Abstract
The International Commission on Radiation Units and Measurements (ICRU) volumes are standardized volume definitions used in radiation oncology practice that have evolved over time to account for advancements in technology and radiation planning. The current definitions have strengths but also practical limitations. The main limitation is related to the process of accounting for tumor motion during treatment. As radiotherapeutic techniques become more precise, motion interplay effects and anatomic changes during treatment must be taken into account to ensure accurate and safe delivery of treatment. Adaptive re-planning can help to mitigate the impact of these uncertainties and widen the therapeutic ratio by maximizing dose to the tumor and protecting critical normal structures. As adaptive re-planning becomes more common, standardization of how adaptive therapy is implemented and reported will become necessary.
Standardized volume definitions are integrated in radiation oncology practice, but this was not always the case. In 1978, the first International Commission on Radiation Units and Measurements (ICRU) volumes were defined with a distinction made between the target and the treated volume[1]. The integration of volumetric imaging into the clinic resulted in updates to the ICRU volumes and the concept of Gross Tumor Volume (GTV), Clinical Target Volume (CTV) and Planning Target Volume (PTV) were introduced in ICRU 50[2]. These definitions were updated in ICRU report 62, where the concept of the Internal Target Volume (ITV) was introduced to account for variations in the CTV shape, size and location inside the patient[3]. The progression of the ICRU target volumes is discussed in detail by Purdy[4].
The standardization of contouring and naming targets and normal structures is essential for advancing the field of radiation oncology. Multi-center clinical trials and the adoption of improved treatment techniques rely upon this standardization. The use of the internal target volume (ITV) varies widely across centers. The ITV assumes that the radiation treatment is delivered based on setup to anatomic landmarks (e.g. bony anatomy, skin tattoos). The role of the ITV when the treatment is aligned to the target remains unclear and lacks standardization.
The strengths and limitations of the current ICRU definitions
The current ICRU definitions take into account advances in technology to more accurately define margins required for tumor motion. The excursion of targets can be highly variable depending on the patient and location in the body. Some patients may take shallower or deeper breaths than others. Tumors close to the diaphragm typically have more motion than tumors located farther away from the diaphragm. Creating an ITV individually tailors respiratory motion margins for each patient and helps to more accurately target tumors. Limitations related to creating an ITV include the ICRU-defined sequence of contouring the ITV as well as uncertainties related to the technique for generating a motion envelope. Das et al. evaluated compliance to ICRU-83, which was released in 2010, to specifically address intensity-modulated radiation therapy (IMRT) and volumetric based planning. Plans from 5094 patients from 10 institutions were analyzed [5]. They found that compliance to ICRU definitions is poor, with only 25% of cases using standard target site prescription names and many (41%) cases using numbered variants of the nomenclature (ex: CTV1). When contouring, the ICRU volume definition starts with creating a GTV followed by a CTV to account for uncertainties in microscopic tumor spread. The CTV is usually generated by first performing a margin expansion and then adjusting or cropping this volume for anatomic boundaries. For example, tumors without bone invasion are unlikely to have microscopic spread into nearby osseous structures. Therefore, the CTV is often cropped off bone.
According to the ICRU volume definition, the ITV should then be created to represent the motion of the CTV. As CTV is a volume that encompasses microscopic tumor spread, it is impossible to visualize the motion of microscopic disease. Therefore, it is difficult to follow the sequencing of target volume generation based on the current ICRU definitions. A more practical alternative margin method is needed.
In practice, radiation oncologists often alter the sequencing of contouring and contour a GTV followed by IGTV based on the tumor motion of the GTV. The IGTV is then generally expanded for microscopic extension and adjusted for anatomic boundaries. The name of this structure is variable: CTV, ITV or ICTV. The name “ICTV” may be most appropriate, as this structure takes into account both motion and microscopic tumor spread. This structure is then expanded for setup uncertainty to create the PTV. This sequencing of GTV to IGTV to ICTV to PTV is, therefore, a more practical approach for target volume definition.
Generating a motion envelope and the uncertainties
Creating a free-breathing ITV requires having an accurate motion envelope that encompasses the moving tumor during radiation therapy. This is achieved by contouring the tumor on four-dimensional CT (4DCT) scans and has replaced previous techniques of estimating motion envelopes based on a slow-CT scan[6], interpolation between end-expiration and inspiration breath-hold scans[7] and expanding the target volume to account for motion[8]. During the acquisition of a 4DCT scan, the patient motion is monitored and recorded using an external surrogate for motion. This surrogate may be a pressure transducer on the patient’s abdomen, an infrared marker, or optical surface scanning systems that characterize the location of the patient’s chest. While the patient motion is monitored, a series of CT scans are acquired. These static scans are sorted by either phase or amplitude [9], and this collection of sorted images makes up the 4DCT image set.
From the 4DCT study, the motion of the target (GTV) and organs at risk can be evaluated and contoured throughout the breathing cycle. The Average Intensity Projection (AIP) image represents the average of all of the images that make up the 4DCT image set. The Maximum Intensity Projection (MIP) represents the maximum voxel value of all of the images that make up the 4DCT image set. Contouring the target on the AIP or the MIP, using appropriate window/leveling, and verifying that the contour is accurate by visually comparing the contour to the 4DCT is commonly done and is less time-intensive than contouring the target on each individual phase of the 4DCT image set. Although time-efficient, there are limitations to using the MIP for targeting. This approach is only useful when the tumor is surrounded by tissue with a large separation in Hounsfield units, such as a peripherally located tumor surrounded by normal lung parenchyma. When the tumor is surrounded by tissue of similar Hounsfield units, as is the case when the tumor is in close proximity to bone, vessels, or the mediastinum, the MIP cannot optimally distinguish between tumor and surrounding normal tissue, leading to potential over- or under-contouring. Therefore, this approach is of limited utility for centrally located lung tumors and tumors in close proximity to the chest wall.
For treatment planning, the assumption is that the motion captured during the imaging study is representative of motion throughout the treatment. Target motion reproducibility has been evaluated through serial CT scans on the same day[10] and through simulations of target motion taken from patient tumor trajectories over multiple days of treatment[11]. It remains a challenge to predict for which patients the target will move outside of the motion envelope.
Device-less 4DCT techniques are currently available that enable scans that do not rely on external surrogates of motion[12, 13]. However, these techniques do not address if the motion captured at the time of simulation adequately represents motion throughout the course of treatment.
Motion and volume adaptation in current radiotherapeutic paradigms
As advanced treatment delivery techniques such as intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), and pencil beam scanning (PBS)-based proton therapy are integrated into the radiotherapeutic armamentariums, concerns are often raised regarding the impact of tumor motion on accurate delivery of dose to the target. The primary concern is that interplay between tumor motion and beam modulation could result in inadvertent underdosing of the tumor or excessive dose to nearby organs at risk. The interplay effect has been extensively reported on in the setting of IMRT[14–17], VMAT[18–20], and PBS[21, 22]. These studies then paved the way for the development of mitigation strategies to minimize the potential impact of motion interplay. Strategies such as volumetric or layer repainting in PBS-based proton delivery [23], as well as segmentation approaches in VMAT-based lung radiotherapy[24] have been shown to increase the robustness of these treatment approaches in the setting of mobile tumors.
Fortunately, despite potential concerns raised from simulations and theoretical models, the clinical outcomes with these technologies have not suffered. In fact, IMRT was recently shown to be associated with lower rates of clinically significant pneumonitis without any compromise in overall survival, progression-free survival, or local control in the secondary analysis of a large phase III randomized clinical trial of standard vs escalated radiation dose in locally advanced non-small cell lung cancer.[25] This is despite the fact that patients receiving IMRT generally had larger tumor volume and more advanced disease. Similarly, VMAT has yielded comparable results for local control with favorable toxicity rates in the setting of stereotactic body radiation therapy when compared with historical rates achieved with 3D-conformal delivery.[26] The data with pencil beam scanning with protons are emerging, but recent clinical data in the setting of esophageal cancer suggests that use of this approach in the setting of esophageal cancer is safe and feasible.[27]
In summary, the quantification of potential interplay effects through carefully controlled phantom and model-based studies are of vital importance prior to deployment of new technologies in the clinic. These studies pave the way for mitigation strategies that ultimately allow for safe and effective utilization of these highly conformal techniques in the setting of mobile tumors.
Adaptive Re-planning
In addition to considering the impact of tumor and patient motion during treatment, it is important to consider anatomic changes that can occur during a prolonged course of therapy. The size of the tumor as well as the anatomic location of the tumor are subject to change during radiation treatment. Multiple studies have demonstrated a median GTV reduction of 40–50% by weeks 3–5 in patients with non-small cell lung cancer (NSCLC)[28, 29]. This reduction in volume in NSCLC has also been shown to occur as early as within the first 2 weeks of treatment and also correlates with outcome [30–32]. Additionally, changes in lung density, due to atelectasis and pleural effusions, are possible during treatment and require re-planning in about 10% of patients [33, 34].
Head and neck cancers and cervical cancers are also particularly sensitive to radiation effects. Barker et al. demonstrated a 69.5% reduction in GTV volume with a median mass displacement of 3.3mm at the end of radiation treatment in patients with head and neck cancers. Cervical cancers have a median mid treatment regression rate of 69% at 36–45Gy when treated with radiation alone and 79% when treated with concurrent chemoradiation [35]. The resulting anatomic changes during radiation treatment can have a significant impact on dosimetric distributions and can result in inadequate dose delivery to the tumor even with 4D CT image-guided radiotherapy planning [28, 36]. Britton et al. evaluated the dosimetric consequences of anatomic changes during conformal photon beam radiotherapy for 10 patients with NSCLC using weekly 4D CT Scans. The authors noted a substantial decrease (−20.5%) in the PTV dose coverage and variable increases in doses to normal tissue structures such as the lung, spinal cord, esophagus and heart [36].
At present, the standard of care is to base the entire course of radiation treatment upon the initial treatment plan, which is derived from the pre-treatment scan. However, in some cases, it is important to use on-board imaging or verification CT scans to assess the need for treatment re-planning to adjust for anatomical changes during the course of radiation treatment. This could widen the therapeutic ratio by maximizing dose to the tumor and protecting critical normal structures. Ideally, one would account for both inter-and intra-fraction variability on a daily basis or after a given number of fractions.
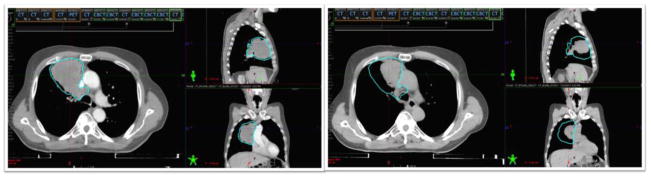
Example cases are noted in Figure 1 and 2. Figure 1 demonstrates CT simulation images of a patient with locally advanced NSCLC undergoing concurrent chemoradiation at the initial simulation (left) and at week 4 of treatment (right). At 4 weeks into treatment, significant regression in tumor size was noted on weekly cone-beam CT. The patient was re-simulated. When the volumes were adjusted for anatomic changes and the initial plan was calculated on the new CT, it was noted that the patient’s lung V20 was over the acceptable range. The patient required re-planning to ensure the radiation treatment would be safe.
Figure 1.
CT simulation images of a patient with locally advanced NSCLC undergoing concurrent chemoradiation at the initial simulation (left) and at week 4 of treatment (right) demonstrating considerable regression in the tumor during treatment.
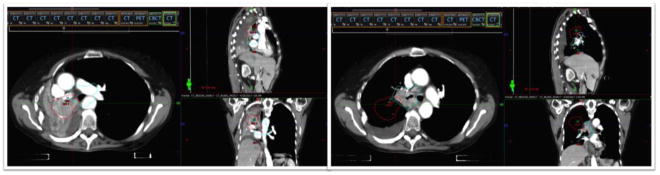
Figure 2.
CT simulation images of a patient with locally advanced NSCLC undergoing concurrent chemoradiation at the initial simulation (left) and after week 1 of treatment (right) demonstrating re-inflation of a collapsed lung.
Figure 2 demonstrates CT simulation images of a patient with locally advanced NSCLC undergoing concurrent chemoradiation at the initial simulation (left) and after week 1 of treatment (right). At the time of initial simulation, her right lung was completely collapsed. After a few fractions of radiation, her right lung re-expanded, causing a dramatic shift in anatomy and displacement of the GTV (red outline is the initial GTV and cyan outline is the GTV on re-simulation). This displacement required a re-simulation and re-planning for accurate targeting.
In addition to anatomic changes, functional changes in the tumor, as assessed by PET scan, have been found during the course of radiation therapy in various cancers, such as lung, head and neck and cervical cancers [37–39]. Mid-treatment PET scans are being investigated as tools to risk-adapt treatment [37]. RTOG 1106 is a randomized phase II trial in patients with locally advanced NSCLC undergoing concurrent chemoradiation. This trial investigated the role of a week 4 PET scan to individually adapt therapy. The control arm treated patients to the standard dose of 60 Gy in 30 fractions without re-planning. The experimental arm escalated the radiation dose to areas of persistent FDG avidity on the week 4 PET scan in an attempt to improve control of areas in the tumor with persistent activity. Results are pending.
Adaptive re-planning comes with some challenges. One concern is that shrinking target volumes could lead to under-coverage of microscopic disease. Berkovic et al., however, noted in a small retrospective study, that with adaptive planning at fractions 15–20, normal tissue structures receive lower doses of radiation and initial CTV volumes that account for initial microscopic disease, still receive adequate microscopic doses of 50 Gy [40]. The safety of re-planning needs to be evaluated in a prospective fashion, as in studies like RTOG 1106. In the experimental arm of this this protocol, 95% of the initial PTV volume was required to receive a radiation dose of 50 Gy, and 95% of the initial CTV volume was required to receive a dose of 60 Gy. These specifications help to ensure adequate coverage of potential microscopic disease, even as tumors evolve during treatment.
Another challenge with adaptive re-planning involves allocation of resources for replanning. Adaptive re-planning requires re-contouring and re-planning, which is currently time consuming. Additional resources to acquire images, such as time on the treatment machine for cone-beam CT images or CT simulator time are also needed. Due to the potential benefits of adaptive re-planning, technology is evolving to accommodate these limitations with automated planning and MR Linac technology [41].
Additionally, another potential burden of adaptive re-planning is standardizing nomenclature to distinguish volumes and targets at different time points. The RTOG 1106 protocol had 10 target volumes in the adaptive arm to distinguish the phases and imaging modalities used to generate the target volumes. With increasing numbers and variations of target volumes, treatment planning becomes more complex. As the field moves in the direction of more frequent adaptive re-planning, ICRU definitions to standardize and formalize treatment-planning approaches will become necessary.
Implications of Tumor Motion and Adaptive Re-planning for Proton Therapy
Tumor motion and normal tissue changes during radiation therapy are of particular concern in proton radiation therapy. Small differences in the amount of tissue that the proton beam passes through can result in large deviations in the dose deposited in the target or surrounding normal tissue. The decrease of a tumor mass as a result of therapy, differences in lung density resulting from radiation therapy[42] and the presence/resolution of a pleural effusion[33] may result in drastic changes to the target coverage and doses to organs at risk if the proton beams pass through these variations and may necessitate adaptive re-planning.
This can be mitigated through the careful choice of beam angles, the use of multiple beams and frequent imaging studies to verify the dose deposited to the target and surrounding organs at risk. The integration of treatment room based volumetric imaging (e.g. cone beam CT) in proton therapy has lagged behind external beam radiation therapy[43] and most adaptive re-planning in proton therapy is based upon follow up CT scans at predetermined intervals. Adaptive re-planning [44–46] in proton therapy has the potential to ensure that the target receives the prescribed radiation dose and normal tissue constraints are met throughout treatment. Future clinical trials evaluating adaptive re-planning will require a standardization of how adaptive therapy is implemented and reported.
Conclusions
In conclusion, while the current ICRU definitions have evolved over time and take into account advances in technology to more accurately define margins and account for tumor motion, the current definitions are not ideal. Revisions of the current ICRU definitions to more practical definitions are recommended. We suggest a more clinically relevant sequencing approach of: GTV → IGTV → ICTV → PTV.
There are different methods of generating a motion envelope. Accounting for motion and motion interplay effects is important, particularly as radiation technology and delivery methods advance and become more precise and in the case of hypofractionated treatments. In addition to tumor motion from respiration, tumor and normal tissue changes during the course of radiation therapy are important to consider. Adaptive re-planning is being investigated as a possible mechanism to widen the therapeutic window. Additionally, mid-treatment functional imaging may be helpful in identifying areas of the tumor that may require higher doses of radiation to achieve better tumor control.
Proton therapy may be particularly sensitive to tumor motion and anatomic changes during treatment, as small differences in the amount of tissue that the proton beam passes through can result in large deviations in the dose deposited. As technology advances and the field moves more towards adaptive and bio-adaptive treatment planning, the ICRU definitions will need to evolve to standardize these more complex approaches to radiation therapy planning and delivery.
Acknowledgments
Acknowledgement of Grant Support: MSKCC Cancer Center Support Grant, P30 CA008748
Footnotes
Disclosure Statement: The authors have no disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annemarie Shepherd, Assistant Attending, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NY.
Sara St James, Affiliate Assistant Professor, Department of Radiation Oncology, University of Washington, WA.
Ramesh Rengan, Professor, Department of Radiation Oncology, University of Washington, WA.
References
- 1.International Commission on Radiation Units and Measurements. 1978. ICRU Report 29. Dose Specification for Reporting External Beam Therapy with Photons and Electrons. [Google Scholar]
- 2.ICRU Report 50. Prescribing, Recording, and Reporting Photon Beam Therapy. International Commission on Radiation Units and Measurements. 1993 [Google Scholar]
- 3.ICRU Report 62. Prescribing, Recording, and Reporting Photon Beam Therapy (Supplement to ICRU Report 50) International Commission on Radiation Units and Measurements. 1999 [Google Scholar]
- 4.Purdy JA. Seminars in radiation oncology: 2004. Elsevier; 2004. Current ICRU definitions of volumes: limitations and future directions; pp. 27–40. [DOI] [PubMed] [Google Scholar]
- 5.Das IJ, Andersen A, Chen ZJ, Dimofte A, Glatstein E, Hoisak J, Huang L, Langer MP, Lee C, Pacella M, et al. State of dose prescription and compliance to international standard (ICRU-83) in intensity modulated radiation therapy among academic institutions. Pract Radiat Oncol. 2017;7(2):e145–e155. doi: 10.1016/j.prro.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Lagerwaard FJ, de Koste JRVS, Nijssen-Visser MR, Schuchhard-Schipper RH, Oei SS, Munne A, Senan S. Multiple “slow” CT scans for incorporating lung tumor mobility in radiotheraphy planning. International Journal of Radiation Oncology* Biology* Physics. 2001;51(4):932–937. doi: 10.1016/s0360-3016(01)01716-3. [DOI] [PubMed] [Google Scholar]
- 7.Hunjan S, Starkschall G, Prado K, Dong L, Balter P. Lack of correlation between external fiducial positions and internal tumor positions during breath-hold CT. International Journal of Radiation Oncology* Biology* Physics. 2010;76(5):1586–1591. doi: 10.1016/j.ijrobp.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Ekberg L, Holmberg O, Wittgren L, Bjelkengren G, Landberg T. What margins should be added to the clinical target volume in radiotherapy treatment planning for lung cancer? Radiotherapy and oncology. 1998;48(1):71–77. doi: 10.1016/s0167-8140(98)00046-2. [DOI] [PubMed] [Google Scholar]
- 9.Lu W, Parikh PJ, Hubenschmidt JP, Bradley JD, Low DA. A comparison between amplitude sorting and phase-angle sorting using external respiratory measurement for 4D CT. Medical physics. 2006;33(8):2964–2974. doi: 10.1118/1.2219772. [DOI] [PubMed] [Google Scholar]
- 10.Guckenberger M, Wilbert J, Meyer J, Baier K, Richter A, Flentje M. Is a single respiratory correlated 4D-CT study sufficient for evaluation of breathing motion? International Journal of Radiation Oncology* Biology* Physics. 2007;67(5):1352–1359. doi: 10.1016/j.ijrobp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 11.StJames S, Mishra P, Hacker F, Berbeco RI, Lewis JH. Quantifying ITV instabilities arising from 4DCT: a simulation study using patient data. Physics in medicine and biology. 2012;57(5):L1. doi: 10.1088/0031-9155/57/5/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Lewis JH, Cervino LI, Jiang SB. 4D CT sorting based on patient internal anatomy. Physics in medicine and biology. 2009;54(15):4821. doi: 10.1088/0031-9155/54/15/012. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Dong J, Shi Y. Google Patents. 2013. Method and system for gated radiation therapy. [Google Scholar]
- 14.Schaefer M, Munter MW, Thilmann C, Sterzing F, Haering P, Combs SE, Debus J. Influence of intra-fractional breathing movement in step-and-shoot IMRT. Physics in medicine and biology. 2004;49(12):N175–179. doi: 10.1088/0031-9155/49/12/n03. [DOI] [PubMed] [Google Scholar]
- 15.Chui CS, Yorke E, Hong L. The effects of intra-fraction organ motion on the delivery of intensity-modulated field with a multileaf collimator. Medical physics. 2003;30(7):1736–1746. doi: 10.1118/1.1578771. [DOI] [PubMed] [Google Scholar]
- 16.Bortfeld T, Jiang SB, Rietzel E. Effects of motion on the total dose distribution. Semin Radiat Oncol. 2004;14(1):41–51. doi: 10.1053/j.semradonc.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.George R, Keall PJ, Kini VR, Vedam SS, Siebers JV, Wu Q, Lauterbach MH, Arthur DW, Mohan R. Quantifying the effect of intrafraction motion during breast IMRT planning and dose delivery. Medical physics. 2003;30(4):552–562. doi: 10.1118/1.1543151. [DOI] [PubMed] [Google Scholar]
- 18.Rao M, Wu J, Cao D, Wong T, Mehta V, Shepard D, Ye J. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using intensity modulated radiotherapy and volumetric modulated arc therapy [corrected] International journal of radiation oncology, biology, physics. 2012;83(2):e251–256. doi: 10.1016/j.ijrobp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Riley C, Yang Y, Li T, Zhang Y, Heron DE, Huq MS. Dosimetric evaluation of the interplay effect in respiratory-gated RapidArc radiation therapy. Medical physics. 2014;41(1):011715. doi: 10.1118/1.4855956. [DOI] [PubMed] [Google Scholar]
- 20.Azcona JD, Xing L, Chen X, Bush K, Li R. Assessing the dosimetric impact of real-time prostate motion during volumetric modulated arc therapy. International journal of radiation oncology, biology, physics. 2014;88(5):1167–1174. doi: 10.1016/j.ijrobp.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seco J, Robertson D, Trofimov A, Paganetti H. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Physics in medicine and biology. 2009;54(14):N283–294. doi: 10.1088/0031-9155/54/14/N01. [DOI] [PubMed] [Google Scholar]
- 22.Dowdell S, Grassberger C, Sharp GC, Paganetti H. Interplay effects in proton scanning for lung: a 4D Monte Carlo study assessing the impact of tumor and beam delivery parameters. Physics in medicine and biology. 2013;58(12):4137–4156. doi: 10.1088/0031-9155/58/12/4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zenklusen SM, Pedroni E, Meer D. A study on repainting strategies for treating moderately moving targets with proton pencil beam scanning at the new Gantry 2 at PSI. Physics in medicine and biology. 2010;55(17):5103–5121. doi: 10.1088/0031-9155/55/17/014. [DOI] [PubMed] [Google Scholar]
- 24.Edmunds K, Bedford J. Assessment of the robustness of volumetric-modulated arc therapy for lung radiotherapy. The British journal of radiology. 2013;86(1023):20120498. doi: 10.1259/bjr.20120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, Bogart JA, Dobelbower MC, Bosch W, Galvin JM, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deodato F, Cilla S, Macchia G, Torre G, Caravatta L, Mariano G, Mignogna S, Ferro M, Mattiucci GC, Balducci M, et al. Stereotactic radiosurgery (SRS) with volumetric modulated arc therapy (VMAT): interim results of a multi-arm phase I trial (DESTROY-2) Clinical oncology. 2014;26(12):748–756. doi: 10.1016/j.clon.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Zeng YC, Vyas S, Dang Q, Schultz L, Bowen SR, Shankaran V, Farjah F, Oelschlager BK, Apisarnthanarax S, Zeng J. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer : Clinical outcome, dosimetry, and feasibility. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2016;192(12):913–921. doi: 10.1007/s00066-016-1034-4. [DOI] [PubMed] [Google Scholar]
- 28.Berkovic P, Paelinck L, Lievens Y, Gulyban A, Goddeeris B, Derie C, Surmont V, De Neve W, Vandecasteele K. Adaptive radiotherapy for locally advanced non-small cell lung cancer, can we predict when and for whom? Acta Oncol. 2015;54(9):1438–1444. doi: 10.3109/0284186X.2015.1061209. [DOI] [PubMed] [Google Scholar]
- 29.Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):341–348. doi: 10.1016/j.ijrobp.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 30.Jabbour SK, Kim S, Haider SA, Xu X, Wu A, Surakanti S, Aisner J, Langenfeld J, Yue NJ, Haffty BG, et al. Reduction in Tumor Volume by Cone Beam Computed Tomography Predicts Overall Survival in Non-Small Cell Lung Cancer Treated With Chemoradiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(3):627–633. doi: 10.1016/j.ijrobp.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo TR, Moon SH, Lim YJ, Kim JY, Kim Y, Kim TH, Cho KH, Han JY, Lee YJ, Yun T, et al. The effect of tumor volume and its change on survival in stage III non-small cell lung cancer treated with definitive concurrent chemoradiotherapy. Radiat Oncol. 2014;9:283. doi: 10.1186/s13014-014-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald P, Mo X, Barney C, Gunderson D, Haglund AK, Bazan J, Grecula J, Chakravarti A, Williams T, Carbone DP, et al. Prognostic value of primary tumor volume changes on kV-CBCT during definitive chemoradiotherapy for stage III non-small cell lung cancer. J Thorac Oncol. 2017 doi: 10.1016/j.jtho.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Moller DS, Khalil AA, Knap MM, Hoffmann L. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol. 2014;110(3):517–522. doi: 10.1016/j.radonc.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Persoon LC, Podesta M, Hoffmann L, Sanizadeh A, Schyns LE, de Ruiter BM, Nijsten SM, Muren LP, Troost EG, Verhaegen F. Is integrated transit planar portal dosimetry able to detect geometric changes in lung cancer patients treated with volumetric modulated arc therapy? Acta Oncol. 2015;54(9):1501–1507. doi: 10.3109/0284186X.2015.1061213. [DOI] [PubMed] [Google Scholar]
- 35.Nam H, Park W, Huh SJ, Bae DS, Kim BG, Lee JH, Lee JW, Lim DH, Han Y, Park HC, et al. The prognostic significance of tumor volume regression during radiotherapy and concurrent chemoradiotherapy for cervical cancer using MRI. Gynecol Oncol. 2007;107(2):320–325. doi: 10.1016/j.ygyno.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Britton KR, Starkschall G, Liu H, Chang JY, Bilton S, Ezhil M, John-Baptiste S, Kantor M, Cox JD, Komaki R, et al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):94–102. doi: 10.1016/j.ijrobp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Kong FM, Ten Haken RK, Schipper M, Frey KA, Hayman J, Gross M, Ramnath N, Hassan KA, Matuszak M, Ritter T, et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollom EL, Song J, Durkee BY, Aggarwal S, Bui T, von Eyben R, Li R, Brizel DM, Loo BW, Le QT, et al. Prognostic value of midtreatment FDG-PET in oropharyngeal cancer. Head Neck. 2016;38(10):1472–1478. doi: 10.1002/hed.24454. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz JK, Lin LL, Siegel BA, Miller TR, Grigsby PW. 18-F-fluorodeoxyglucose-positron emission tomography evaluation of early metabolic response during radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2008;72(5):1502–1507. doi: 10.1016/j.ijrobp.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 40.Berkovic P, Paelinck L, Gulyban A, van Eijkeren M, Surmont V, Lievens Y, Vandecasteele K. Adaptive radiotherapy for locally advanced non-small cell lung cancer: dosimetric gain and treatment outcome prediction. Acta Oncol. 2017:1–4. doi: 10.1080/0284186X.2017.1352103. [DOI] [PubMed] [Google Scholar]
- 41.Kontaxis C, Bol GH, Stemkens B, Glitzner M, Prins FM, Kerkmeijer LGW, Lagendijk JJW, Raaymakers BW. Towards fast online intrafraction replanning for free-breathing stereotactic body radiation therapy with the MR-linac. Phys Med Biol. 2017;62(18):7233–7248. doi: 10.1088/1361-6560/aa82ae. [DOI] [PubMed] [Google Scholar]
- 42.Kwint M, Conijn S, Schaake E, Knegjens J, Rossi M, Remeijer P, Sonke J-J, Belderbos J. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiotherapy and Oncology. 2014;113(3):392–397. doi: 10.1016/j.radonc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Veiga C, Janssens G, Teng CL, Baudier T, Hotoiu L, McClelland JR, Royle G, Lin L, Yin L, Metz J, Solberg TD, Tochner Z, Simone CB, 2nd, McDonough J, Teo BK. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. International Journal of Radiation Oncology* Biology* Physics. 2016;95(1):549–559. doi: 10.1016/j.ijrobp.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 44.Szeto YZ, Witte MG, van Kranen SR, Sonke J-J, Belderbos J, van Herk M. Effects of anatomical changes on pencil beam scanning proton plans in locally advanced NSCLC patients. Radiotherapy and Oncology. 2016;120(2):286–292. doi: 10.1016/j.radonc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann L, Alber M, Jensen MF, Holt MI, Møller DS. Adaptation is mandatory for intensity modulated proton therapy of advanced lung cancer to ensure target coverage. Radiotherapy and Oncology. 2017;122(3):400–405. doi: 10.1016/j.radonc.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Kraan AC, van de Water S, Teguh DN, Al-Mamgani A, Madden T, Kooy HM, Heijmen BJ, Hoogeman MS. Dose uncertainties in IMPT for oropharyngeal cancer in the presence of anatomical, range, and setup errors. International Journal of Radiation Oncology* Biology* Physics. 2013;87(5):888–896. doi: 10.1016/j.ijrobp.2013.09.014. [DOI] [PubMed] [Google Scholar]