ± 6.2 | .37 |
| BMI, median (IQR) | 28.0 (24.4–32.3) | 25.3 (23.5–31.5) | 28.4 (25.2–33.4) | .07 |
| BMI category | .19a | |||
| Underweight | 0 | 0 | 0 | |
| Normal | 20 (27.8) | 10 (45.5) | 10 (20.0) | |
| Overweight | 26 (36.1) | 5 (22.7) | 21 (42.0) | |
| Obesity grade I | 15 (20.8) | 5 (22.7) | 10 (20.0) | |
| Obesity grade II | 7 (9.7) | 1 (4.5) | 6 (12.0) | |
| Obesity grade III | 4 (5.6) | 1 (4.5) | 3 (6.0) | |
| Type of HSCT | .07 | |||
| Autologous | 52 (72.2) | 12 (54.5) | 40 (80.0) | |
| Allogeneic | 14 (19.4) | 8 (36.4) | 6 (12.0) | |
| MUD | 6 (8.3) | 2 (9.1) | 4 (8.0) | |
| Diagnosis | ||||
| Multiple myeloma | 30 (41.7) | 10 (45.5) | 20 (40.0) | .04b |
| Lymphoma | 26 (36.1) | 3 (13.6) | 23 (46.0) | |
| Acute leukemia | 13 (18.1) | 7 (31.8) | 6 (12.0) | |
| Myelofibrosis | 2 (2.8) | 1 (4.5) | 1 (2.0) | |
| CLL/SLL | 1 (1.4) | 1 (4.5) | 0 | |
| Conditioning regimen | .015c | |||
| Melphalan | 29 (40.3) | 10 (45.5) | 19 (38.0) | |
| BEAM | 17 (23.6) | 1 (4.5) | 16 (32.0) | |
| Fludarabine/melphalan | 13 (18.1) | 7 (31.8) | 6 (12.0) | |
| BendaEAM | 5 (6.9) | 1 (4.5) | 4 (8.0) | |
| Busulfan/fludarabine | 4 (5.6) | 3 (13.6) | 1 (2.0) | |
| Total-body irradiationd | 3 (4.2) | 0 | 3 (6.0) | |
| Ara-C | 1 (1.4) | 0 | 1 (2.0) |
Values are presented as number (%) unless otherwise indicated. BEAM, BCNU (carmustine), etoposide, Ara-C (cytarabine), and melphalan; BendaEAM, bendamustine, etoposide, cytarabine, and melphalan; BMI, body mass index; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; HSCT, hematopoietic stem cell transplant; IQR, interquartile range; MUD, matched unrelated donor.
χ2 Analysis between normal, overweight, and obese; P > .05.
χ2 Analysis between lymphoma, multiple myeloma, and acute leukemia; P < .05.
χ2 Analysis between melphalan, BEAM, and fludarabine/melphalan; P < .05.
Total-body irradiation plus additional chemotherapy agents, including fluarabine, cytoxan, and/or cyclophosphamide.
Dietary Intake
Total patients
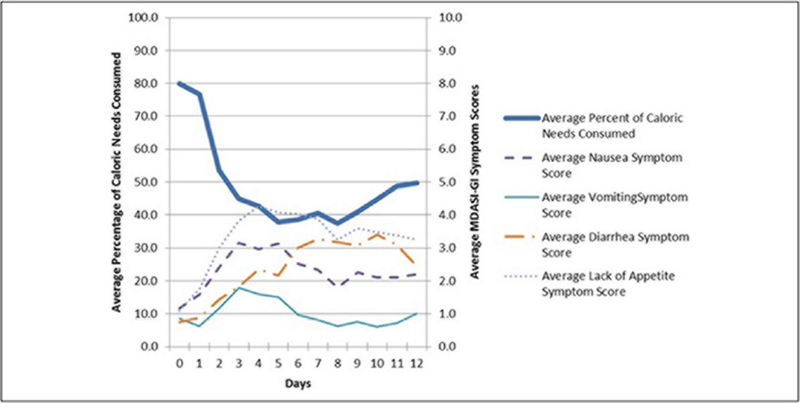
Most patients consumed an oral diet without nutrition support (97.2%; n = 70). Two patients (2.2%) received EN secondary to being intubated. The median (IQR) percentage of caloric needs met for all patients was 49.2% (35.1%–66.6%) (Table 2). Most patients (n = 50; 69.4%) consumed <60% of their caloric needs from the time of transplant to time of engraftment. Calories were also reported as kcal/kg/d. The median (IQR) intake for all subjects was 10.5 (7.0–14.8) kcal/kg/d, approximately one-third of their estimated needs (Table 2). As with calories, protein was examined as the percentage of protein needs consumed and grams of protein/kg/d. The median (IQR) percentage of protein needs met for all patients was 38.3% (27.1%–53.0%), and the median (IQR) intake of protein for all patients was 0.4 (0.3–0.6) g of protein/kg/d. As seen in Figure 1, calorie intake decreased from baseline to transplant day 8 as severity of GI symptoms increased. Average calorie intake fell to below 60% of requirements by transplant day 3.
Table 2. Median (IQR) Calorie and Protein Intake Over Time in HSCT Patients by Type of Transplant.
| Energy | Protein | |||||
|---|---|---|---|---|---|---|
| Type of HSCT | Percent Caloric Needs |
kcal/kg/d | Caloric Deficit/d |
Percent Protein Needs |
Grams of Protein/kg/d |
Protein Deficit/d |
| Total (N = 72) | 49.2 (35.1 to 66.6) |
10.5 (7.0 to 14.8) |
−1098 (−1435 to −640) |
38.3 (27.1 to 53.0) |
0.4 (0.3 to 0.6) |
−68.7 (−80.2 to −40.8) |
| Autologous (n = 52) | 44.5 (30.8 to 56.6)a |
13.4 (9.3 to 17.0)a |
−1135 (−1462 to −813)a |
34.9 (24.9 to 44.9)a |
0.5 (0.4 to 0.7)a |
−70.7 (−82.6 to −50.9)a |
| Allogeneic (n = 14) | 66.5 (49.8 to 97.8)b |
20.0 (14.9 to 29.3)b |
−557 (−1168 to −53)b |
55.9 (38.1 to 76.9)b |
0.6 (0.3 to 1.2)b |
−39.0 (−75.9 to −27.3)b |
| MUD (n = 6) | 58.1 (46.4 to 65.1) |
17.4 (13.9 to 19.5) |
−1084 (−1267 to −920) |
45.5 (40.5 to 60.4) |
0.7 (0.6 to 0.9) |
−68.9 (−73.5 to −51.9) |
HSCT, hematopoietic stem cell transplant; IQR, interquartile range; MUD, matched unrelated donor.
Values bearing different superscripts in the same column are significantly different on the basis of Bonferroni-corrected Mann-Whitney U tests.
Values bearing different superscripts in the same column are significantly different on the basis of Bonferroni-corrected Mann-Whitney U tests.
Figure 1.
Average MD Anderson Symptom Inventory–Gastrointestinal (MDASI-GI) scores and percentage of caloric needs consumed by hematopoietic stem cell transplant patients (n = 72). Day 0 = day of transplant.
Type of HSCT and conditioning regimen
A comparison of caloric and protein intake in HSCT patients receiving different types of transplants is also shown in Table 2. Patients were stratified by type of transplant: autologous, allogeneic, and MUD. Allogeneic HSCT patients consumed a significantly higher percentage of their calorie (P = .005) and protein needs (P = .004) than did autologous HSCT patients (Table 2). There were no other significant differences in median percentage of caloric needs or protein needs met between the other groups. A post hoc analysis of calorie and protein intake between conditioning regimens was performed, but there were no significant differences.
GI symptoms
There were no significant differences in median MDASI-GI component scores or MDASI-GI symptom scores between those who consumed adequate calories compared with those who consumed inadequate calories. However, patients who consumed ≥60% of caloric needs had significantly lower median diarrhea scores (1.7; IQR, 0.7–2.7) compared with those who consumed <60% of caloric needs (2.7; IQR, 1.6–2.7; P < .05). When stratified by type of HSCT, this was no longer significant. An inverse relationship between percentage of caloric needs met by total patients and MDASI-GI component score (rs = −0.28, P = .019), MDASI-GI symptom score (rs = −0.26, P = .029), and lack of appetite score (rs = −0.35, P < .003) was observed.
Discussion
Since many of the side effects of treatment experienced by HSCT patients may impair their ability to consume an adequate amount of calories and protein, those who consumed <60% of their caloric needs were predicted to report more severe GI symptoms compared with those who were able to consume ≥60% of their caloric needs.
The amount of calories and protein that HSCT patients consume as reported in the literature varies and is limited. The caloric and protein intake for patients in this study was consistent with other reports in HSCT patients.11,12 In a study conducted by Szeluga et al,11 the researchers reported an average intake of 20–25 kcal/kg/d and 0.5–1.0 g of protein/kg/d. The larger intakes in the study by Szeluga et al are likely due to inclusion of patients receiving supplemental EN or PN. In the current study, 70 patients (97.2%) consumed an oral diet without any nutrition support. Patients in our study consumed an average of 1109.9 ± 501.7 kcal/d and 44.3 ± 20.9 g of protein/d, which is greater than the intake reported for patients in a study conducted by Roberts et al.12 Patients in the oral diet group of the Roberts et al study consumed 951 ± 191 kcal/d and 36 ± 10 g of protein/d. However, 50% (n = 14) of the patients in their oral diet group received PN due to 10 consecutive days of oral intake <40% of nutrient needs. This may account for the lower intake in these patients and help explain the discrepancy in the results between the 2 studies. Differences in intake may also be due to differences in the study populations. Roberts et al included only patients with breast cancer receiving autologous HSCT, while the current study and Szeluga et al included patients with various diagnoses receiving both autologous and allogeneic HSCT.
Habschmidt et al13 recently conducted a study surveying registered dietitians who practice within the area of HSCT. The purpose of this study was to determine the current medical nutrition therapy (MNT) provided to adult patients undergoing HSCT and examine the current and desired role of dietitians in providing MNT to HSCT patients. Most survey respondents reported that oral intake was the main form of nutrition therapy used in HSCT patients as they identified barriers to nutrition support, including GI toxicities (38%), physician and medical staff agreement with care (30%), mucositis (13%), and a lack of research data and protocols for practice (8%). Similarly, most patients who consented in the present study consumed an oral diet (82.4%; n = 70).
Our findings show that most HSCT patients consumed <60% of caloric and protein needs from time of transplant to time of engraftment. Many of the GI side effects experienced by HSCT patients may affect their ability to consume an adequate amount of calories and protein; however, little supportive evidence exists in the literature. A study conducted by Leistra et al14 investigated predictors for achieving protein and energy requirements on the fourth day of admission in undernourished hospitalized patients. Negative predictors for achieving energy and protein requirements included nausea (odds ratio [OR], 0.18; 95% confidence interval [CI], 0.06–0.53; P <.001) and patients having cancer (OR, 0.57; 95% CI, 0.35–0.93; P < .001); nausea was commonly reported in the present study as well.
Calorie and protein intake also varied depending on the type of transplant the patients received. Allogeneic and MUD HSCT patients consumed more calories and protein than did autologous HSCT patients. Age was considered a contributing factor to this difference since autologous HSCT patients tend to be older than allogeneic and MUD HSCT patients.15 However, age was not significantly different between autologous and allogeneic/MUD HSCT patients in this study. The small sample size of the current study could have influenced the findings. Limited information is available in the literature examining differences in caloric intake by type of HSCT and conditioning regimen received.
A number of factors may have affected the MDASI-GI scores, resulting in very few differences between intake groups. There was a high amount of missing data, which may have biased the results. Twenty-five patients (34.7%) had more than 10% of data missing, which demonstrates the burden on patients to complete daily surveys. It appeared that the number of missing surveys increased as symptom severity increased, and thus the scores may be underestimated. Patients who consumed ≥60% of caloric needs had less diarrhea than those who consumed <60% of caloric needs. Adequate nutrients help heal the endothelial lining of the GI tract, which promotes regrowth of the endothelial lining and could possibly be related to better oral intake.16 However, many people may self-restrict their oral intake when they are experiencing diarrhea with the belief that food will worsen the effect. This may explain why those patients in the current study who consumed <60% had more diarrhea. A study conducted by Mulder et al17 compared the number of days that autologous HSCT patients who received either PN or partial parenteral plus EN by tube feeding (PPN/EN) experienced diarrhea. The percentage of days with diarrhea was lower in the PPN/EN group (26.8 ± 16.8 days) compared with the PN group (53.6 ± 20.4 days) (P < .005).
Our findings showed a significant inverse relationship between intake and MDASI-GI component, MDASI-GI symptom score, and lack of appetite scores, which was expected. As these symptom scores increased, oral intake decreased. However, the relationships were weak, indicating that very little of the variance in intake was explained by the GI symptoms.
Limitations
Several limitations should be considered when deriving conclusions from this study. Due to the small sample size, particularly across types of transplants and conditioning regimens, there may not have been enough power to detect actual differences in outcomes measured. Another limitation of the current study is that nil per os (NPO) status was not documented. Therefore, if no oral intake was reported, this could have indicated that patients were either choosing not to eat or were unable to eat due to NPO status. Possible reasons patients could have been NPO include procedures, tests, or neutropenic enterocolitis. Finally, the fact that severity of GI symptoms was based on self-report is a limitation of the current study.
Conclusion
Most patients consumed <60% of their caloric needs from time of transplant to time of engraftment. An inverse relationship between percentage of caloric needs met and MDASI-GI component score, MDASI-GI symptom score, and lack of appetite score was observed. There were few differences in self-reported GI symptoms between the groups, but those who consumed >60% of caloric needs had significantly lower median diarrhea scores. More research is needed to describe the oral intake of HSCT patients by type of transplant and type of conditioning regimen. If consuming an adequate amount of calories and protein can demonstrably reduce the frequency and severity of GI symptoms in HSCT patients and positively affect clinical outcomes, maximizing nutrient delivery via EN support may become an important part of treatment for these patients. There appears to be a gap in the literature with respect to adequacy of intake and clinical outcomes, survival, and quality of life in this population. Future research investigating the association between nutrition adequacy and outcomes would make a positive contribution to the field.
Footnotes
Financial disclosure: None declared
References
- 1.Cetin T, Arpaci F, Dere Y, et al. Total parenteral nutrition delays platelet engraftment in patients who undergo autologous hematopoietic stem cell transplantation. Nutrition. 2002;18(7–8):599–603. [DOI] [PubMed] [Google Scholar]
- 2.Sheean P, Freels S, Helton WS, Braunschweig C. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(6):656–664. [DOI] [PubMed] [Google Scholar]
- 3.Allen D, Huhmann MB; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoetic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33: 472–500. [DOI] [PubMed] [Google Scholar]
- 4.Sefcick A, Anderton D, Byrne JL, Teahon K, Russell NH. Naso-jejunal feeding in allogeneic bone marrow transplant recipients: results of a pilot study. Bone Marrow Transplant. 2001;28(12):1135–1139. [DOI] [PubMed] [Google Scholar]
- 5.Seguy D, Berthon C, Micol J, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006;82(6):835–839. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Agriculture. National nutrient database for standard reference. 2011. http://ndb.nal.usda.gov/ndb/search/list. Accessed November 15, 2011.
- 7.Wilkens K. Adjustment for obesity. ADA Renal Practice Group Newsletter. 1984;3:6. [Google Scholar]
- 8.Russell M, Andrews M, Brewer C, Rogers J, Seidner D. Standards for specialized nutrition support: adult hospitalized patients. Nutr Clin Pract. 2002;17(6):384–391. [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland C. The MD Anderson Symptom Inventory user guide. 2012. http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/m-d-anderson-symptom-inventory.html. Accessed September 25, 2011.
- 11.Szeluga DJ, Stuart RK, Brookmeyer R, Utermohlen V, Santos GW. Energy requirements of parenterally fed bone marrow transplant recipients. JPEN J Parenter Enteral Nutr. 1985;9(2):139–143. [DOI] [PubMed] [Google Scholar]
- 12.Roberts S, Miller J, Pineiro L, Jennings L. Total parenteral nutrition vs oral diet in autologous hematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32(7):715–721. [DOI] [PubMed] [Google Scholar]
- 13.Habschmidt M, Bacon C, Gregoire M, Rasmussen H. Medical nutrition therapy provided to adult hematopoietic stem cell transplantation patients. Nutr Clin Pract. 2012;27(5):655–660. [DOI] [PubMed] [Google Scholar]
- 14.Leistra E, Willeboordse F, van Bokhorst M. Predictors for achieving protein and energy requirements in undernourished hospital patients. Clin Nutr. 2011;30(4):484–489. [DOI] [PubMed] [Google Scholar]
- 15.Rush University Medical Center. Blood and marrow information network. http://www.bmtinfonet.org/node/6722. Accessed November 12, 2011.
- 16.Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: executive summary. Crit Care Med. 229;37(5):1757–1761. [DOI] [PubMed] [Google Scholar]
- 17.Mulder PO, Bouman JG, Gietema JA, et al. Hyperalimentation in autologous bone marrow transplantation for solid tumors: comparison of total parenteral versus partial parenteral plus enteral nutrition. Cancer. 1989;64(10):2045–2052. [DOI] [PubMed] [Google Scholar]