Abstract
Lung cancer is a clinically difficult disease with rising disease burden around the world. Unfortunately, most lung cancers present at a clinically advanced stage. Of these cancers, many also present with brain metastasis which complicates the clinical picture. This review summarizes current knowledge on the molecular basis of lung cancer brain metastases. We start from the clinical perspective, aiming to provide a clinical context for a significant problem that requires much deeper scientific investigation. We review new research governing the metastatic process, including tumor cell signaling, establishment of a receptive tumor niches in the brain and evaluate potential new therapeutic options that take advantage of these new scientific advances.
Lung cancer remains the largest single cause of cancer mortality in the United States (Siegel et al., 2015). This continues to be the clinical picture despite significant advances in therapy, including the advent of targeted molecular therapies and newly adopted immunotherapies for certain subtypes of lung cancer. In the vast majority of cases, lung cancer presents as advanced disease; in many instances, this advanced disease state is intimately associated with micro and macrometastatic disease (Goldberg et al., 2015). For both non-small cell lung cancer and small cell lung cancer patients, the predominant metastatic site is the brain, with up to 68% of patients with mediastinal lymph node metastasis eventually demonstrating brain metastasis (Wang et al., 2009). The frequency (incidence) of brain metastasis is highest in lung cancers, relative to other common epithelial malignancies (Schouten et al., 2002). Other studies have attempted to predict the risk of brain metastasis in the setting of previously non-metastatic disease. One of the largest studies to do this, analyzing historical data from 1973 to 2011 using the SEER database revealed a 9% risk of patients with previously non-metastatic NSCLC developing brain metastasis over the course of their disease, while 18% of small cell lung cancer patients without previous metastasis went on to develop brain metastasis as their disease progressed (Goncalves et al., 2016). The reasons underlying this predilection for the central nervous system, as well as the recent increase in the frequency of brain metastasis identified in patients remain important questions for both clinicians and basic scientists. More than ever, the question of how brain metastasis develop and how they can be treated and managed requires the involvement of interdisciplinary teams—and more importantly—scientists who are capable of thinking like clinicians and clinicians who are capable of thinking like scientists. This review aims to present a translational perspective on brain metastasis. We will investigate the scope of the problem of brain metastasis and the current management of the metastatic disease process in lung cancer. From this clinical starting point, we will investigate the literature surrounding the molecular underpinnings of lung tumor metastasis and seek to understand the process from a biological perspective to generate new hypotheses.
Keywords: Lung cancer, Metastasis, Brain metastasis of lung cancer, Non-small cell lung cancer, Small cell lung cancer
1. Brain metastasis in lung cancer
The phenomenon of lung cancer metastasis to the brain is not a new one; it has long been noted that lung tumors have a predilection to spread to and within the central nervous system (Goldberg et al., 2015; Wang et al., 2009; Schouten et al., 2002; Goncalves et al., 2016). However, as imaging modalities have improved and as clinicians have worked to anticipate the spread of disseminated lung cancer to the brain, the instance of lung cancer metastasis in the brain has increased dramatically in recent decades. It is unclear whether this increase is related in to changing treatment options and modalities or whether it can be attributed solely to improved diagnostics. Increasingly, evidence suggests that the increased incidence of clinically validated lung tumor metastases to the brain is the result of a confluence of factors, including improved detection owing to improved imaging modalities and clinical awareness as well as changes in the treatment of lung cancers, particularly those susceptible to targeted molecular therapies.
2. Current clinical management of lung tumor brain metastasis
Clinical management of lung metastasis to the brain and CNS is currently guided by a number of factors, including the performance status and the overall health of the patient. Aggressive clinical management, typified by surgical interventions, whole brain radiation and stereotactic radiosurgery improve survival times for patients, although these interventions themselves are also associated with morbidities (Baykara et al., 2014). Other studies however, such as the recent QUARTZIII trial failed to reveal significant improvements in survival or quality of life in non-small cell lung cancer patients receiving whole brain irradiation. However, these results have not yet been parsed by stratifying patient data based on disease subtypes, and the results represent an interim study endpoint; the possibility remains that patients with certain performance status or disease characteristics could still benefit from whole brain irradiation. Other studies have evaluated a broader subset of cancers, defined histologically. One recent study found a slight, but statistically non-significant increase in survival in small cell lung cancer patients receiving whole brain irradiation (Nieder et al., 2013). In all cases where brain metastases are symptomatic, management with corticosteroids is the current standard of care, although steroids by themselves do not significantly prolong survival times (Gallego Perez-Larraya and Hildebrand, 2014). The goal in this instance is to manage the vasculogenic brain edema associated with lung tumor cell infiltration of brain and CNS tissues and to provide relief to patients.
Typically, patients present with headache or occasionally with altered mental status and even more rarely with personality changes or increased emotional lability. Many of the latter are highly subjective and can be difficult to assess clinically, especially given the traumatic nature of a cancer diagnosis in and of itself. However, frequent headaches or severe headaches can often be an early warning sign of potential brain metastasis in cancer patients that warrants diligent clinical follow up.
2.1. Detecting brain lesions
Clarifying actual brain lesions from generalized brain edema can be difficult; to that end, multiple imaging modalities are often preferred. Currently, magnetic resonance imaging with contrast enhancement represents the gold standard for the detection of brain metastasis, although this may be supplemented with other modalities and techniques, including diffusion tensor imaging (DTI) to increase confidence in the diagnosis (Fink and Fink, 2013). In some instances, biopsy of the lesion is indicated, although open brain biopsy is also associated with risks and potential morbidity. Early studies revealed that MRI, and longitudinal monitoring with MRI may represent a more sensitive approach than CT scan alone (Yokoi et al., 1999). In the case of lung cancers, brain lesions are typically multiple and disseminated, although interestingly, they do tend to localize in different regions of the brain than other cancer types- a fact that can have diagnostic significance. Specifically, lung cancer metastases to the brain tend to develop focal lesions in “watershed” regions of the brain- these are the regions where the vasculature is finest and most narrow (Takano et al., 2016). This suggests a potential role for hematogenous spread of lung tumor cells to the brain and their subsequent entrapment in fine vessels and extravasation into the tissue. Although it has generally been assumed that dissemination of lung tumor cells to the brain is mediated hematogenously, new evidence that a robust lymphatic system may actually exist in CNS tissues may lead to future work that could potentially indicate other mechanisms (Louveau et al., 2015). Generally speaking, lung tumor metastasis presents as multiple focal lesions, rather than distinct solitary lesions which may be indicative of a different tumor type, although if detected early, lesions can be solitary in nature—and this is associated with better response to surgical resection (Ali et al., 2013).
2.2. Treatment of brain lesions
Once metastatic lesions are detected, treatment can follow several courses, which are detailed in Table 1. If patients are experiencing symptoms, or there is evidence of substantial edema, treatment with corticosteroids may be warranted. This often presents clinically with complaints of headache. In patients with brain metastasis broadly speaking where corticosteroid treatment is initiated, clinical evidence suggests that this strategy is effective; however, current systemic reviews are limited to only two major studies (Ryken et al., 2010), which only provide evidence that corticosteroids are useful once patients present with physical sequelae of cerebral edema (headache). In other cases where lesions are well-defined, stereotactic radiosurgery may be used to delivery high dose radiation to the lesion site. Stereotactic radiosurgery has some advantages over whole brain irradiation, particularly given the ability to deliver much higher doses of radiation to more localized regions (Bowden et al., 2015). This may be more difficult, particularly with lung cancer metastases, which tend to be multiple and more diffuse in localization, necessitation whole brain irradiation. Chemotherapy regimens have also been attempted to control the growth of distant metastatic sites in lung cancer. In general, these approaches tend to show some efficacy, although they are ultimately insufficient. This may be partially driven by the pharmacology of traditional, cytotoxic chemotherapeutics; most are relatively large in size and not capable of freely diffusing across the tight junctions of the blood brain barrier (BBB) (reviewed in (Schuette, 2004)). However, some newer targeted agents are smaller in size and have superior uptake into CNS tissues given their ability to cross the BBB. The prototypic agents in this class are small molecule EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib (and subsequent drugs designed to counter EGFR-resistance mutations). Indeed, treatment with these drugs, especially in patients with primary lung disease that demonstrates EGFR mutation shows superior efficacy versus traditional chemotherapies in shrinking brain metastases, with 60–80% response rates (reviewed in (Dempke et al., 2015)). Interestingly, patients with EGFR mutant disease treated with EGFR inhibitors show somewhat different patterns of brain metastasis, including metastases that are less deeply seeded within the brain (Takano et al., 2016).
Table 1.
Current Treatment Modalities for Lung Cancer Brain Metastasis.
| Clinical Presentation | Modalities | Efficacy | Reference |
|---|---|---|---|
| Solitary Lesion | Surgery | Dependent on aggressiveness of tumor; generally effective with solitary lesions | Reviewed in Lippitz et al. (2014) |
| Solitary Lesion | Whole Brain Irradiation; Stereotactic Radiosurgery | Most effective against single lesions. Stereotactic radiosurgery associated with less morbidity. Combination stereotactic radiosurgery and whole brain irradiation may further improve survival. | Wegner et al. (2011) |
| Multifocal Lesion | Whole Brain Irradiation | Generally less effective; multiple lesions indicative of more advanced disease. Can be palliative. Often used in combination with stereotactic radiosurgery or local surgical resection. | Khan and Dicker (2013); Soffietti et al. (2013) |
| Multifocal Lesion | Stereotactic Radiosurgery | May be indicated if lesions are well-defined; generally palliative. Combination with whole brain irradiation may provide additional benefit. | Wegner et al. (2011) |
| Multifocal Lesion | Systemic chemotherapy | Difficulty with many cytotoxic chemotherapeutics in crossing the blood brain barrier. Targeted small molecules, such as anti-EGFR drugs may be effective in some instances. Eventual tumor regrowth as resistance to therapy develops. | Fan et al. (2014) |
| EGFR or ALK Mutant NSCLC (Confirmed) | Targeted therapy with small molecule TKIs/ALK inhibitor | Highly effective in shrinking brain metastasis bearing mutations matching the primary tumor; evolution of resistance remains a clinical problem as in primary-site disease. | Jamal-Hanjani and Spicer (2012); Costa et al. (2015) |
3. Treatment considerations depending on histological subtype
It should be noted that treatment regimens may vary depending on the histologic subtype of the presenting lung cancer. Broadly speaking, lung cancers are divided into small cell and non-small cell lung cancers. Small cell cancers tend to be much more diffuse throughout the lung and have a higher overall mortality and shorter disease course. This histologic subtype represents approximately 15–20% of overall lung cancer cases in the United States, with some variation depending on the epidemiologic study cited (reviewed in Molina et al., 2008). In the case of small cell lung cancer, which is especially characterized by distant spread and brain metastasis at the time of presentation, many clinicians may elect to pursue prophylactic whole brain radiation as part of the treatment course, even in the absence of confirmed brain lesions; in fact, this is considered standard of care (Nosaki and Seto, 2015). Recent studies have found that in many patients who appear to respond fully to conventional first-line therapies in SCLC, subsequent brain metastasis without clinical symptoms continues to be a serious cause of recurrent disease (Auperin et al., 1999). Use of prophylactic cranial irradiation (PCI) in the absence of detectable brain metastasis in the SCLC patient population resulted in significantly fewer brain metastases over the course of three years, with brain metastasis having a 33% incidence in patients undergoing PCI, versus 59% in matched patients not receiving PCI (Auperin et al., 1999).
By contrast, tumors bearing lesions which are targetable by TKIs tend to be non-small cell lung cancers. When non-small cell lung cancers bear EGFR mutations, EGFR TKIs have been shown to be highly effective agents in the treatment of both primary and metastatic disease. In patients with molecularly confirmed EGFR mutations, an objective response rate of 70–80% has been noted in patients with brain metastasis (Jamal-Hanjani and Spicer, 2012). As a result, treatment of EGFR-mutant primary tumors which have metastasized to the brain in the setting of NSCLC with EGFR TKIs should be considered standard of care. In addition, tumors with other validated molecular mutations that have metastasized to the brain, such as those bearing ALK mutation have proven highly sensitive to small molecule inhibition of ALK with crizotinib (Costa et al., 2015). The experiences with both EGFR inhibition in EGFR mutant NSCLC and ALK inhibition in ALK re-arranged NSCLC suggest that targeted molecular therapy should be standard of care in NSCLC metastatic to brain when these mutations can be identified in either the primary or metastatic tumor. However, as with primary tumors, evolution of resistance to small molecule, targeted agents is a frequent feature of disease progression and can limit their efficacy after disease recurrence.
4. Molecular basis of brain metastasis
The dismal prognosis associated with disseminated disease metastatic to the brain has not been addressed by existing clinical management strategies and therapies. Efforts to develop better therapies and clinical management processes must be driven by an understanding of the mechanistic basis of this disease process. In this section, we provide a general overview of the metastatic process, as well as specific factors that may be at play in lung cancers metastatic to the brain.
4.1. Seed and soil
As a clinical phenomenon, metastasis has beguiled physicians for centuries. In 1889, one physician, Stephen Paget, posited that metastasis is driven by two interrelated yet semi-independent processes, encapsulated by his “soil and seed hypothesis.” In broad terms, Paget hypothesized that tumor cells (the seed) must have the capacity to germinate in distant target tissues, and the ability to disseminate themselves. However, in Paget’s view, this represented an oversimplification. Tumor metastasis in many different cancers could not be explained by anatomy alone- specific cancers appear to have a predilection to migrate and establish metastasis in specific organs and organ systems, even those that are sometimes anatomically distant or not obvious. Therefore, Paget hypothesized that not only must the tumor cell itself be able to migrate; it must also land in fertile soil- a specific microenvironment established within the target tissue that allows the tumor cell (seed) to fully germinate into a growing tumor. This concept continues to be a somewhat useful way of conceptualizing the metastatic process on a global level- therefore, we will consider both the seed and the soil of lung tumor metastasis.
4.1.1. Seed
The seed or specific cells that are capable of undergoing metastasis are an important consideration. Repeated studies have demonstrated that not all tumor cells are capable of metastasis- in fact, only a small minority are. This suggests that the programming that is required and the adaptations intrinsic to allow metastasis represent a high evolutionary hurdle for cancer cells to overcome. Tumor cells must develop the ability to infiltrate through basement membrane, intravasate, or enter the vasculature or lymphatic system, arrest their spread through the vasculature or lymph network, extravasate, establish within a brand new microenvironment and then begin to proliferate (reviewed in (Valastyan and Weinberg, 2011). This is understandably a tall order, and the means by which cancer cells accomplish this process is still not well understood, even more than a century after Paget’s initial seed and soil hypothesis.
The stimuli that underlie metastasis have been investigated, but detailed understanding remains elusive. Rapid tumor growth often leads to the development of a necrotic tumor core, and the production of localized hypoxia. This in turn favors cell populations capable of inducing angiogenesis and increasing the vascular supply to the tumor. This phenomenon has been observed in many different tumor types clinically (Rofstad et al., 2000). As the “soil” becomes increasingly depleted of nutrients and filled with increasing metabolic waste products within the initial tumor site, this may also serve as a signal or a cue that drives the outgrowth of “seed” capable of selectively adapting to these environmental conditions by leaving. However, this again may be more nuanced-recent data from lung cancer models suggests that hypoxia may increase tumor cell invasiveness and ultimately metastasis by influencing the immune composition of the tumor microenvironment (Zhang et al., 2014).
Increasing evidence suggests that metastasis is a selective process that favors the acquisition of metastatic capabilities, driven by environmental stressors. However, other evidence also suggests that subpopulations of tumor cells may be uniquely “qualified” to metastasize. In particular, tumor initiating cells (TICs) or cancer stem-like cells (CSCs) as they are alternately defined in the literature represent one cell type with high metastatic propensity. TICs/CSCs are defined by the ability to self-renew, as well as expression of genes and developmental programs that bear similarity to those present with embryonic stem cells. Increased expression of stem-associated genes is associated with enhanced cell motility, as well as increased ability of cells to survive under conditions of hypoxic and genotoxic stress. Indeed, in tumor xenograft models, cancer stem-like cells have been found to be capable of engrafting in the host and establishing tumors at extremely low density. In some animal models, as few as 500 cells enriched for cancer stem-like cell markers are capable of initiating disseminated disease, versus hundreds of thousands or millions of cells required from parental cell lines not enriched for cancer-stem like cells. (reviewed in Reya et al., 2001). This suggests that the hypothesis that metastasis is a dynamic evolutionary process driven by environmental stressors and the hypothesis that select subpopulations of tumor cells have intrinsic properties that favor metastasis both have merit. In fact, recent work has revealed that the cancer stem-like cell state may in fact be a plastic one; CSCs appear to be dynamically regulated within the tumor itself- non-CSCs are capable of acquiring CSC-like markers and properties (Chaffer et al., 2011). Additionally, recent work with cell lines established from brain metastasis in lung cancer models indicates that the metastatic cells contain a population that expresses stem-associated markers and exhibit stem-like phenotypes (Nolte et al., 2013).
The plasticity and cross talk between tumor cells, coupled with the high degree of heterogeneity that can be detected between tumor cells, even in cell lines that are supposedly clonal in nature highlights another element of the “seed” that bears consideration: tumor cells do not exist in a vacuum (reviewed in Meacham and Morrison, 2013). Not only are they influenced and regulated by environmental stressors that occur as a result of rapid tumor growth- they are also regulated and influenced by crosstalk with surrounding elements of the tumor. Indeed, recent work has revealed that some unique tumor cells within the heterogenous mishmash that is a tumor are capable of transdifferentiating into leaky endothelial cells, directly bringing nutrients to the tumor (Soda et al., 2011). This degree of cooperativity present within a tumor suggests that a simple understanding of a metastatic “seed” as an individual cell driven solely by an evolutionary process that favors individual cell growth may be too simplistic. By contrast, increasing evidence suggests that tumor cells at the very least exhibit some characteristics that suggest cooperation and may even suggest that a tumor itself has a type of multicellular, organismic character. All of these data influence our understanding of what drives the production of “seed” capable of embarking on the metastatic cascade.
4.1.2. Preparing the seed
Understanding the factors that motivate the development of tumor “seed” capable of metastasizing is useful, but does not provide the level of knowledge required to effectively target these cells. How is it that selected populations of tumor cells that expand in response to environmental cues are capable of metastasizing on a mechanistic level? Several recent studies have attempted to look for “metastasis mutations” or unique metastatic signaling that plays a role in this process. However, despite detailed genomic studies, mutations that underlie metastasis appear to be few and far between. However, epigenetic changes that lead to activation of a host of new metastatic programs and overexpression of metastasis-related genes are commonplace in metastatic cells (Wang and Shang, 2013). On a teleological level, this may suggest that there are in fact programs present that enable cells to metastasize and invade tissues. In fact, this is the case in the instance of a number of immune components. Neutrophils and macrophages, as well as lymphocytes and other immune cell subtypes have the ability to traffic widely throughout the body and to undergo extravasation at target sites. These cells can then traffic to the site of injury or infection and respond to environmental stimuli to address the underlying insult that triggered their metastasis. In some cases, this can involve proliferation of the infiltrating cells at the local site. In some sense, this sounds similar to the process that can occur in tumor cell metastasis. It has long been known that in the case of tumors of epithelial origin, cells that move on to migrate undergo a transition away from epithelial terminal differentiation, and toward increased expression of mesenchymal markers (Xiao and He, 2010). Mesenchymal cells, such as those that compose the bone marrow are characterized by limited cell–cell connection, loss of tight junctions and ability to migrate and invade through extracellular matrix. Given the epigenetic changes present in tumor cells and the plasticity that is a central element of cancerous cells, it is plausible that re-activation of latent programs that play a role in normal morphogenesis during embryonic development could lead to the acquisition of a mesenchymal-like state and increased capacity for tumor cells to migrate and ultimately metastasize. Alternatively, additional work has suggested that tumor cells may be capable of significant crosstalk with the immune system that may influence metastasis. For instance, lung tumor cells appear capable of triggering the release of resistin from dendritic cells, which then promotes increased migration by tumor cells and cancer progression (Kuo et al., 2013). Recent studies have suggested that tumor cells may secrete soluble factors that draw in infiltrating neutrophils and components of the innate immune system. These multiple theories and multiple lines of supportive evidence provide further support for the concept that tumor cell metastasis is a complicated and multi-faceted process that may require a multi-pronged and multimodal approach in order to be addressed.
4.1.3. Metabolic reprogramming of metastatic tumor cells
Another of the defining features of cancer is disordered metabolic process. Increasingly, additional evidence suggests that metabolic changes within tumor cells may contribute to metastasis, and may in fact be linked to the epigenetic shifts and acquisition of mesenchymal-like and de-differentiated states that occur in cells that are preferentially capable of metastasis. A recent study revealed that circulating tumor cells express significantly higher levels of genes involved in mitochondrial biogenesis—and concomitantly, greater overall levels of mitochondrial mass. In addition, oxidative phosphorylation is significantly greater in circulating tumor cells. This is not the case within the primary tumor; in fact, the opposite is often true. Downregulation of oxidative phosphorylation and the use of aerobic glycolysis by primary tumor cells has been documented for almost a century. This phenomenon may be due to the need to produce precursors essential for rapid proliferation (reviewed in (Vander Heiden, 2009)). However, these findings may suggest that tumor cell migration may require additional energy levels that can only be attained through reactivation or increased use of oxidative phosphorylation. Recent work revealed that genetic knockdown of PGC1alpha, a key mediator of mitochondrial production does not actually impact the ability of tumor cells to proliferate. However, PGC1alpha knockdown significantly attenuates the development of metastasis- a striking example from an in vivo study that supports this hypothesis (LeBleu et al., 2014). Mediators produced by mitochondria may also promote metastasis (Porporato et al., 2014). Moreover, these findings may suggest that both anti-glycolytic agents as well as agents that target mitochondria may be useful in the treatment of primary and metastatic cancer, or potentially as a means of preventing metastasis.
4.2. Soil
The preceding sections provide general background on potential mechanisms at work within tumor cells that lead to migration, and ultimately metastasis. However, they only tangentially address why tumor cells from specific primary disease sites appear to have a predilection for specific metastatic sites within the body. Paget was one of the first to observe that tumors tend to colonize specific, distal organ sites depending on the nature of the primary tumor. For decades, scientific opinion has continued to shift regarding the relative contributions of anatomy versus intrinsic biological differences within the tumor that impact and ultimately guide where metastases will take root. The liver is one of the largest metastatic sites for cancers globally, and this may make sense in terms of an anatomical hypothesis; the liver ultimately receives and filters all venous circulation, making it a natural target site for tumor cells. Likewise, the nature of the circulation may also make the lung a primary metastatic site for many disease types. However, interestingly, some disease types do not migrate exclusively to organ sites that might seem the most anatomically convenient; rather distal metastases are present at certain sites and not at others (reviewed in Nguyen et al., 2009). Fig. 1 illustrates in broad terms the dynamic interplay that occurs between the tumor and the metastatic site.
Fig. 1. Tumor-stroma crosstalk: priming the metastatic site.
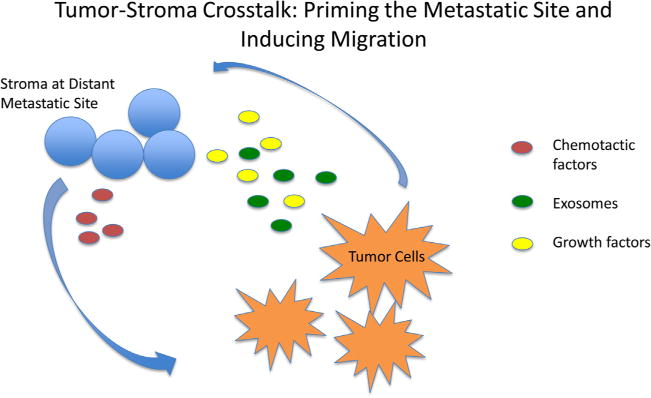
Tumor cells secrete soluble factors and exosomally encapsulated factors that condition stroma at the metastatic site to support engraftment of mobile tumor cells. A chemotactic gradient produced by target tissue specific factors guides circulating tumor cells to the niche.
Increasingly, accumulating evidence suggests that the soil itself may also shape the nature of brain metastasis. Recent work conducted primarily in breast cancer models (many breast cancers are also highly metastatic to brain) revealed that the brain microenvironment alters the phenotype of tumor cells. Specifically, brain, and CNS tissues appear to downregulate expression of the tumor suppressor PTEN, which may then result in accelerated growth and establishment of a growing lesion at the metastatic site. Strikingly, metastasis to other organ sites does not result in downregulation of PTEN- this feature appears, at least in these model systems to be unique to brain metastasis. The mechanism by which the microenvironment alters gene expression patterns within metastatic tumor cells remains somewhat of a mystery, although there are convincing clues that soluble mediators may play a role—although these soluble mediators are not limited to cytokines and other “traditional” exogenous mediators of cell signaling. Rather, it appears that microRNAs released from astrocytes in exosomes near the tumor colonization site, specifically miR-19 may play a direct role in downregulating PTEN-expression in infiltrating cells. Downregulation of PTEN may then lead to changes in soluble mediators secreted by the brain-colonizing, proliferating tumor cells. Increased production of chemokines such as CCL2 may then draw myeloid cells and brain-resident microglia to the tumor site. These cell types further add to the tumor microenvironment, creating a permissive space within the brain for tumor proliferation to continue, while also directly accelerating proliferation and reducing tumor cell apoptosis (Zhang et al., 2015). This fascinating example is another instance that suggests that our understanding of basic cell biology, crosstalk and the metastatic process was far too simplistic. Whether this mechanism is actually active in lung cancer remains an open question; still, this example is illustrative of the systems-level thinking and appreciation of complexity that is required to fully understand metastasis generally and specifically to the brain.
Increasing evidence also suggests that metastatic tumor cells may in some way alter the immune microenvironment surrounding them, although it is worth noting that some of the observed effects could be due to organ-specific differences between primary and metastatic sites. Of note, in a series of lung cancer patients, staining for infiltrating lymphocytes revealed dramatically reduced CD8+ T cell infiltration in metastatic sites, as well as relative increases in other cell types that are suggestive of a tolerogenic environment, which may speed metastatic cell growth upon organ site colonization (Hoshino et al., 2015).
The underlying predilection for this organ/CNS tissues appears to be driven by factors greater than anatomy alone, which will be discussed in detail below. First, it is necessary to understand in more general terms why tumors might preferentially colonize distal organs despite the seeming anatomic difficulty. This is in large part driven by the second portion of Paget’s hypothesis: the soil.
4.2.1. How do tumor cells find the right soil?
Cellular movement and trafficking of large distances is generally guided by chemokines and chemotactic factors- small, bioactive peptides that occur as gradients and are capable of guiding target cells with cognate receptors to specific locations. This is most vividly seen during development, where gradients of morphogens direct cellular movement to form basic organ structures. Within the adult, it is a fixture of healthy immune trafficking; specific classes and subtypes of immune cells possess chemokine receptors in a cell-dependent manner. As ligand is produced, specific classes of cells migrate in response to the gradient, ultimately accumulating at the focal point of ligand production where the gradient ceases. Increasing evidence suggests that these same gradients and receptors can also play a role in migration to specific tissues. Fig. 2 outlines in conceptual terms the timeline, integrated with various tumor and site-specific factors that drive tumors to the proper soil enabling the establishment of metastatic tumors.
Fig. 2. Conceptual overview of the metastatic cascade.
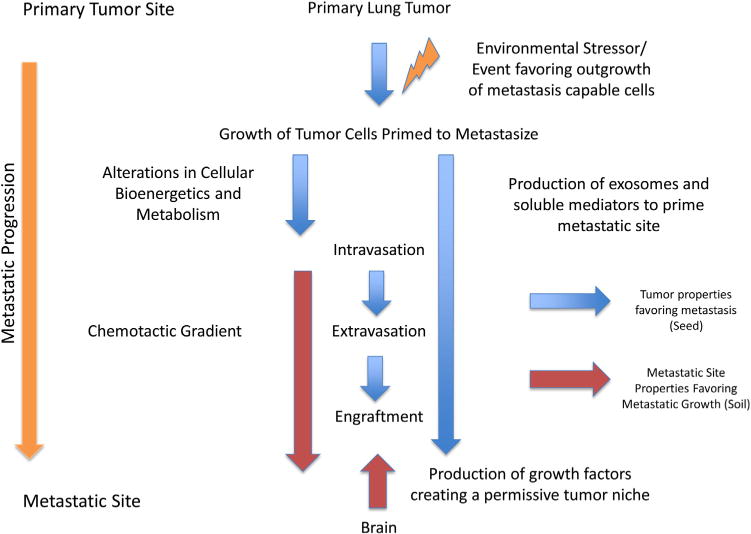
Changes within the primary tumor microenvironment either lead to the development of metastasis-capable cells or favor the outgrowth of metastasis-capable clones. This is associated with metabolic changes that enable metastasis. Metastatic tumor cell produced factors/events are illustrated by blue arrows, while target tissue (brain) elements that influence metastasis are depicted by red arrows.
Recent work has also suggested that chemokine gradients may do more than simply direct migrating cells to tissue targets. In fact, pancreatic cancer cells, when exposed to different levels of the chemokine CXCL12 behave in very different ways; certain doses of the chemokine suppress oxidative phosphorylation through an AMPK-dependent mechanism, while other doses of the chemokine increase oxidative phosphorylation. Decreases in oxidative phosphorylation are linked to decreases in cell motility and invasiveness, while increases in motility are linked to increases in oxidative phosphorylation and mitochondrial activity (Roy et al., 2015). These studies provide some evidence that soluble chemical mediators produced by the soil are not only guiding trafficking of tumor cells, but also influencing their biology in ways that make metastasis more or less feasible. As a result, many chemokines are now under investigation as both metastasis-promoting and metastasis-limiting agents, depending on the context and dose of their administration. The larger point that this example illustrates is the delicate and complicated interplay that occurs between the seed and the soil in order to enable metastatic progression.
In the context of lung cancer metastasis, the role of chemokines may be less clear than in some other cancer types. However, a number of studies have established an important role for these soluble factors in driving soil-seed communication. CXCR4 and CXCL12 have both been implicating in metastasis through recruitment of endothelial cells and neo-angiogenesis at the metastatic site (Liang et al., 2007). Recent work has also suggested that expression of various chemokine receptors and ligands may also vary based on the molecular drivers of the tumor and the histologic subtype. Specifically, the chemokine receptor CXCR6 is dramatically overexpressed in lung adenocarcinoma tissues, but not in lung squamous cell carcinomas. The only known ligand for CXCR6, CXCL16 is also dramatically increased in the serum of patients with lung adenocarcinomas (Mir et al., 2015).
In the instance of lung cancer metastases to the brain, over-expression of the protein ADAM9, a matrix metalloproteinase, is frequently observed. Beyond its’ role as matrix metalloproteinase, ADAM9 also is capable of modulating expression of integrins on the surface of tumor cells, ultimately resulting in adhesion to certain endothelial cells. It also appears that overexpression of ADAM9 is also capable of triggering tumor cells to migrate in response to certain specific growth factors secreted by neural tissues. In a seminal experiment, overexpression of ADAM9 in parental cells was shown to trigger lung tumor cell migration in response to a neural growth factor (NGF) gradient. In vivo, ADAM9 overexpressing cells migrate to and take up residence in the brain, while parental cells that express this protein do not migrate to the brain. Interestingly, using monoclonal antibodies directed against integrin 1 significantly decreased brain metastasis of cell lines engineered to overexpress ADAM9, suggesting that not only is the ability to migrate in response to NGF or other neural growth factors key to brain-tropism, but that the mechanism extends further to overexpression of specific integrins as a result of this signaling (Shintani et al., 2004). Other data also suggests that ADAM9 may increase the activity of tissue plasminogen activator (tPA), which is capable of cleaving and activating the protein CDPC1, frequently expressed on the surface of lung cancers. Activation of this protein leads to increased migration and brain metastasis (Lin et al., 2014).
New work also suggests that metastatic tumor cells are capable of preparing the soil for seeding from afar. Tumor cells shed a number of soluble factors, as well as factors incorporated into exosomes, which are small lipid vesicles shed from cells. Interestingly, exosomes from different tumors are coated with unique patterns of integrins that regulate the cells that the exosomes interact with and eventual fuse to. Recent work has shown that the pattern of the integrins present on exosomes secreted by tumor cells strongly predicts where the tumor cells will ultimately migrate and engraft. Furthermore, these exosomes are packed with mediators that increase phosphorylation of Src and induce an inflammatory environment at the target site. In the instance of brain-tropic cancers, secreted exosomes were revealed to express a unique integrin pattern that allows tumor-secreted exosomes to preferentially fuse with endothelial cells located in the brain (Hoshino et al., 2015). This study provides further evidence of the complicated interplay between both tumor cells themselves and the target organ site.
5. Moving basic science insights into the clinic
Although much remains to be elucidated about tumor cell metastasis and specifically regarding lung tumor cell metastasis to the CNS, the clinic does not and cannot wait. Given what is known now regarding the mechanisms behind brain metastasis in non-small cell lung cancer, there are several potential “low-hanging fruit” approaches that could be employed to develop therapies aimed at targeting metastasis in this disease. Broadly speaking, those potential approaches are likely to cluster into one of three general approaches: (1) modulation of chemokines and soluble mediators that drive migration and angiogenesis; (2) targeting cellular bioenergetics known to be key in the metastatic cascade; (3) targeting the “seed” directly to prevent metastasis by altering cell signaling pathways.
6. Modulation of chemokines and soluble mediators
Recent work has suggested that cytokines and chemokines can be manipulated to alter metastatic potential, although much of this work has not been done in lung cancer. Overexpression of CXCR4 and downregulation of CXCL12 are features common in pancreatic cancer that metastasizes. A group recently discovered that CXCL12 can exist in both monomeric and dimeric forms; interestingly, dimeric CXCL12 triggers different signaling pathways than monomeric cytokine. Dimeric CXCL12, rather than promoting metastasis, locks cells into an “ataxic” state and prevents metastasis of tumor cells in vivo (Roy et al., 2015; Drury et al., 2011). These data and studies such as these further illustrate that there is much about cytokine/chemokine biology that is not yet understood, but approaches such as this also suggest that there may, in the future, be clinical potential to design rational strategies to manipulate chemokines to prevent metastasis in cancer.
7. Targeting cellular bioenergetics
Work to alter chemokine signaling revealed that bioenergetics are also massively dysregulated in metastatic cells. More direct means of targeting cellular bioenergetics may be effective as inhibitors of metastasis. This makes sense, especially in light of work linking chemokine-mediated metastasis to changes in cellular bioenergetics (Roy et al., 2015). A number of efforts have been undertaken to evaluate the potential role of natural compounds known to modulate cellular energy balance as tools to prevent or treat metastasis. One promising natural compound in this arena is honokiol, an extract produced from magnolia bark long used in traditional medicines in Asia. Recent work has shown that honokiol can inhibit EMT and metastasis through modulation of Stat3 signaling (Avtanski et al., 2014). Further work has revealed that this may ultimately be accomplished through modulation of cellular bioenergetics (Pan et al., 2014).
8. Targeting cell signaling pathways in metastatic cells
Recent clinical data strongly suggest that EGFR tyrosine kinase inhibitors have a role to play in treating and potentially preventing brain metastasis of lung cancers, as reviewed above. However, EGFR represents only one target; as basic science continues to evolve, new agents designed to target overexpressed or mutated elements within tumor cells themselves that are responsible for metastasis will make rational sense as tools to fight this deadly phenomenon.
9. Perspective
Lung cancer metastasis is an incredibly challenging clinical problem with enormous implications for patients. Much work remains to be done in this area. It is important to realize that our current understanding is fragmented and incomplete. Truly developing therapies that target lung cancer brain metastasis will require significant additional basic science research and likely highly interdisciplinary clinical teams to implement these findings in the translational setting. At the same time, existing understanding of metastasis and clinical studies indicating that tyrosine kinase inhibitors and other molecularly targeted therapies that cross the blood brain barrier are effective adjuncts to treat brain metastasis must be heeded. Clinical practice must increasingly match the scientific understanding of metastasis, which should lead to broad adoption of TKI and molecular therapy to treat metastasis in lung cancer patients with identified mutations. In addition, the clinical community should place renewed emphasis on pursuing studies such as the QUARTZ trials which are designed to shed light on the ability of broadly used clinical strategies, including whole-brain irradiation to actually produce clinically meaningful improvements in survival and quality of life. In clinical practice, the weight of the evidence must be considered; at this moment, the evidence strongly suggests broader use of molecular therapies to target metastatic sites and reaffirms the need to conduct more extensive basic and clinical research to better understand the true efficacy of current standard of care, while simultaneously developing the next generation of agents capable of better targeting brain metastasis.
Acknowledgments
The authors thank and acknowledge the National Institutes of Health for grant support (R01CA175360).
References
- Ali A, et al. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20(4):e300–6. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auperin A, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission: prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- Avtanski DB, et al. Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis. Mol Oncol. 2014;8(3):565–580. doi: 10.1016/j.molonc.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykara M, et al. Management of brain metastases from non-small cell lung cancer. J Cancer Res Ther. 2014;10(4):915–921. doi: 10.4103/0973-1482.137939. [DOI] [PubMed] [Google Scholar]
- Bowden G, et al. Gamma knife radiosurgery for the management of cerebral metastases from non-small cell lung cancer. J Neurosurg. 2015;122(4):766–772. doi: 10.3171/2014.12.JNS141111. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108(19):7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DB, et al. Clinical experience with crizotinib in patients with advanced ALK-Rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempke WC, et al. Brain metastases in NSCLC – are TKIs changing the treatment strategy? Anticancer Res. 2015;35(11):5797–5806. [PubMed] [Google Scholar]
- Drury LJ, et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci U S A. 2011;108(43):17655–17660. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Xu X, Xie C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: a pooled analysis of published data. Onco Targets Ther. 2014;7:2075–2084. doi: 10.2147/OTT.S67586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(4):S209–19. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego Perez-Larraya J, Hildebrand J. Brain metastase. Handb Clin Neurol. 2014;121:1143–1157. doi: 10.1016/B978-0-7020-4088-7.00077-8. [DOI] [PubMed] [Google Scholar]
- Goldberg SB, et al. Lung cancer brain metastases. Cancer J. 2015;21(5):398–403. doi: 10.1097/PPO.0000000000000146. [DOI] [PubMed] [Google Scholar]
- Goncalves PH, et al. Risk of brain metastases in patients with nonmetastatic lung cancer: analysis of the Metropolitan Detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer. 2016;122(12):1921–1927. doi: 10.1002/cncr.30000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938–944. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- Khan AJ, Dicker AP. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;31(1):11–13. doi: 10.1200/JCO.2012.46.0410. [DOI] [PubMed] [Google Scholar]
- Kuo CH, et al. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/twist pathway. Carcinogenesis. 2013;34(11):2600–2609. doi: 10.1093/carcin/bgt281. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. 1–15. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359(3):716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, et al. ADAM9 promotes lung cancer metastases to brain by a plasminogen activator-based pathway. Cancer Res. 2014;74(18):5229–5243. doi: 10.1158/0008-5472.CAN-13-2995. [DOI] [PubMed] [Google Scholar]
- Lippitz B, et al. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir H, et al. CXCR6 expression in non-small cell lung carcinoma supports metastatic process via modulating metalloproteinases. Oncotarget. 2015;6(12):9985–9998. doi: 10.18632/oncotarget.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JR, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nieder C, et al. Radiotherapy versus best supportive care in patients with brain metastases and adverse prognostic factors. Clin Exp Metastasis. 2013;30(6):723–729. doi: 10.1007/s10585-013-9573-x. [DOI] [PubMed] [Google Scholar]
- Nolte SM, et al. A cancer stem cell model for studying brain metastases from primary lung cancer. J Natl Cancer Inst. 2013;105(8):551–562. doi: 10.1093/jnci/djt022. [DOI] [PubMed] [Google Scholar]
- Nosaki K, Seto T. The role of radiotherapy in the treatment of small-cell lung cancer. Curr Treat Options Oncol. 2015;16(12):56. doi: 10.1007/s11864-015-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, et al. Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer Prev Res (Phila) 2014;7(11):1149–1159. doi: 10.1158/1940-6207.CAPR-14-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato PE, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8(3):754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- Reya T, et al. Stem cells: cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rofstad EK. Microenvironment-induced cancer. Int J Radiat Biol. 2000;76(5):589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- Roy I, et al. Pancreatic cancer cell migration and metastasis is regulated by chemokine-biased agonism and bioenergetic signaling. Cancer Res. 2015;75(17):3529–3542. doi: 10.1158/0008-5472.CAN-14-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryken TC, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten LJ, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast: colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45(Suppl. 2):S253–S257. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- Shintani Y, et al. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 2004;64(12):4190–4196. doi: 10.1158/0008-5472.CAN-03-3235. [DOI] [PubMed] [Google Scholar]
- Siegel RL, et al. Cancer death rates in US congressional districts. CA Cancer J Clin. 2015;65(5):339–344. doi: 10.3322/caac.21292. [DOI] [PubMed] [Google Scholar]
- Soda Y, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108(11):4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- Takano Koji, Kinoshita Manabu, Takagaki Masatoshi, Sakai Mio, Tateishi Souichirou, Achiha Takamune, Hirayama Ryuichi, Nishino Kazumi, Uchida Junji, Kumagai Toru, Okami Jiro, Kawaguchi Atsushi, Hashimoto Naoya, Nakanishi Katsuyuki, Imamura Fumio, Higashiyama Masahiko, Yoshimine Toshiki. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol. 2016;18(5):716–724. doi: 10.1093/neuonc/nov266. http://dx.doi.org/10.1093/neuonc/nov266, first published online October 31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shang Y. Epigenetic control of epithelial-to-mesenchymal transition and cancer metastasis. Exp Cell Res. 2013;319(2):160–169. doi: 10.1016/j.yexcr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Wang SY, et al. Risk of cerebral metastases for postoperative locally advanced non-small-cell lung cancer. Lung Cancer. 2009;64(2):238–243. doi: 10.1016/j.lungcan.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Wegner RE, et al. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):e21–e27. doi: 10.1016/j.ijrobp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Xiao D, He J. Epithelial mesenchymal transition and lung cancer. J Thorac Dis. 2010;2(3):154–159. doi: 10.3978/j.issn.2072-1439.2010.02.03.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, et al. Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest. 1999;115(3):714–719. doi: 10.1378/chest.115.3.714. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. Tumor hypoxia enhances non-small cell lung cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5(20):9664–9677. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]


