Abstract
We previously developed a distress tolerance-based (DT) treatment that showed promising results for smokers with a history of early lapse. In the current study, we conducted a randomized controlled trial (RCT) of this DT treatment for a general population of smokers not limited to those with a history of early lapse. We randomized 116 participants (41% female) to DT or standard treatment (ST). Both treatments included one individual session during week 1 followed by seven group sessions during weeks 2-9 (quit date at session 4), two 20-minute phone sessions, and 8 weeks of transdermal nicotine patch. Results indicated no significant differences between conditions in the primary outcome of biochemically verified 7-day point prevalence smoking abstinence or in time to first lapse. Verified abstinence rates in DT were 38.7%, 38.7%, 46.77%, 40.32%, 20.9% and 17.7% vs. 40.7%, 37.0%, 53.7%, 44.4%, 33.3%, and 22.2% in ST at 1-, 2-, 4-, 8-, 13-, and 26-weeks post-quit, respectively. Additionally, we found no significant moderators of treatment efficacy and few differences in treatment process variables. These findings stand somewhat in contrast to our previous study and other recent studies of similar acceptance-based treatments. However, differences in methodology, inclusion of nicotine replacement therapy in both treatment conditions, and strict inclusion/exclusion criteria that excluded many smokers with affective vulnerabilities may underlie this discrepancy. Future research should evaluate the utility of DT and other acceptance-based treatments in populations with affective vulnerabilities who might specifically benefit from a DT-based approach.
Keywords: smoking cessation, tobacco cessation, distress tolerance, Acceptance and Commitment Therapy, nicotine dependence
INTRODUCTION
Although 70% of smokers want to quit (CDC, 2011), approximately 95% who quit unassisted (Hughes, Keely, & Naud, 2004) and 70-80% of those who receive pharmacological and/or behavioral treatment relapse within one year (Fiore et al., 2008). Accumulating evidence suggests that initial lapses occur very early regardless of treatment, with at least 50% smoking within the first week of a quit attempt (Ashare, Wileyto, Perkins, & Schnoll, 2013; Hughes et al., 2004). In a recent large study of 1429 smokers, 44% failed to quit for 7 consecutive days (Japuntich et al., 2011).
Roles of Distress Tolerance and Experiential Avoidance in Smoking Lapse
Negative affect, which is a prominent feature of nicotine withdrawal (Hendricks, Ditre, Drobes, & Brandon, 2006; Hughes, Higgins, & Hatsukami, 1990), has long been thought to be a key factor in the maintenance of substance use behavior (e.g., Khantzian, 1997) including tobacco use (Baker, Piper, McCarthy, Majeskie, & Fiore; Leventhal & Zvolensky, 2015). Numerous empirical studies have confirmed that negative affect, even independent of nicotine withdrawal, is a critical factor in smoking treatment outcomes and a primary precipitant of smoking lapse and relapse. In early studies, smokers consistently reported that relapse to smoking often occurred in situations involving negative moods such as anxiety, anger, and depression (Bliss, Garvey, Heinold, & Hitchcock, 1989; Brandon, Tiffany, Obremski, & Baker, 1990; Marlatt & Gordon, 1980; Shiffman, 1982), and lapses in negative affect situations were more likely to lead to complete relapses (O’Connell & Martin, 1987). Later prospective studies suggested that the severity of negative affect (Baker, Brandon, & Chassin, 2004; Covey, Glassman, & Stetner, 1990; Ginsberg, Hall, Reus, & Muñoz, 1995; Kinnunen, Doherty, Militello, & Garvey, 1996; Piasecki, Kenford, Smith, Fiore, & Baker, 1997; West, Hajek, & Belcher, 1989) and temporal variability in affective symptoms are more robustly related to smoking cessation outcomes than are severity or variability in other withdrawal factor domains (al’Absi, Hatsukami, Davis, & Wittmers, 2004; Brandon et al., 2003; Kenford et al., 2002; McCarthy, Piasecki, Fiore, & Baker, 2006; Piasecki et al., 2000; Strasser et al., 2005). More recent work has used methodology such as ecological momentary assessment (EMA) to sample participants’ mood and smoking behavior throughout the day in real time, which has revealed acute temporal relationships between negative affect and smoking lapse and relapse (e.g., Lam et al., 2014; Minami, Frank, Bold, & McCarthy, 2018; Minami, Yeh, Bold, Chapman, & McCarthy, 2014; Shiffman, 2005; Shiffman et al., 2007)
Extending this work, further research has demonstrated that one’s ability to tolerate affective distress (i.e., distress tolerance), that is, to persist at maintaining abstinence despite encountering various states of affective discomfort, may also be a key determinant of successful long-term cessation (e.g., Brandon et al., 2003; Brown, Lejuez, Kahler, & Strong, 2002). From this perspective, it is not simply the absolute magnitude of affective distress experienced, but rather how one responds to this distress that may be critical in determining the outcome of a quit attempt. Indeed, evidence suggests that smokers are less persistent than nonsmokers on tasks that produce affective and/or physical distress (e.g., breath-holding, mental arithmetic, tracing geometric figures from the perspective of a mirror) (Brown et al., 2002; Quinn, Brandon, & Copeland, 1996) and within smokers, the duration of persistence on these tasks prospectively predicts abstinence following a quit attempt (Brandon et al., 2003; Brown et al., 2009).
Other recent research has focused on the related concept of experiential avoidance, which “occurs when a person is unwilling to remain in contact with particular private experiences (e.g., bodily sensations, emotions, thoughts, memories, behavioral dispositions) and takes steps to alter the form or frequency of these events and the contexts that occasion them, even when doing so creates harm” (Hayes, 2004, p.14). Efforts to control or suppress private experience may be a problematic method of coping for smokers who, in an effort to escape the uncomfortable negative affect and symptoms of nicotine withdrawal, may readily resort to the calming effects of nicotine (Piasecki, Fiore, & Baker, 1998) for relief. Recent work has demonstrated that higher levels of smoking-specific experiential avoidance are associated with greater reliance on cigarettes and affect-regulatory smoking outcome experiences (Farris, Zvolensky, DiBello, & Schmidt, 2015). Additionally, smoking-specific experiential avoidance is associated with greater negative affect, craving, and nicotine withdrawal at the initiation of smoking cessation treatment (Farris, Zvolensky, & Schmidt, 2015). Further, in the context of experiencing internal distress while quitting (e.g., negative affect, physical withdrawal symptoms), high experientially avoidant smokers are at an increased likelihood of smoking lapse, even after smoking cessation treatment, relative to smokers with low levels of experiential avoidance (Minami, Bloom, Reed, Hayes, & Brown, 2014). Reductions in experiential avoidance by quit date are also associated with increased likelihood of quit date abstinence, and predictive of lower levels of internal distress on quit day (Farris, Zvolensky, & Schmidt, 2015).
Distress Tolerance Treatment for Smoking Cessation
Taken together, evidence from research findings in distress tolerance and experiential avoidance indicate that how one responds to affective distress is a significant predictor of smoking relapse. These findings are distinct in their emphasis on the regulation of and sensitivity to affect, rather than solely on the level of affective symptoms being reported or expressed, and led us to develop a distress tolerance-based (DT) treatment for smoking cessation. We have conducted a small, randomized controlled trial of this DT treatment in a sample of smokers with a history of early lapses (all participants had not attained more than 3 days of abstinence during the previous 10 years) (Brown et al., 2008; Brown et al., 2013). Results showed that compared to standard treatment, participants who received the DT treatment showed a larger decrease in smoking-specific experiential avoidance between baseline and quit date, were 6.46 times more likely to be abstinent at the end of treatment (66.7% vs. 31.8%), and also much more likely to recover from early lapses (Brown et al., 2013). However, smoking outcomes were not significantly different between conditions at post-treatment follow-ups (8, 13, and 26 weeks post-quit).
This distress tolerance treatment has at its core, the systematic and repeated exposure to increasingly lengthy periods of smoking abstinence prior to quit date. This was accomplished by prescribing specific periods of smoking abstinence of increasing duration over time. Treatment sessions were focused on the practice of distress tolerance skills used to manage the affective distress experienced. Participants needed to demonstrate a willingness to engage fully in this exposure experience. An acceptance of the discomfort and distress involved as they worked toward their desired goal of quitting smoking was encouarged. Attempts to use distraction procedures or “control strategies” that promote experiential avoidance were discouraged given these strategies have been shown to be significantly less effective than focused exposure in the treatment of anxiety disorders (Craske, Street, & Barlow, 1989; Grayson, Foa, & Steketee, 1982). Therefore, we also provided training in skills derived from Acceptance and Commitment Therapy (ACT) (Hayes, Strosahl, & Wilson, 1999), which is designed to facilitate acceptance behaviors aimed at private events that have interfered with accomplishing life goals and approach of psychologically aversive or troubling internal stimuli while behaving adaptively (Gifford, 1994; Gifford & Hayes, 1997). Ideally, smokers learned that controlling negative affect and avoiding thinking certain thoughts may simply not be a feasible permanent solution.
The Current Study
While it is evident that a low level of distress tolerance is predictive of early smoking lapse (Brandon et al., 2003; Brown et al., 2002; Brown et al., 2009), it is not as evident that this translates to the identification of individuals who, across multiple quit attempts over time, are characterized consistently by a history of early lapses. Furthermore, recent trials of ACT-based treatment for smoking cessation that were not limited to smokers with a history of early lapses have shown positive outcomes (Bricker, Wyszynski, Comstock, & Heffner, 2013; Bricker, Bush, Zbikowski, Mercer, & Heffner, 2014; Bricker, Mann, Marek, Liu, & Peterson, 2010; Gifford et al., 2004; Gifford et al., 2011; Hernandez-Lopez, Luciano, Bricker, Roales-Nieto, & Montesinos, 2009). Therefore, in the current study, we conducted a randomized controlled trial of our DT treatment in a general population of cigarette smokers (i.e., not restricted only to smokers with a history of early lapses), with standard behavioral treatment (ST) matched for contact time serving as the comparison condition. All participants in both groups also received a standard 8-week course of transdermal nicotine patch (TNP). We hypothesized that the behavioral exposure and training in distress tolerance skills in our DT treatment would more efficacious in ameliorating withdrawal-related relapse risk than ST. We also tested the hypotheses that participants randomized to the DT treatment would experience 1) greater increases in task persistence and greater decreases in emotional avoidance during the pre-quit period, and 2) lower levels of negative affect on quit date.
METHOD
Participants
Adult smokers were recruited from the local community via newspaper and radio advertisements. Eligible individuals were between 18-65 years old, smoked at least 10 cigarettes per day for the past 3 years, and were motivated to quit smoking (at least 5 on a 10-point scale). Exclusion criteria were current DSM-IV Axis I disorder, non-nicotine substance abuse or dependence within the past 6 months, current use of psychotropic medication, history of a significant medical condition (e.g., cardiovascular, neurologic, gastrointestinal, other systemic illness) or deemed as currently unhealthy in the context of a brief physician screening, pregnancy or breastfeeding, or current use of pharmacotherapy for smoking cessation or other tobacco products.
Interested individuals were first screened via telephone; those who met preliminary criteria were scheduled for a more comprehensive baseline assessment at our Butler Hospital research laboratory during which they provided informed consent and were administered a diagnostic interview (Mini-International Neuropsychiatric Interview, M.I.N.I.Sheehan et al., 1998) to confirm their eligibility. The CONSORT flow diagram is shown in Figure 1. The Institutional Review Board at Butler Hospital approved this study.
Figure 1.
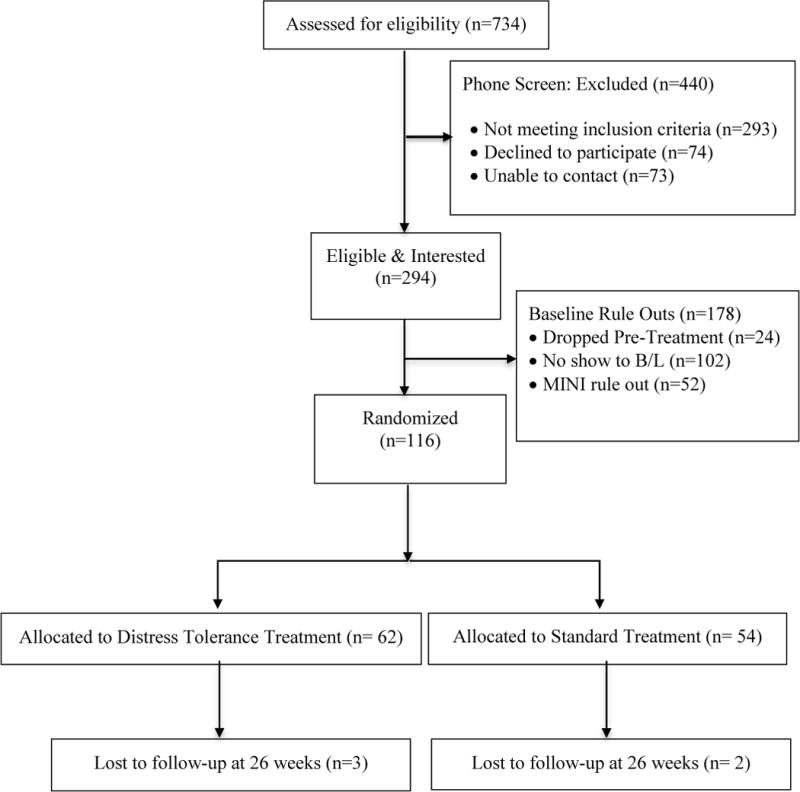
CONSORT flow diagram.
Procedure
The anticipated number and size of the behavioral treatment groups were determined in advance and each group’s treatment assignment was randomly selected from the fixed pool of possible assignments by an individual who had no contact with the participants. Participants were informed of their treatment assignment upon arrival to their first session; therefore, all randomized participants received their assigned treatment. Detailed therapist manuals were used to ensure standardized delivery of the treatments. The therapists were masters’ level clinicians (social worker, nurse) and psychology trainees (pre-doctoral students). Therapists were paired; each pair served as co-therapists for groups in both treatment conditions. The senior author (RAB) and second author (KMPR) trained the therapists and conducted weekly group supervision sessions to ensure standardization of protocol delivery. All sessions were conducted in our research laboratory at Butler Hospital and videotaped to facilitate supervision; therapists were provided detailed written feedback each week by KMPR.
Treatment Conditions
The standard treatment (ST) and distress tolerance treatment (DT) conditions were matched on time and structure. Both were delivered over a nine-week period beginning with one 1-hour individual session during week 1, followed by seven 2-hour group sessions during weeks 2-9, with quit date at group session 4. Additionally, all participants received two 20-minute phone sessions: between session 4 (the quit date session) and session 5, and between sessions 6 and 7 (during the week without a group session). All participants (ST and DT) began using transdermal nicotine patch (TNP) on their quit date and continued for four weeks on 21mg patch, then tapered to 14mg for two weeks, and finally 7mg for two weeks.
Standard Smoking Cessation Treatment (ST)
ST was based upon a standard behavioral protocol (Brown, 2003) that has yielded positive outcomes in controlled trials (Brown et al., 2001), and was consistent with the most recent clinical practice guideline from the U.S. Department of Health and Human Services (Fiore et al., 2008). During the individual session, therapists established rapport, provided support and encouragement, engaged participants in a discussion of previous quit experiences, and provided an overview of ST. The pre-quit and quit date group sessions focused on preparation for quit date, reinforcement and support for quitting, discussion of past quit experiences, initiation of self-monitoring, identification of triggers, development of self-management skills as coping strategies for triggers (three “A’s”: avoid, alter, alternative), avoidance of alcohol use, enlisting social support, and instruction in use of TNP. During the quit date session, participants received individual support and the opportunity for more tailored and elaborate discussions of quitting experiences and coping strategies. After quit date, remaining sessions focused on relapse prevention, including ongoing discussion of quitting experiences, anticipation of high-risk situations, developing social support, and initiating lifestyle changes that support abstinence.
Distress Tolerance Treatment (DT)
A detailed description of our original DT treatment for smokers with history of early lapse has been published previously (Brown et al., 2008; Brown et al., 2013). The DT treatment in the current study included the same elements, but the structure and duration of the sessions were changed to match the ST protocol as described above (one 1-hour individual session, seven 2-hour group sessions, and two 20-minute phone sessions). Participants in DT engaged in exercises aimed at increasing their tolerance of distress while maintaining a focus on the valued life goals associated with quitting smoking. The individual session served to increase motivation and foster the therapeutic relationship through values assessment and clarification and assessment of the phenomenology of participants’ past quit attempts. Pre-quit date sessions focused on increasing exposure to withdrawal-related distress via nicotine fading(Foxx & Brown, 1979) and increasing periods of scheduled abstinence, along with exercises and metaphors to illustrate the concepts of acceptance and defusion. DT also included the major elements of ST (e.g., self-management skills, instruction in use of NRT, increasing social support, identifying high-risk situations, and avoiding lapses). However, given that DT and ST were matched on time, the amount of time devoted to some ST elements was shortened in DT to make room for the additional metaphors and experiential exercises drawn from ACT to demonstrate concepts of willingness, assertiveness, values, and committed action. Furthermore, some ST elements in DT were conveyed with slightly different wording to ensure consistency and smooth transitions with the ACT-based content.
When originally developing DT (Brown et al., 2008; Brown et al., 2013), we had some concern that reducing withdrawal-related distress via pharmacotherapy (i.e., TNP) might paradoxically reduce the efficacy of DT given its focus on tolerating distress. However, we also recognized that there would be abundant distress to be experienced prior to quit date (i.e., prior to the use of TNP) via nicotine fading. Furthermore, pharmacotherapy is recommended for all smokers by the current clinical practice guideslines (Fiore et al., 2008). Finally, given that we found a significant treatment effect in our previous trial in which all participants received TNP (Brown et al., 2013), we retained TNP in the current study.
Measures
Assessment of the primary outcome (smoking status) occurred at baseline, during behavioral treatment (1-, 2-, and 4-weeks post-quit), and at 8-, 13-, and 26-week post-quit follow-ups, with 96%, 96%, and 92% follow-up completion rates, respectively. Negative affect was also measured at these timepoints. Distress tolerance was measured at baseline and at a “pre-quit” assessment during the week prior to the scheduled quit date. Other process measures described below were administered at baseline, pre-quit, end of behavioral treatment (4 weeks post-quit), and follow-ups. Participants were paid $40 for the pre-quit assessment, $25 for the end of treatment, 8-, and 13-week post-quit assessments, and $50 for the 26-week post-quit assessment. They received an additional $30 for providing biochemical verification of abstinence (expired carbon monoxide and/or saliva cotinine, see below) at 8, 13, and 26 weeks. Assessors were blinded to treatment condition at the baseline assessment but could not be blinded for follow-ups because they were present at the beginning of group sessions to administer assessments. Nevertheless, during follow-ups the assessors would only be aware of the participant’s condition if they specifically remembered it or the participant said something that revealed condition. The participant’s condition was not written on any assessment materials.
Smoking Status
Self-reports of past-week abstinence (7-day point prevalence) were verified by expired carbon monoxide (CO, less than 8 ppm) at 1-, 2-, 4-, 8-, 13-, and 26-weeks post-quit, and by saliva cotinine (10 ng/ml or less) at 13 - and 26-weeks. When CO was unavailable for an assessment, self-reported abstinence was verified by informants identified by participants prior to quitting when possible (n = 1 at week 8 and 13). Unverified reports of abstinence were considered to be smoking.
Process Measures
Distress Tolerance
As in previous studies, distress tolerance was operationalized as duration of persistence on the Computerized Mirror-Tracing Persistence Test (C-MTPT), which requires participants to trace geometric figures from the perspective of a mirror (Strong et al., 2003) and the Paced Auditory Serial Audition Test (PASAT), which requires participants to engage in increasingly difficult mental arithmetic (Lejuez, Kahler, & Brown, 2003). Participants were asked to complete tasks to the best of their ability, but were not provided with any incentives to do so.
Experiential Avoidance
General tendency toward experiential avoidance was assessed using the 10-item version of the Acceptance and Action Questionnaire II (AAQ-II)(Bond et al., 2011) (Cronbach’s α = .80) and the 8-item Avoidance/Rumination subscale of the Behavioral Activation for Depression Scale (BADS-AR)(Kanter, Mulick, Busch, Berlin, & Martell, 2007) (Cronbach’s α = .87). We also assessed smoking-specific experiential avoidance with the Avoidance and Inflexibility Scale (AIS) (Cronbach’s α = .89), a 13-item measure designed to assess the likelihood that smoking-related internal experiences (e.g., thoughts of smoking, cravings, physical withdrawal) will lead one to smoke and the degree to which one believes that reduction in the frequency and intensity of these internal experiences (i.e., avoidance of them) is necessary in order not to smoke (Gifford, Antonuccio, Kohlenberg, Hayes, & Piasecki, 2002).
Negative Affect
Measures of negative affect included the affective withdrawal subscale of the Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes & Hatsukami, 1986) (Cronbach’s α = .77) to assess withdrawal-related negative affect and the Negative Affect subscale of the Positive and Negative Affect Scale (PANAS) (Cronbach’s α = .93 and .78, respectively) to assess general negative affect(Watson, Clark, & Tellegen, 1988).
Treatment Adherence
A modified version of the ACT Tape Rating Scale (Gifford & Hayes, 1998), created to map onto the DT treatment manual, was used to rate adherence for DT group sessions. A rating scale for ST was developed in a similar manner. All groups sessions were videotaped, and 35% of the tapes (both the DT and ST group sessions) were randomly selected for rating. Two raters independently assessed the presence or absence of therapists’ behaviors that were categorized as “prescribed” (e.g., in-session exposure exercise, discussion of awareness, acceptance, and willingness to experience – for the DT sessions) and “proscribed” (e.g., discussion of values and goals, experiential acceptance, discussing how efforts to control thoughts and feelings do not work – for the ST sessions) (Waltz, Addis, Koerner, & Jacobson, 1993). Treatment adherence ratings indicated that 100% of prescribed elements were present in the DT tapes and the ST tapes that were rated while no proscribed behaviors in ST session tapes was observed, indicating good treatment integrity in both treatment conditions. There was no disagreement between the two raters.
Data Analysis
Sample Size Estimation
We wanted to ensure an ability to detect a clinically meaningful increase in quit rates for DT of 10% over ST. To increase power and accurately reflect the planned analysis for the longitudinal design, we estimated sample size based on an analysis of all planned assessments of the primary outcome (7-day point prevalence smoking abstinence) through the last follow-up. We relied on outcomes from our previous trial (Brown et al., 2013) to estimate effects during the active treatment period and extrapolated long-term outcomes for the ST comparison condition using data from the current Clinical Practice Guideline for the Treatment of Tobacco Dependence (Fiore et al., 2008). Our original power analysis indicated that 101 participants per group would be required. However, after the study was initiated, the sponsor reduced the budget and we reached an agreement to reduce the target sample size to 128. We were able to recruit a final sample of 116 within the budget and time constraints determined by the sponsor.
Primary Outcome
The effects of DT treatment on verified 7-day point-prevalence abstinence through 26-week follow-up was tested using Generalized Estimating Equations (GEE). All randomized participants were included in these analyses; participants lost to follow-up were considered smoking. Planned baseline covariates included gender, age, baseline nicotine dependence, and time (weeks from quit date). An autoregressive (AR-1) correlation structure was specified in the GEE models because model fit indices (quasi-likelihood under the independence model criterion, QIC) indicated that AR-1 correlation structure fit the data best, compared to either an “independence” or “unstructured” correlation structure. Furthermore, we explored potential moderators of treatment efficacy by including baseline values of process measures (i.e., AAQ-II, AIS, MTPT, PASAT) as well as treatment X process measure interaction terms (e.g., treatment X AAQ-II) in the GEE primary outcome model separately, controlling for the same planned covariates.
Secondary Outcomes
Cox proportional-hazards regression analyses were conducted to test whether latency to first smoking lapse and latency to relapse (7 consecutive smoking days) differed between treatment conditions, controlling for gender, age and nicotine dependence. All randomized participants were included in the analysis; those who never lapsed or relapsed were treated as censored in analyses. We used linear regression analysis to evaluate effects of treatment on process measures (task persistence, emotional avoidance, and negative affect), controlling for the corresponding baseline score. Sample sizes for these analyses varied based on available data, and ranged from 75 (quit date affective withdrawal) to 112 (moderator analysis with AAQ-II).
RESULTS
Preliminary Analyses
A total of 116 participants (41% female, 90.5% White, mean age 46.06 years) were randomized to receive Distress Tolerance Treatment (DT, n = 62) or Standard Treatment (ST, n = 54). Prior to treatment, participants reported smoking 20.10 (SD = 7.85) cigarettes per day and had a mean FTND of 5.7 (SD =2.04) indicating a moderate level of nicotine dependence. Table 1 presents demographic and baseline characteristics for each treatment group. Treatment groups did not differ on any baseline variables except that the DT group reported marginally higher baseline general emotional avoidance (AAQ-II) compared to the ST group (M = 57.9, 54.9 SD = 7.8, 9.6, respectively, p = .066).
Table 1.
Participant Demographics and Baseline Characteristics
| DT (N = 62) | ST (N = 54) | |
|---|---|---|
|
| ||
| n (%) | n (%) | |
| Female (%) | 26 (41.9%) | 22 (40.7%) |
| Race/Ethnicity | ||
| Hispanic | 2 (3.2%) | 1 (1.9%) |
| White | 58 (93.5%) | 47 (87.0%) |
| African-American | 2 (3.2%) | 6 (11.1%) |
| Other | 2 (3.2%) | 1(1.9%) |
|
| ||
| M (SD) | M (SD) | |
|
| ||
| Age | 44.97 (11.45) | 47.31(10.98) |
| Nicotine Dependence (FTND) | 5.73 (2.00) | 5.76(2.10) |
| Cigarettes per day | 20.11 (8.46) | 20.09 (7.16) |
| Computerized Mirror Tracing Persistence Test (C-MTPT) | 192.32 (103.24) | 174.71 (108.46) |
| Paced Auditory Serial Addition Task (PASAT) | 155.23 (132.77) | 149.31 (122.70) |
| General Experiential Avoidance (AAQ-II) | 57.91 (7.81) | 54.87(9.57) |
| Smoking-Specific Experiential Avoidance (AIS) | 44.05 (10.49) | 45.36 (8.30) |
| Avoidance and Rumination (BADS-AR) | 15.37 (7.92) | 14.35 (6.63) |
| General Negative Affect (PANAS) | 11.87 (2.95) | 12.61 (3.84) |
| Affective Withdrawal (MNWS) | 1.38 (0.60) | 1.26 (0.38) |
Smoking Outcomes
The GEE models first examined the effects of planned covariates on smoking outcome along with the linear effect of time. Age (AOR =1.04, 95%CI = 1.01 – 1.07, p = 0.004) and level of nicotine dependence (AOR =0.81, 95CI = 0.69 – 0.96, p = 0.013), but not gender (AOR =0.98, 95%CI = 0.60 – 1.97, p = 0.97), predicted smoking outcome, such that older participants and those with a lower level of dependence were more likely to be abstinent. Moreover, the significant linear effect of time (AOR =0.96, 95%CI = 0.94 – 0.98, p = 0.000) on smoking outcome indicated declines in abstinence rates over time. We also tested the curvilinear effect of time (time was centered at 8-weeks post-quit) on smoking outcome by including a quadratic term in the models; however, no such effect was found (p = .32).
After controlling for the primary set of covariates, GEE models found no differences in verified abstinence rates at 1-, 2-, 4-, 8-, 13-, and 26-weeks after quit date for smokers in DT vs. ST (AOR = 0.88, 95%CI = 0.48 – 1.60, p = .68). Verified abstinence rates for participants in the DT condition were 38.7%, 38.7%, 46.77%, 40.32%, 20.9% and 17.7% vs. 40.7%, 37.0%, 53.7%, 44.4%, 33.3% and 22.2% in the ST condition. A linear or quadratic effect of time (again, time centered at 8-weeks post-quit) by treatment was not significant (ps > .19). Additionally, abstinence rates were not significantly different for DT and ST participants at any of the individual assessment points (ps > .05).
Latency to First Lapse and Relapse
We also examined the effects of treatment on latency to first smoking lapse and relapse using Cox proportional hazard regression, controlling for the primary covariates of age, gender, and nicotine dependence. The observed median time to first lapse was 11.5 days (M = 57.8 days; SD = 73.5) in DT and 20 days (M = 68.9 days; SD = 79.2) in ST while median time to relapse was 63 days (M = 84.4 days; SD = 76.5) in DT and 110.5 days (M = 104.5 days; SD = 76.1) in ST. However, no statistically significant difference in latency to first lapse (Hazard Ratio [HR] = 1.20, 95%CI = 0.79 - 1.83, p = .400) or relapse (HR = 1.35, 95%CI = 0.84 - 2.16, p = .210) was observed between treatment conditions.
Group Session Attendance and Nicotine Patch Use
A total of 53 (45.7%) participants (DT = 29, 46.8%; ST=24, 44.4%) attended all 7 group sessions during the study (mean = 5.34, SD = 2.22) and two-thirds (n = 87) of the participants (DT = 48, 77.4%; ST=39, 72.2%) attended at least 5 group sessions. No difference was found between treatment conditions in the proportion of those who attended all 7 or at least 5 sessions (ps > 0.58). Including an adherence variable (1= attended all 7 groups sessions, 0 = missed any sessions) in the primary outcome model above showed that those who attended all 7 group sessions were more likely to be abstinent (AOR = 5.6, 95%CI = 2.8 – 11.1, p = 0.000). However, a subsequent model with an adherence X treatment interaction term revealed no moderating effect of adherence on treatment efficacy (AOR =1.21, 95%CI = 0.35 - 4.14, p = .76).
On average, during the first 8 weeks, participants in DT and ST groups used nicotine patches on 34.4 days (61.3%) and 35.3 days (63.0%), respectively. A total of 9 participants (14.5%) in DT group and 10 (18.5%) in ST group never used patches during the study while the majority of participants in both DT and ST groups (64.5% and 61.1%, respectively) used at least 4 weeks (50%). In addition, 16 participants (25.8%) in DT group and 13 (24.1%) in ST group reported using patches after the initial 8 weeks post-quit (for 13.8 days and 20.2 days on average, respectively). No significant differences across conditions were found in any of these adherence rates (ps > 0.30).
Potential Moderators
We also explored baseline scores on AAQ-II, AIS, BADS-AR, MTPT, and PASAT as potential moderators in separate GEE models. However, no moderating effect of any of the variables on treatment efficacy was found (ps > .41).
Treatment Effects on Process Variables
Task Persistence – Pre-Quit Changes
No difference in change from baseline to the pre-quit assessment in task persistence measured by the MTPT (range = 14 - 376) (B = 2.17, SE = 16.17, p= .89) or PASAT (range = 1 - 347) (B = 23.76, SE = 23.25, p = .31) was found between conditions, controlling for the baseline score of the corresponding measure.
Experiential Avoidance – Pre-Quit Changes
No difference in change from baseline to the pre-quit assessment in general experience avoidance (AAQ-II: B = −1.16, SE = 1.56, p = .46) or smoking-specific experiential avoidance (AIS: B = 1.04, SE = 2.11, p = .63) was found between conditions, controlling for baseline score of the corresponding measure. However, those in the ST condition reported significantly greater increases in rumination and avoidance (BADS-AR: B = 3.17, SE = 1.42, p = .029), compared to those in the DT treatment (Figure 2).
Figure 2.
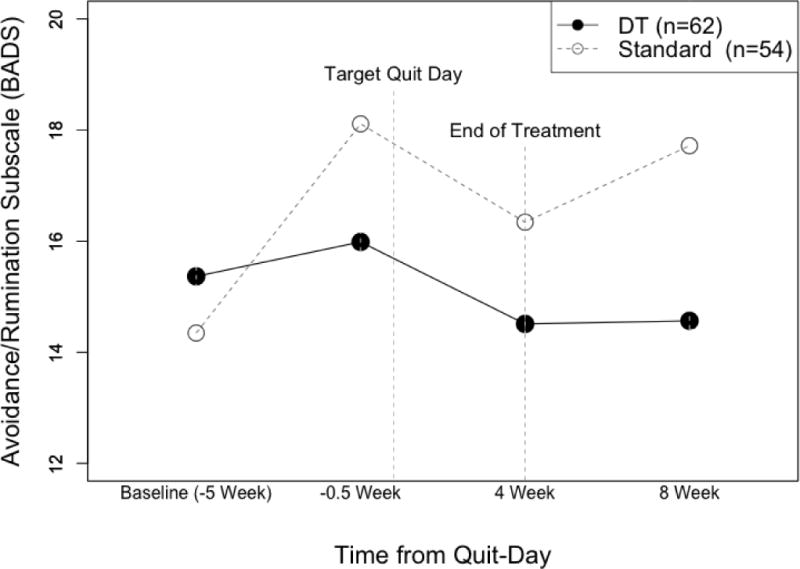
Changes in Avoidance and Rumination (BADS-AR) pre- and post-quit by treatment condition (Distress Tolerance vs. Standard Treatment).
Experiential Avoidance – Post-Quit Changes
No difference in change from baseline to end of behavioral treatment (4 weeks post-quit) in general experience avoidance (AAQ-II: B = 1.88, SE = 1.62, p = .25), smoking-specific experiential avoidance (AIS: B = 0.95, SE = 2.76, p = .73), or rumination and avoidance (BADS-AR: B = 2.04, SE = 1.50, p = .18) was found between conditions, controlling for baseline score of the corresponding measure.
Negative Affect on Quit Date
Results showed that there was no significant difference between conditions in either affective withdrawal (MNWS) or negative affect (PANAS) on quit date (ps > 0.25), controlling for the corresponding baseline score.
Effects of Process Measures on Smoking Outcomes
Next, we examined whether changes in process measures (from baseline to the end of behavioral treatment at 4 weeks post-quit) predicted smoking outcome at the end of pharmacotherapy (8 weeks post-quit), controlling for age, gender, nicotine dependence, treatment condition, and baseline values of the corresponding variable. Logistic regression models showed that decreases in smoking-specific experiential avoidance (AIS: B = −0.072, SE = 0.023, p = 0.002) at the end of behavioral treatment significantly predicted increased odds of abstinence at 8 weeks post-quit. However, changes in general experiential avoidance (AAQ-II: B = 0.054, SE = 0.034, p = 0.11) and avoidance and rumination (BADS-AR: B = 0.012, SE = 0.037, p = 0.73) were not associated with abstinence rates at 8-weeks post-quit.
Exploratory Analysis: Early Lapsers
Finally, we explored whether the pattern of results for the primary smoking outcome changed when only early lapsers (defined as reporting no previous periods of abstinence longer than 72 hours prior to participating in this study) were included. Although these GEE models did not reach statistical significance due to the small sample size (n = 18), they revealed that early lapsers in the DT treatment had almost twice the odds of abstinence over the first 4 weeks post-quit, compared to early lapsers in the ST treatment (AOR = 1.97, 95%CI=0.55-7.04, p = .299), but not after 8 weeks post-quit (Figure 3). This pattern of results is consistent with our previous study (Brown et al., 2013), which demonstrated the short-term efficacy of the DT treatment, compared to the ST treatment, among smokers with a history of early lapse (in that study, defined as no periods of abstinence longer than 72 hours in the previous 10 years).
Figure 3.
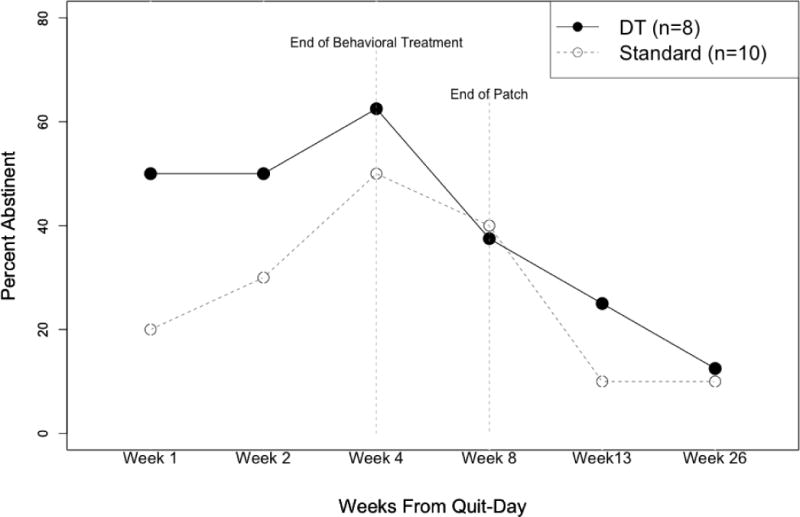
Abstinence rates for early lapsers by treatment condition (Distress Tolerance vs. Standard Treatment).
DISCUSSION
A Distress Tolerance (DT) treatment for smoking cessation that combined elements of exposure, distress tolerance, and Acceptance and Commitment Therapy (ACT)(Hayes et al., 1999) along with pharmacotherapy in the form of transdermal nicotine patch (TNP), did not improve short-term or prolonged smoking outcomes relative to standard behavioral treatment (ST) with TNP. There were no significant differences in abstinence rates between the two treatment groups at any timepoint. Furthermore, there were few group differences in hypothesized process measures, but decreases in smoking-specific experiential avoidance between baseline and end of treatment (4 weeks post-quit) did predict successful cessation at 8 weeks post-quit. In contrast, our previous study (Brown et al., 2013) in smokers with a history of early lapse found a significant treatment effect. Also, in our previous study, individuals who received DT reported larger decreases in smoking-specific experiential avoidance between baseline and quit date, but pre-quit changes in smoking-specific experiential avoidance did not relate to smoking cessation outcomes at the end of treatment. At the same time, exploratory analysis in the current study suggested that the DT treatment may have had greater short-term efficacy than ST within the small (n = 18) subsample of participants who had a history of early lapse (had never quit for more than 72 hours prior to study participation). This finding is consistent with results from our previous trial in which all participants were early lapsers who had never quit for more than 72 hours in the past 10 years (Brown et al., 2008; Brown et al., 2013) and leaves open the possibility that our DT treatment may be more effective than ST for smokers with a history of early lapse but not for those without such a history. Furthermore, in the current study, the DT and ST treatments were matched on total contact time and structure (i.e., number of sessions), whereas in our previous trial with early lapsers, the DT treatment had more total contact time than ST (six 50-minute individual sessions and nine 2-hour group sessions vs. six 90-minute group sessions, respectively). Thus, we are unable to determine with confidence whether the superior short-term efficacy of DT was due to differences in the treatment content or contact time, but it is possible that our DT treatment is likely to have benefit for early lapsers but not for a general population of smokers.
The literature is not yet extensive enough to directly compare our DT treatment to related treatments, including ACT-based treatments for general populations of smokers (i.e., not limited to early lapsers) (Bricker et al., 2013; Bricker et al., 2014; Bricker et al., 2010; Gifford et al., 2004; Gifford et al., 2011; Hernandez-Lopez et al., 2009). Excluding small pilots (Bricker et al., 2010) and non-randomized studies,(2009) two prior studies have compared ACT to a medication-only treatment (Gifford et al., 2004; Gifford et al., 2011). When ACT has been compared to another established behavioral treatment, some studies have not included pharmacotherapy (Bricker et al., 2013; Hernandez-Lopez et al., 2009), which make direct comparison difficult since medication may have improved outcomes in the comparison conditions. A study that evaluated telephone-based ACT and provided 2 weeks of NRT (Bricker et al., 2014) echoes some of the findings in the present study. It likewise did not find a significant difference in quit rate between the two treatments at 6-month follow-up (although the overall ACT quit rate was somewhat higher at 6 months, 33%). It also found outcome differences in subgroups that overlap with our findings for early lapsers. For example, there was a significant difference in outcomes at follow up for those with a low initial acceptance of cravings. This similarity in the pattern of results suggests that researchers might usefully explore the impact of DT and other acceptance-based approaches with particularly difficult to treat subgroups.
Moderation of Treatment Effects and Treatment Process
Previous studies have found that ACT-based treatment resulted in greater decreases in smoking-specific experiential avoidance (AIS) that mediated the effects of the treatment on smoking outcome (Bricker et al., 2013; Gifford et al., 2004; Gifford et al., 2011). Other research has shown that smoking abstinence elicits reductions in smoking-specific EA(Farris et al., 2016). In the current study, participants in the ST condition had significantly greater increases in a measure of rumination and avoidance (BADS-AR) compared to those in the DT treatment, but no differences in changes in AIS or other process measures were observed across treatments. With respect to the relationship between experiential avoidance and smoking outcomes, decreases in smoking-specific experiential avoidance at the end of behavioral treatment were significantly associated with abstinence at 8 weeks post-quit. However, this relationship did not differ between treatment conditions, suggesting that the DT condition did not differentially affect smoking-specific experiential avoidance relative to ST, unlike in our previous DT study (Brown et al., 2013) and therefore it is not surprising that smoking outcomes did not differ between DT and ST.
Limitations
The current study had a number of strengths including a rigorous, randomized controlled trial design with treatment conditions matched on time and structure; however, there were also limitations that warrant acknowledgment. First, the study may have been underpowered because we had to reduce the sample size from the original target. However, we believe it is very unlikely that lack of power explains why we did not find group differences in abstinence rates, given our effect sizes. Second, we excluded smokers with current psychiatric and substance use comorbidities. Relative to the general population, smoking prevalence among individuals with these comorbidities is substantially higher (CDC, 2013; Lasser et al., 2000). Furthermore, these individuals have more difficulty in quitting successfully compared to the general population of smokers (Prochaska, Delucchi, & Hall, 2004), indicating that many smokers with these comorbidities are likely to have a history of early lapse. Gifford and colleagues excluded smokers with psychiatric and/or substance use comorbidities (Gifford et al., 2004; Gifford et al., 2011), whereas other studies of ACT-based treatments for smoking cessation did not (Bricker et al., 2013; Bricker et al., 2014; Bricker et al., 2010; Hernandez-Lopez et al., 2009). Some of these studies have shown stronger differential outcomes in psychologically-distressed participants, suggesting the need for more research in this area. Third, the DT treatment we tested in the current study (1 60-min individual session and 7 2-hr group sessions) differed in total duration and structure from our prior DT treatment (6 50-minute individual sessions and 9 2-hr group sessions) for reasons previously described. Although in revising the duration and structure we intended to retain all the major content elements, it is possible that one or more critical elements were unintentionally removed. Finally, including TNP in the DT treatment may have conveyed an inconsistent message and reduced participants’ opportunities for exposure and practice of DT skills, as the purpose of using TNP is to decrease discomfort and intensity of nicotine withdrawal symptoms and cravings. Indeed, long-term quit rates for ACT-based treatments in previous studies that did not include pharmacotherapy (Gifford et al., 2004; Hernandez-Lopez et al., 2009) were somewhat higher than in the current study. Finally, we did not collect compliance data on the extent to which participants engaged in the scheduled abstinence or nicotine fading procedures, thus limiting our ability to examine outcomes according to these treatment compliance indices.
Conclusion
In conclusion, in the current study we conducted a randomized controlled trial of distress tolerance treatment (DT) vs. standard behavioral treatment (ST) for smoking cessation in a general population sample of healthy adult daily smokers. Results indicated no significant differences in efficacy between the two treatment conditions, no significant moderators of treatment efficacy, and few differences in treatment process variables. There was some indication of better outcomes for early lapsers, however, which comports with previous research and suggests a useful avenue of research exploration.
Acknowledgments
This study was partially supported by grant R01 DA017332 from the National Institute on Drug Abuse to Richard A. Brown.
Richard A. Brown has equity ownership in Health Behavior Solutions, Inc., which is developing products for tobacco cessation although not products directly related to this publication. The terms of this arrangement have been reviewed and approved by the University of Texas at Austin in accordance with its policy on objectivity in research. Erika Litvin Bloom is a paid consultant for WayBetter, Inc., which develops Internet-based games to motivate health behavior change including smoking cessation; this work is unrelated to this publication.
The authors wish to thank Chris Faria, Jacki Hecht, Steve Matsko, Jennifer Rogers and Jessica Wolfe for their contributions to this research.
References
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73(3):267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med. 2013;7(4):249–254. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology. 1989;57(3):443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Zettle RD. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy. 2011;42(4):676–688. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112(3):448–456. doi: 10.1037/0021-843X.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine & Tobacco Research. 2013;15(10):1756–1764. doi: 10.1093/ntr/ntt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob Res. 2014;16(11):1446–1454. doi: 10.1093/ntr/ntu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, Peterson AV. Telephone-delivered Acceptance and Commitment Therapy for adult smoking cessation: a feasibility study. Nicotine & Tobacco Research. 2010;12(4):454–458. doi: 10.1093/ntr/ntq002. [DOI] [PubMed] [Google Scholar]
- Brown RA. Intensive behavioral treatment. In: Abrams RSNDB, Brown RA, Emmons KM, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press; 2003. pp. 118–177. [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Miller IW. Cognitive-behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69(3):471–480. doi: 10.1037/0022-006X.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behavior Modification. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Hayes SC. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research. 2013;15(12):2005–2015. doi: 10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Quitting smoking among adults — United States, 2001-2010. Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- CDC. Vital signs: Current cigarette smoking among adults aged ≥ 18 years with mental illness – United States, 2009-2011. Morbidity and Mortality Weekly Report. 2013;62(05):81–87. [PMC free article] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990 Jul-Aug;31(4):350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Craske MG, Street L, Barlow DH. Instructions to focus upon or distract from internal cues during exposure treatment of agoraphobic avoidance. Behavior Research and Therapy. 1989;27(6):663–672. doi: 10.1016/0005-7967(89)90150-2. [DOI] [PubMed] [Google Scholar]
- Farris SG, DiBello AM, Heggeness LF, Reitzel LR, Vidrine DJ, Schmidt NB, Zvolensky MJ. Sustained smoking abstinence is associated with reductions in smoking-specific experiential avoidance among treatment-seeking smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2016;51:51–57. doi: 10.1016/j.jbtep.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, DiBello AM, Schmidt NB. Validation of the Avoidance and Inflexibility Scale (AIS) among treatment-seeking smokers. Psychological Assessment. 2015;27(2):467–477. doi: 10.1037/pas0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Schmidt NB. Smoking-specific experiential avoidance cognition: explanatory relevance to pre- and post-cessation nicotine withdrawal, craving, and negative affect. Addictive Behaviors. 2015;44:58–64. doi: 10.1016/j.addbeh.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. May, Treating Tobacco Use and Dependence: 2008 Update. 2008. [Google Scholar]
- Foxx RM, Brown RA. Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. Journal of Applied Behavior Analysis. 1979;12(1):111–125. doi: 10.1901/jaba.1979.12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EV. Setting a course for behavior change: The verbal context of acceptance. In: Hayes SC, Jacobson NS, Follette VM, Dougher MJ, editors. Acceptance and change: Content and context in psychotherapy. Reno, NV: Context Press; 1994. pp. 218–222. [Google Scholar]
- Gifford EV, Antonuccio DO, Kohlenberg BS, Hayes SC, Piasecki MM. Paper presented at the Association for Advancement of Behavioral Therapy. Reno, NV: 2002. Combining Bupropion SR with acceptance and commitment-based behavioral therapy for smoking cessation: Preliminary results from a randomized controlled trial. [Google Scholar]
- Gifford EV, Hayes SC. Paper presented at the Paper presented at the meeting of the Association for Behavior Analysis. Chicago, IL: 1997. Discrimination training and the function of acceptance. [Google Scholar]
- Gifford EV, Hayes SC. ACT tape rating scale. 1998 Unpublished manuscript. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behavior Therapy. 2004;35:689–705. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy. 2011;42(4):700–715. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Munoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3(4):389–395. [Google Scholar]
- Grayson JB, Foa EB, Steketee G. Habituation during exposure treatment: distraction vs attention-focusing. Behaviour Research and Therapy. 1982;20(4):323–328. doi: 10.1016/0005-7967(82)90091-2. [DOI] [PubMed] [Google Scholar]
- Hayes SC. Acceptance and commitment therapy and the new behavior therapies: Mindfulness, acceptance, and relationship. In: Hayes SC, Follette VM, Linehan MM, editors. Mindfulness and acceptance. New York: The Guilford Press; 2004. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York: The Guilford Press; 1999. [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors. 2009;23(4):723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, Israel Y, Kalant H, Sellers EM, Vingilis ER, editors. Research Advances in Alcohol and Drug Problems. Vol. 10. 1990. pp. 317–398. [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB. Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine. 2011;40(3):286–294. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): Psychometric properties and factor structure. Journal of Psychopathology and Behavioral Assessment. 2007;29:191–202. [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70(1):216–227. doi: 10.1037/0022-006X.70.1.216. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine dependence. Journal of Consulting and Clinical Psychology. 1996;64(4):791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Lam CY, Businelle MS, Aigner CJ, McClure JB, Cofta-Woerpel L, Cinciripini PM, Wetter DW. Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res. 2014;16(5):569–575. doi: 10.1093/ntr/ntt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. Behavior Therapist. 2003;26:290–293. [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141(1):176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral Medicine: Changing Health Lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Minami H, Bloom EL, Reed KMP, Hayes SC, Brown RA. The Moderating Role of Experiential Avoidance in the Relationships Between Internal Distress and Smoking Behavior During a Quit Attempt. Psychology of Addictive Behaviors. 2014 doi: 10.1037/adb0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami H, Frank BE, Bold KW, McCarthy DE. Ecological momentary analysis of the relations among stressful events, affective reactivity, and smoking among smokers with high versus low depressive symptoms during a quit attempt. Addiction. 2018;113(2):299–312. doi: 10.1111/add.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami H, Yeh VM, Bold KW, Chapman GB, McCarthy DE. Relations among affect, abstinence motivation and confidence, and daily smoking lapse risk. Psychology of Addictive Behaviors. 2014;28(2):376–388. doi: 10.1037/a0034445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1987;55(3):367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology. 1998;107(2):238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, Baker TB. Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science. 1997;8(3):184–189. doi: 10.1111/j.1467-9280.1997.tb00409.x. [DOI] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109(1):74–86. doi: 10.1037/0021-843X.109.1.74. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and sustance use? Applying learned industriousness theory to addictive behaviors. Experimental and Clinical Psychopharmacology. 1996;4:186–190. doi: 10.1037/1064-1297.4.2.186. [DOI] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91(2–3):159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Kaufmann V, Jepson C, Perkins KA, Pickworth WB, Wileyto EP, Lerman C. Effects of different nicotine replacement therapies on postcessation psychological responses. Addictive Behaviors. 2005;30(1):9–17. doi: 10.1016/j.addbeh.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Strong DR, Lejuez CW, Daughters SB, Marinello M, Kahler CW, Brown RA. The computerized mirror tracing task version 1. 2003 Unpublished manual. [Google Scholar]
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. Journal of Consulting and Clinical Psychology. 1993;61(4):620–630. doi: 10.1037/0022-006X.61.4.620. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]


