Abstract
Background
Atrial fibrillation (AF) with rapid ventricular rate (RVR; HR>100) in non-cardiac post-operative (NCPO) surgical patients is associated with poor outcomes. The objective of this study was to evaluate the practice patterns of AF management in a surgical ICU to determine practices associated with rate and rhythm control and additional outcomes.
Materials and Methods
Adult patients (≥ 18 years) admitted to the SICU from June 2014–June 2015 were retrospectively screened for the development of new onset AF with RVR. Demographics, hospital course, evaluation and treatment of AF with RVR, and outcome were evaluated and analyzed.
Results
1070 patients were admitted to the SICU during the study period; 33 met inclusion criteria (3.1%). Twenty-six patients (79%) had rate and rhythm control within 48 hours of AF with RVR onset. β-blockers were the most commonly used initial medication (67%), but were successful at rate and rhythm control in only 27% of patients (6/22). Amiodarone had the highest rate of success if used initially (5/6, 83%) and secondarily (11/13, 85%). Failure to control rate and rhythm was associated with a greater likelihood of comorbidities (100% vs 57%; p=0.06).
Conclusions
New onset AF with RVR in the NCPO patient is associated with a high mortality (21%). Amiodarone is the most effective treatment for rate and rhythm control. Failure to establish rate and rhythm control was associated with cardiac co-morbidities. These results will help to form future algorithms for the treatment of AF with RVR in the SICU.
Keywords: atrial fibrillation, mortality, surgical intensive care unit, non-cardiac surgery
Introduction
Post-operative atrial fibrillation (POAF) with rapid ventricular rate (RVR) in noncardiac surgical, trauma1 and ICU patients2 is of significant clinical concern. Mortality is twice as great in critically-ill surgical patients who develop atrial fibrillation when compared to those patients who do not3. Further, new onset POAF may be a marker for future stroke or myocardial infarction4.
Advanced Cardiac Life Support (ACLS) guidelines provide recommendations for the treatment of acute tachyarrhythmias, including AF. For post-operative cardiac patients, there have been numerous investigations into the optimal treatment of atrial fibrillation,5 but beyond ACLS recommendations, there are no evidence-based guidelines for the treatment for post-operative non-cardiac surgical patients who develop AF6,7. Because post-operative patients develop AF for a variety of reasons, management can be complex and often involves a variety of measures of evaluation and treatment8.
The objective of this study was to evaluate the practice patterns of POAF management in a surgical ICU without a protocol for management of AF with RVR and to then assess associated outcomes specifically looking at rate and rhythm control and mortality. The practice patterns of treatment for these patients and those associated with the best outcomes will help to better frame future treatment algorithms.
Methods and Material
This is a retrospective study of critically ill non-cardiac and non-thoracic post-operative, adult patients (≥ 18 years old) admitted to the surgical ICU (SICU) at Loyola University Medical Center (LUMC) between June 1, 2014 and June 1, 2015. The study protocol was approved by the Institutional Review Board of LUMC. Consent was waived because of the observational nature of the study. The primary outcome of this study was to determine the treatment strategies associated with 1) rate and rhythm control within 48 hours of POAF with RVR onset and 2) correlation of treatments with mortality. Both rate and rhythm control were measured as our patient population had no history of AF prior to surgery, developed both POAF and RVR and were not post-cardiac surgery patients. Secondary outcomes included rate and rhythm medication used at 7 days following diagnosis or at time of discharge if before 7 days, anticoagulation used at 7 days following diagnosis or discharge, in-hospital morbidity (myocardial infarction, stroke, deep venous thrombosis or pulmonary emboli), readmission to the SICU for treatment of AF, readmission to SICU for recurrent AF, 30 day readmission to SICU for recurrent AF, 30 day readmission after discharge, 30 day emergency department visit, discharge disposition (home, dead, skilled nursing facility or inpatient rehabilitation, long-term acute care (LTAC), transfer to other hospital), and scheduled follow up with either primary care or cardiology.
Development of POAF was determined by documentation of electrocardiogram (ECG) interpretation by a cardiologist. New onset POAF was defined as development of AF in any patient with no prior history of AF as documented in the patient history or review of available medical records. AF with RVR was defined as a heart rate >100 beats per minute based on the highest recorded rate during the period of AF9. Patients without RVR were excluded from the study. Patients who had recent cardiac surgery within 30 days were also excluded from the study. In summary, patients included were non-cardiac post-operative patients ≥ 18 years old with development of new-onset POAF with RVR as documented by cardiologist-interpreted ECG.
Once patients were identified, the electronic medical record was reviewed for demographics (age, sex, race, and body mass index), home medications, co-morbidities (hypertension, diabetes mellitus type II, coronary artery disease, asthma/COPD, stroke and peripheral vascular disease). Other data collected were post-operative day at which AF occurred, hemodynamics during AF, fluid balance and electrolytes, development of hypotension (systolic blood pressure < 90) within 2 hours of AF onset, requirement of vasopressors within 24 hours of AF onset, incidence of surgical complications (surgical site infection, anastomotic leak, reoperation), and laboratory values. Medication management (β-blocker, calcium channel blocker, amiodarone) was recorded as first, second, or third agent used as well as the success of these medications. Finally, we recorded ICU and hospital length of stay, discharge location, death, and medical follow up.
Statistical Analysis
All data were analyzed using SPSS (version 22.0, SPSS Inc., Chicago, IL). For continuous variables, the median [interquartile range] are reported, whereas for categorical variables, the frequency and the corresponding percentage are given. When appropriate for bivariate categorical data, statistical analysis was performed by the chi-square or Fisher’s exact test. Markov chain analysis was used to determine the treatment success of each medication over time10. Markov chain analysis can reliably compare a desired outcome (rate and rhythm control) over a desired time frame (48 hours) while accounting for those patients who have already achieved the desired outcome. In addition, by assessing the desired outcome overtime, Markov chain analysis allows for recognition that the desired outcome was already achieved with a different medication. For all analyses, a p value of < 0.05 was considered to be significant.
Results
Overall SICU patient population
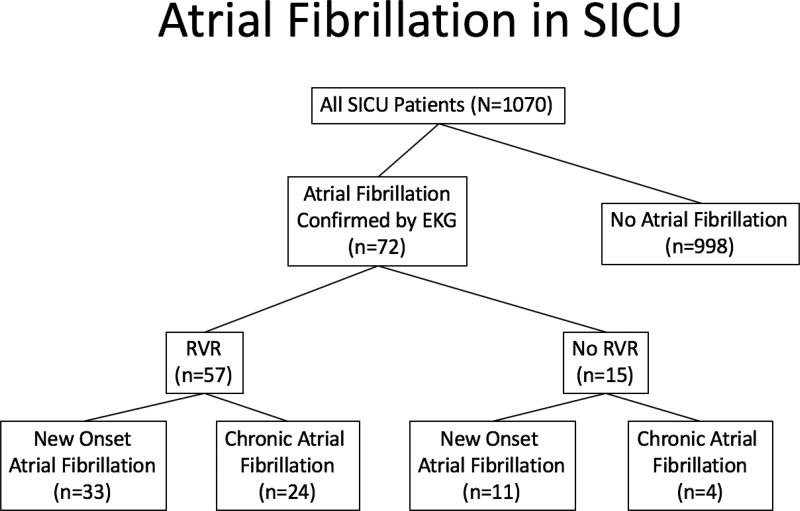
During the 13 month study period, 1070 adult patients were admitted to the SICU (Figure 1). Seventy-two patients (6.7%) developed POAF. Fifty-seven of these 72 patients had RVR (79%), while the remainder (n=14; 21%) did not. Of the patients with POAF with RVR, 24 (42%) had chronic AF while 33 (57.9%) had new onset POAF with RVR, our target population. Overall, 3.1% of patients admitted to the SICU had new onset POAF with RVR. All 33 patients underwent surgery with the most common being esophagectomy (8/33) and the remaining being mostly intraabdominal (cystectomy (1), pancreaticoduodenectomy (1), HIPEC (1), colectomy (1), small bowel resection (2), gastrojejunostomy (1), nephrectomy (1), aortic aneurysm repair (1)). Other cases included craniotomy, thyroidectomy, inguinal lymphadenectomy, wound debridement, thromboembolectomy and head and neck cancer resection. Seven (21%) patients were involved in trauma of which one had chest trauma requiring thoracotomy. Sixteen (48.5%) patients had the diagnosis of sepsis at the time of POAF onset.
Figure 1.
Patient screening for new onset POAF with RVR in the SICU from all patients admitted for 1 year. RVR= Rapid Ventricular Rate (heart rate > 100).
Patient characteristics
Of patients who developed POAF with RVR, most tended to be old (median age= 71 [64,80]), male (19; 58%) and white (27; 82%). Most suffered from hypertension (n=20, 61%). Only six (18%) had coronary artery disease. The majority of patients had at least one cardiac risk factor: hypertension, coronary artery disease, diabetes mellitus, stroke, peripheral vascular disease, or COPD/asthma (n=22, 67%). (Table 1) No patient died within the first 48 hours of POAF onset.
Table 1.
Patient Demographics
| N = 33 median [intraquartile range] or n (%) |
|
|---|---|
| Age | 71 [64, 80] |
| Sex | |
| Male | 19 (58) |
| Female | 14 (42) |
| Race | |
| White | 27 (82) |
| Black | 4 (12) |
| Hispanic | 1 (3) |
| Asian | 1 (3) |
| Body Mass Index (BMI) | 27.59 [24, 30] |
| Co-Morbidities | |
| HTN | 20 (61) |
| CAD | 6 (20) |
| Stroke | 4 (12) |
| PVD | 3 (9) |
| Asthma/COPD | 6 (20) |
| DM | 8 (24) |
Patient Trends at POAF with RVR Onset
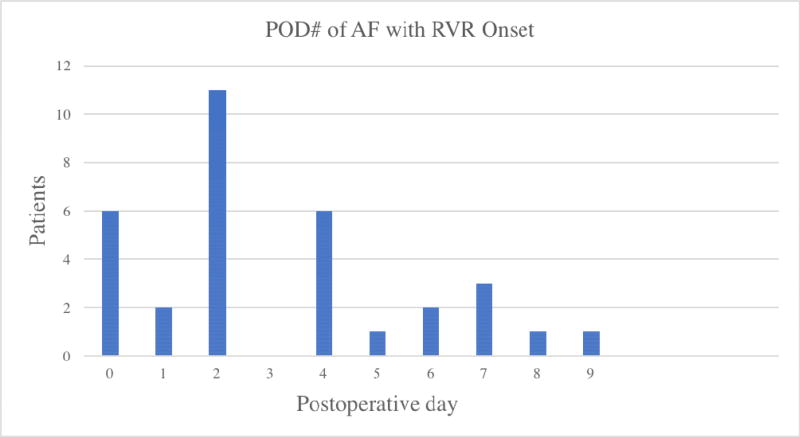
POAF most commonly occurred on postoperative day 2 (n=11, 33%) or earlier (Figure 2). Only 54% (7/13) of patients who had a β-blocker as a home medication had it started within 24 hours of AF onset (Table 2). The median fluid balance at the time of onset of AF was 4865 mL [2450, 9225]. Forty-eight percent of patients developed hypotension within 2 hours of AF onset, and 36% of patients required the use of vasopressors within 24 hours. Electrolyte abnormalities included hypokalemia (K+ < 4.0 mEq/L) in 15 (45%) patients and magnesium < 2.0 mEq/L in 13 (39%) patients.
Figure 2.
Post-operative Day of Onset of POAF with RVR
Table 2.
Patient Trends at POAF with RVR Onset
| N = 33 median [intraquartile range] or n (%) |
|
|---|---|
| POD of AF onset | 2 [2, 4.25] |
| Fluid balance at AF onset (mL) | 4865 [2450, 9225] |
| Hypotension at AF onset (SBP < 90 within 2 hours of onset) | 16 (48) |
| Vasopressor use within 24 hours of AF onset | 12 (36) |
| New intubation within 24 hours of AF onset | 5 (15) |
| Beta blocker started within first 24 hours of AF onset if home medication (n=13) | 7 (54) |
| Patients with K < 4 on first lab after AF onset | 15 (45) |
| Patients with Mg < 2 on first lab after AF onset | 13 (39) |
| Surgical site infection during hospitalization | 5 (15) |
| Anastomotic leak during hospitalization | 5 (15) |
| Return to OR during hospitalization | 16 (48) |
Diagnostic Evaluation of POAF
Within 4 hours of POAF, 27 patients had a basic metabolic panel measured (82%), 25 patients had a troponin level (76%), 16 patients had an arterial blood gas (48%) and 5 patients had a lactate level drawn (15%). Fifty-eight percent of patients had an echocardiogram obtained with 24 hours of AF onset (n=19). Fifty-five percent of patients had a cardiology consult within 24 hours of AF onset (n=18) and 58% at any time after the AF event (n=19).
Treatment for Patients with POAF with RVR
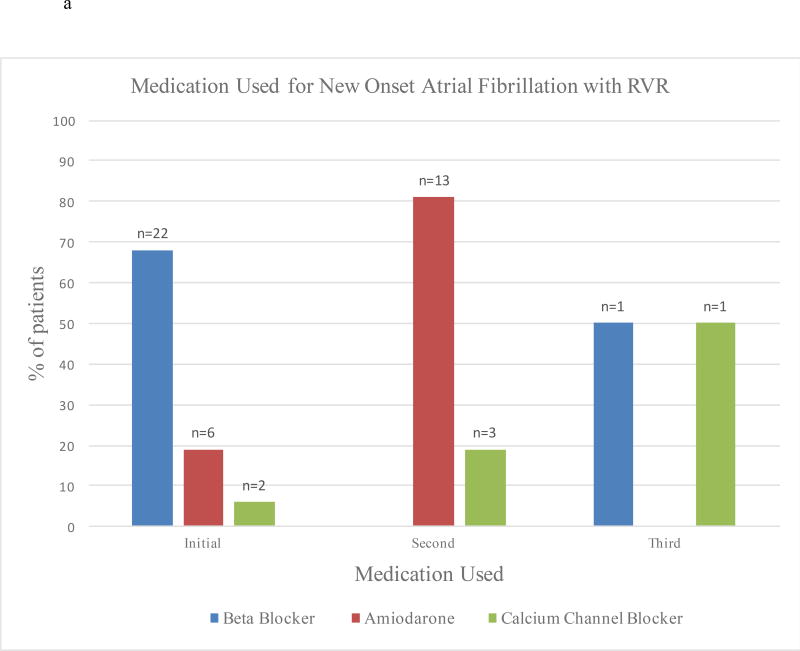
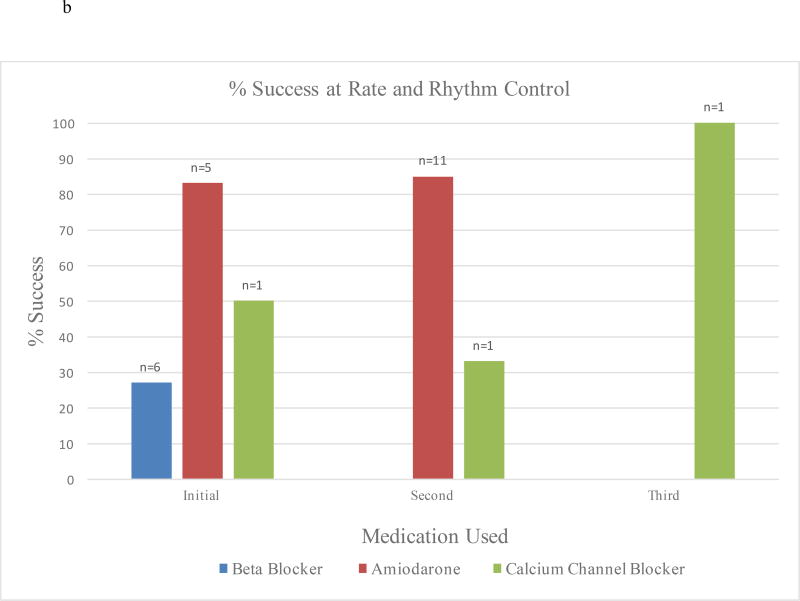
Most patients were initially treated with β-blockers (n=22, 67%) (Table 3). Only 6 (18%) patients received amiodarone, 2 (6%) received calcium channel blockers, and 3 (9%) were not medically treated as AF resolved spontaneously. Almost half (n=16, 48%) of patients received a second medication due to failure of rate or rhythm control, with amiodarone being the most common (n=13, 81%). Only two patients received a third medication (Figure 3a). Amiodarone was the most successful at achieving rate and rhythm control when used as an initial or secondary drug at 83% and 85% respectively (Figure 3b). Only 27% of patients who initially received β-blocker achieved rate control. When comparing patients who received β-blocker to those who received amiodarone as a first medication, amiodarone was significantly more likely to result in rate and rhythm control (p=0.022). When analyzed using Markov chains11, amiodarone as a first, second, or third medication was significantly more likely to result in rate and rhythm control (p=0.001) than were β-blockers (p=0.001). Cardioversion was done in 4 (12%) patients, and successful in 3 of the 4 cases.
Table 3.
Medication Comparison for Rate and Rhythm Control at 48 hours
| Rate and Rhythm Control within 48 hours of AF onset (N = 26) n (%) |
No Rate and Rhythm Control within 48 hours of AF onset (N = 7) n (%) |
|
|---|---|---|
| Initial medication used | ||
| None | 2 (8) | 0 |
| Beta blocker | 19 (73) | 4 (57) |
| Calcium channel blocker | 1 (4) | 1 (14) |
| Amiodarone | 4 (15) | 2 (29) |
| Digoxin | 0 | 0 |
| Patients requiring 2nd medication | 12 (46) | 4 (57) |
| If unsuccessful, 2nd medication used | ||
| Beta blocker | 0 | 0 |
| Calcium channel blocker | 1 (8) | 2 (50) |
| Amiodarone | 11 (92) | 2 (50) |
| Digoxin | 0 | 0 |
| Patients requiring 3rd medication | 1 (4) | 1 (14) |
| If unsuccessful, 3rd medication used | ||
| Beta blocker | 0 | 1 (100) |
| Calcium channel blocker | 1 (100) | 0 |
| Amiodarone | 0 | 0 |
| Digoxin | 0 | 0 |
| Rate of success if used first* | ||
| Beta blocker (n = 22) | 7 (37) | 0 |
| Calcium channel blocker (n = 2) | 1 (100) | 0 |
| Amiodarone (n = 6) | 4 (100) | 1 (50) 82 hrs. |
| Digoxin | - | - |
| Rate of success if used second* | ||
| Beta blocker | - | - |
| Calcium channel blocker (n = 3) | 1 (100) | 0 |
| Amiodarone (n = 13) | - | 1 (50) 62 hrs. |
| Digoxin | - | - |
| Rate of success if used third* | ||
| Beta blocker (n = 1) | - | 0 |
| Calcium channel blocker (n = 1) | 1 (100) | - |
| Amiodarone | - | - |
| Digoxin | - | - |
| Cardioversion necessary | 4 (15) | 0 |
| Cardioversion success (n = 2) | 3 (12) | - |
| Lasix given within 8 hours of AF onset | 7 (27) | 0 |
Figure 3.
a: Medications Used for Patients with POAF with RVR
b: Percent Success at Rate and Rhythm Control for Medications Used for Patients with POAF with RVR
Outcome Measures for Patients with POAF with RVR in the SICU
Median SICU length of stay was 7 days [IQR 5,18] while hospital length of stay was 13 days [8,22] (Table 4). Seventy-nine percent of patients were both rate and rhythm controlled within 48 hours of AF with RVR onset. Six (18%) patients died within 7 days of POAF onset. Of the remaining 26 patients, 17 continued to receive β-blockers, 5 amiodarone, 2 no medications, 1 calcium channel blocker and 2 a combination of medications at 7 days or at discharge if before 7 days. Therapeutic anticoagulation was used at discharge or within 7 days of onset of AF with RVR in 46% of patients. In terms of new cardiovascular morbidities, 5 patients had a deep vein thrombosis/pulmonary embolism, 2 patients had a stroke and 1 patient a myocardial infarction (MI). Seventy-five percent of patients were discharged without having an in-hospital cardiovascular morbidity (myocardial infarction, stroke, or deep vein thrombosis/ pulmonary embolism).
Table 4.
Outcome Measures for Patients with POAF with RVR in the SICU
| N = 27 median [intraquartile range] or n (%) |
|
|---|---|
| Medication used at 7 day following diagnosis or discharge if before 7 days (n=27, 6 died before day 7) | |
| None | 2 (7) |
| Beta blocker | 17 (63) |
| Calcium channel blocker | 1 (4) |
| Amiodarone | 5 (19) |
| Digoxin | 0 |
| Amiodarone and Beta blocker | 1 (4) |
| Digoxin, Beta blocker, and calcium channel blocker | 1 (4) |
| Anticoagulation used at 7 days following diagnosis or discharge if before 7 days | |
| None | 18 (54) |
| Warfarin | 4 (12) |
| Rivaroxaban | 1 (3) |
| Dabigatran | 1 (3) |
| Heparin | 9 (27) |
| Hospital length of stay | 13 [8, 22] |
| SICU length of stay | 7 [5, 18] |
| ICU Scoring System | |
| APACHE II | 14 [10, 18] |
| SOFA | 4 [3, 6] |
| SAPS II | 32 [21, 36] |
| ICU readmission after AF treatment | 3 (9) |
| Readmission to SICU for recurrent AF | 2 (6) |
| 30 day readmission (n = 26) | 8 (31) |
| 30 day ED visit (n = 18) | 1 (6) |
| Discharge Disposition | |
| Home / home health service | 15 (45) |
| Dead | 7 (21) |
| Skilled Nursing Facility/ Inpatient Rehabilitation | 6 (18) |
| Long Term Acute Care Center | 3 (9) |
| Transfer to other hospital | 2 (6) |
| Rate/Rhythm controlled at 48 hours from AF onset | 26 (79) |
| Rate controlled at discharge/death | 31 (94) |
| Rhythm controlled at discharge/death | 27 (82) |
| Primary Care physician follow-up (n = 26) | 10 (38) |
| Cardiology follow-up scheduled (n = 26) | 6 (23) |
Of the 26 (79%) patients who survived to hospital discharge, just over half (n= 15, 58%) were discharged home. Almost all patients (94%) were both rate and rhythm controlled by discharge or death. Eight patients (31%) were readmitted within 30 days. Only 38% of patients had scheduled follow up with their primary care physician and only 23% were scheduled for cardiology follow up.
Characteristics of Patients Based on Rate and Rhythm Control at 48 hours
Patients who achieved rate or rhythm control at 48 hours were less likely to have diabetes mellitus but more likely to have earlier onset POAF. (Table 5). Using Spearman correlation coefficient, time to rate or rhythm was not related to LOS (hospital LOS or SICU), charges, or severity of illness scores (p>0.05, for all).
Table 5.
Characteristics of Patients Based on Rate and Rhythm Control at 48 hours
| Rate and Rhythm control within 48 hours of AF onset (N = 26) median [intraquartile range] or n (%) |
Rate and Rhythm control within 48 hours of AF onset (N = 7) median [intraquartile range] or n (%) |
p- value | |
|---|---|---|---|
| Age | 70.5 [62.5, 79.75] | 76 [67, 81] | 0.747 |
| Sex | 0.106 | ||
| Male | 17 (65) | 2 (29) | |
| Female | 9 (35) | 5 (72) | |
| Race | 0.899 | ||
| White | 21 (81) | 6 (86) | |
| Black | 3 (12) | 1 (14) | |
| Hispanic | 1 (4) | 0 | |
| Asian | 1 (4) | 0 | |
| BMI | 25.4 [23.2, 28.7] | 31.6 [29.3, 33.4] | 0.781 |
| Co-Morbidities | |||
| HTN | 14 (58) | 6 (86) | 0.202 |
| CAD | 4 (15) | 2 (29) | 0.584 |
| Stroke | 3 (12) | 1 (14) | 0.999 |
| PVD | 3 (12) | 0 | 0.999 |
| Asthma/COPD | 4 (15) | 2 (29) | 0.584 |
| DM | 4 (15) | 4 (57) | 0.041 |
| POD of AF onset | 2 [2, 5] | 4 [0.5, 4] | 0.018 |
| Fluid balance at AF onset (mL) | 6500 [3000, 10300] | 2300 [1618, 3900] | 0.161 |
| Hypotension at AF onset (SBP < 90 within 2 hours of onset) | 14 (54) | 2 (29) | 0.398 |
| Vasopressor use within 24 hours of AF onset | 10 (38) | 2 (29) | 0.999 |
| New intubation within 24 hours of AF onset | 4 (15) | 1 (14) | 0.999 |
| Patients with K < 4 on first lab after AF onset | 12 (46) | 3 (43) | 0.999 |
| Patients with Mg < 2 | 9 (35) | 4 (57) | 0.393 |
| Surgical site infection | 3 (12) | 2 (29) | 0.282 |
| Anastomotic leak | 3 (12) | 2 (29) | 0.282 |
| Unplanned return to OR | 13 (50) | 3 (43) | 0.999 |
Discussion
This study describes the practice patterns of a SICU without protocols for the management of new onset, non-cardiac surgery POAF with RVR. As expected, evaluation and treatments varied and certain medications were associated with improved rate and rhythm control. While this is a small, single centered retrospective review, these findings may prompt future study of AF management in the critically-ill surgical patient.
New onset POAF following noncardiac surgery is a rare event but is associated with poor short-term and long-term outcomes. In this study, only 5.3% of patients admitted to the SICU developed POAF with RVR (3.1% new onset), consistent with previously reported incidences of 3.0%12 to 10.5%1,13,14(4.5% of these being new onset)9. Although a rare development in SICU patients, new onset POAF should heighten the concern for the critical care team. In this study of only SICU patients, new onset AF was associated with a mortality of 21% and 30-day readmission rate of 31%. In all surgical patients (ICU and non-ICU), POAF after non-cardiac surgery has been associated with mortality of 12%15 to 37.5%16,13,1. In addition, if the patient does survive their hospitalization, a new diagnosis of AF following surgery has been associated with a 3 fold increased risk of a cardiovascular event following gastrectomy17 and radical cystectomy4 and a 13 month decrease in long term survival following esophagectomy18. Given the high rate of cardiac co-morbidities in these patients (Table 1) and the catecholamine surges associated with surgery and sepsis, the development of POAF is probably a harbinger of impending cardiac complications.
Future studies in providing prophylaxis in high risk patients for developing POAF is needed15. It is difficult to determine who is at greatest risk for POAF. Admission NT-proBNP level elevation may predict future AF in SICU19 and cardiothoracic surgery patients20,21, and this may prove to be a useful screening lab. In cardiothoracic surgery, prophylaxis with amiodarone22 or metoprolol23 can decrease POAF rates. However, in general surgery, POAF prophylaxis may actually worsen outcome as the POISE study showed that perioperative β-blockade with metoprolol slightly decreased the risk of POAF but increased the risk of stroke and mortality23.
Given the high mortality and cardiovascular events associated with non-cardiac POAF, prediction tools and prophylaxis to prevent non-cardiac surgery POAF warrant further study. Typically, a β-blocker, calcium channel blocker or amiodarone is used to medically treat new onset POAF. A β-blocker was used most often as the first medication (Table 4) but was successful in rate and rhythm control in only 27% of cases. Conversely, amiodarone was rarely used as a first agent but had the greatest success rate (83%). Amiodarone was highly successful at rate and rhythm control when used as the second agent with an 85% success rate as well. This finding is consistent with another study that found an 87% conversion rate with amiodarone18. In a study of patients who developed new onset AF following lung resection, diltiazem and amiodarone had similar rates of sinus rhythm control at 48 hours (80%)24. In a recent summary of noncardiac, POAF treatment, β-blockers were recommended as the first line agent6. However, these authors recommend this with rate control alone as the primary goal. In this study, we used both rate and rhythm control as the primary outcome as rhythm control should lead to less need for post-operative cardiac medication and the need for anticoagulation. Our work suggests that amiodarone may be effectively used as a first line agent for new onset AF with RVR in the post-operative patient.
Due to lack of guidance for the management of these patients, conventional thinking is that rate and rhythm control should be the primary goal. However, as this study has shown, traditional strategies to achieve rate and rhythm control often fail in new onset POAF with RVR. This can be for a variety of reasons including the patient being unable to tolerate beta blockers or calcium channel blockers due to hemodynamic instability. As amiodarone had the most success at achieving rate and rhythm control when used as first or second line agent, it appears to be a reasonable initial choice in the management of new onset POAF with RVR. However, the greatest success rate (92%) was with beta-blockers first, follow by amiodarone which may suggest an additive effect of the two medications. Future studies are needed to further explore this and determine many unknowns including optimal dosing and route, need for anticoagulation, and duration of treatment.
There is no clear evidence to recommend for or against therapeutic anticoagulation in patients with new onset POAF that resolves while hospitalized. In this study, over half of the patients did not receive any anticoagulation, but 79% resolved POAF within 48 hours. In other studies, as few as 16% of POAF patients received anticoagulation for new onset AF9. The exact risk of future embolic events is unclear. Future study is needed to determine, if, for how long, and with which anticoagulant noncardiac surgery patients who develop POAF should be treated. The answer to these questions cannot be found in this work but would be of significant clinical impact for these high-risk patients.
It is well-established that age and cardiac co-morbidities place patients at higher risk for POAF1. Traditionally, atrial stretch from fluid overload or post-operative fluid mobilization was felt to lead to AF26,27. While this may be the case, we found that patients with a higher fluid balance at the time of AF onset had similar likelihood of rate and rhythm controlled by 48 hours following POAF onset (p=0.161) (Table 5). Typically, assessment and treatment of AF involves assessing intravascular volume status and diuresis as indicated or allowed. Theoretically, as the atrial stretch from hypervolemia resolves, the impetus for AF is gone27. This may be why patients with higher fluid balances responded to treatment/diuresis within 48 hours and the AF resolved. On the other hand, the patients who did not have rate or rhythm control had higher BMIs and comorbidities which may reflect worse cardiac function at baseline and therefore inherent susceptibility to the cardiac stresses of surgery. In fact, new onset POAF may unveil undiagnosed cardiac disease and the propensity for future cardiac events26.
The cause of AF may be related to catecholamines16 and inflammation1 especially because higher rates of AF are found in patients who undergo emergency surgery and those with higher SAPSII and ISS scores1,16. In fact, in a population of medical Medicare beneficiaries with sepsis, 7.3% developed AF during their hospitalization28,13. In addition, prior studies comparing ICU patients with and without AF have linked mortality in this patient population not to the arrhythmia but to increased rates of cancer, sepsis and shock1,13. In this study, 48% of patient who developed new onset AF with RVR returned to the OR at some point either for a planned second look operation or for control of sepsis. Therefore, the development of AF with RVR may be reflective of underlying sepsis, catecholamine surge or persistent and overwhelming inflammation. Developing AF with RVR in the setting of surgical sepsis should heighten the concern of an ICU practitioner as prior work has shown that the mortality for sepsis with AF is much greater than with sepsis alone13.
This study focused on patients with new onset AF with RVR as opposed to chronic AF or new onset AF without RVR. We chose this population as, anecdotally, these patients seemed to have the greatest complications, mortality and treatment variety. Our study is novel in that it examined only patients with new onset POAF with RVR. Other studies also included patients with chronic AF9,6,13. Treatments and success rates may differ in patients with chronic AF. Therefore, while the results of this study should be viewed in the context of AF with RVR rather than just AF in general, it supports the use of amiodarone as the treatment for POAF with RVR. Future study should focus on optimal treatment regimens, comparison between RVR and non-RVR, comparison between new onset and chronic AF and ensuring adequate follow up.
This study has several limitations. The study is retrospective, small and single centered, and therefore, any conclusions cannot be generalized to patient care or for care in other scenarios or centers. In one year, only 3.1% of SICU patients developed new onset AF with RVR. With this low rate, prospective studies would need to be multi-centered. Another limitation is that the definition of RVR varies in literature, which limits the direct comparison of studies. While some other studies used RVR defined as >1101, we used 100 beats per minute.
A strength of our study is that we used Markov chains, which enabled us to show that amiodarone was, despite the small study size and the use of multiple medications, associated with better rate and rhythm control.
In conclusion, the development of new onset, non-cardiac, POAF with RVR is a rare but clinically significant event in the SICU. We found that while beta-blockers were most commonly used, amiodarone was better at rate and rhythm control. We also found that many patients were discharged without anticoagulation. Prospective study is needed to determine the best treatments for POAF.
Acknowledgments
Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health and the NHLBI under award numbers 1T35HL120835. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
Study Concept and Design: Brown, Blackwell, Posluszny
Acquisition of Data: Brown, Nassoiy, Posluszny, Chaney
Analysis and interpretation of data: Brown, Nassoiy, Plackett, Chaney, Blackwell, Luchette, Engoren, Posluszny
Drafting of the manuscript: Brown, Nassoiy, Plackett, Chaney, Blackwell, Luchette, Engoren, Posluszny
Statistical Analysis: Engoren, Posluszny
Administrative, technical, and material support: Brown, Nassoiy, Engoren, Posluszny
Study Supervision: Posluszny
Presented as Oral Presentation at the Academic Surgical Congress 2016 Jacksonville, FL
Disclosure of Financial Interest and Potential Conflicts of Interest: None
References
- 1.Seguin P, Laviolle B, Maurice A, Leclercq C, Mallédant Y. Atrial fibrillation in trauma patients requiring intensive care. [Accessed Aug 9, 2017];Intensive Care Med. 2006 32(3):398–404. doi: 10.1007/s00134-005-0032-2. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 2.Makrygiannis SS, Margariti A, Rizikou D, et al. Incidence and predictors of new-onset atrial fibrillation in noncardiac intensive care unit patients. [Accessed Aug 9, 2017];J Crit Care. 2014 29(4):5. doi: 10.1016/j.jcrc.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Seguin P, Signouret T, Laviolle B, Branger B, Mallédant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. [Accessed Aug 9, 2017];Crit Care Med. 2004 32(3):722–726. doi: 10.1097/01.ccm.0000114579.56430.e0. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 4.Blackwell RH, Ellimoottil C, Bajic P, et al. Postoperative atrial fibrillation predicts long-term cardiovascular events after radical cystectomy. [Accessed Aug 9, 2017];J Urol. 2015 194(4):944–949. doi: 10.1016/j.juro.2015.03.109. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 5.Omae T, Kanmura Y. Management of postoperative atrial fibrillation. [Accessed Aug 9, 2017];J Anesth. 2012 26(3):429–437. doi: 10.1007/s00540-012-1330-9. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danelich IM, Lose JM, Wright SS, et al. Practical management of postoperative atrial fibrillation after noncardiac surgery. [Accessed Aug 9, 2017];J Am Coll Surg. 2014 219(4):831–841. doi: 10.1016/j.jamcollsurg.2014.02.038. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 7.Vallurupalli S, Shanbhag A, Mehta JL. Controversies in postoperative atrial fibrillation after noncardiothoracic surgery: Clinical and research implications. Clinical Cardiology. 2017;40(5):329–332. doi: 10.1002/clc.22652. http://onlinelibrary.wiley.com/doi/10.1002/clc.22652/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacs RJ, Flaker GC, Saxonhouse SJ, et al. Practical management of anticoagulation in patients with atrial fibrillation. [Accessed Aug 9, 2017];J Am Coll Cardiol. 2015 65(13):1340–1360. doi: 10.1016/j.jacc.2015.01.049. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 9.Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. Journal of critical care. 2012;27(3):326.e1. doi: 10.1016/j.jcrc.2011.10.011. http://www.ncbi.nlm.nih.gov/pubmed/22226423. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993 Oct-Dec;13(4):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 11.Hamra G, MacLehose R, Richardson D. Markov chain monte carlo: An introduction for epidemiologists. International journal of epidemiology. 2013;42(2):627–634. doi: 10.1093/ije/dyt043. http://www.ncbi.nlm.nih.gov/pubmed/23569196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. [Accessed Aug 9, 2017];Am Heart J. 2012 164(6):918–924. doi: 10.1016/j.ahj.2012.09.004. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. [Accessed Aug 9, 2017];Chest. 1998 114(2):462–468. doi: 10.1378/chest.114.2.462. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 14.Shaver C, Chen W, Janz D, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Critical Care Medicine. 2015;43(10):2104–2111. doi: 10.1097/CCM.0000000000001166. http://www.ncbi.nlm.nih.gov/pubmed/26154932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christians KK, Wu B, Quebbeman EJ, Brasel KJ. Postoperative atrial fibrillation in noncardiothoracic surgical patients. [Accessed Aug 9, 2017];Am J Surg. 2001 182(6):713–715. doi: 10.1016/s0002-9610(01)00799-1. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 16.Sohn GH, Shin D, Byun KM, et al. The incidence and predictors of postoperative atrial fibrillation after noncardiothoracic surgery. [Accessed Aug 9, 2017];Korean Circ J. 2009 39(3):100–104. doi: 10.4070/kcj.2009.39.3.100. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassoiy SP, Blackwell RH, Kothari AN, et al. New onset postoperative atrial fibrillation predicts long-term cardiovascular events after gastrectomy. [Accessed Aug 9, 2017];Am J Surg. 2016 211(3):559–564. doi: 10.1016/j.amjsurg.2015.10.024. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mc Cormack O, Zaborowski A, King S, et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: Incidence, management, and impact on short- and long-term outcomes. [Accessed Aug 9, 2017];Ann Surg. 2014 260(5):778. doi: 10.1097/SLA.0000000000000960. discussion 778. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 19.Chokengarmwong N, Yeh DD, Chang Y, et al. Admission N-terminal pro-brain natriuretic peptide (NT-proBNP) level predicts the development of atrial fibrillation in general surgical intensive care unit patients. [Accessed Aug 9, 2017];J Trauma Acute Care Surg. 2017 doi: 10.1097/TA.0000000000001552. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed]
- 20.Sinner MF, Stepas KA, Moser CB, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: The CHARGE-AF consortium of community-based cohort studies. [Accessed Aug 9, 2017];Europace. 2014 16(10):1426–1433. doi: 10.1093/europace/euu175. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardinale D, Colombo A, Sandri MT, et al. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. [Accessed Aug 9, 2017];Circulation. 2007 115(11):1339–1344. doi: 10.1161/CIRCULATIONAHA.106.647008. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw SM, Galbraith PD, Mitchell LB, Sauve R, Exner DV, Ghali WA. Prophylactic amiodarone for prevention of atrial fibrillation after cardiac surgery: A meta-analysis. [Accessed Aug 9, 2017];Ann Thorac Surg. 2006 82(5):1927–1937. doi: 10.1016/j.athoracsur.2006.06.032. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 23.POISE Study Group. Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. [Accessed Aug 9, 2017];Lancet. 2008 371(9627):1839–1847. doi: 10.1016/S0140-6736(08)60601-7. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 24.Bobbio A, Caporale D, Internullo E, et al. Postoperative outcome of patients undergoing lung resection presenting with new-onset atrial fibrillation managed by amiodarone or diltiazem. [Accessed Aug 9, 2017];Eur J Cardiothorac Surg. 2007 31(1):70–74. doi: 10.1016/j.ejcts.2006.10.020. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. [Accessed Aug 9, 2017];J Am Coll Cardiol. 2014 64(21):1. doi: 10.1016/j.jacc.2014.03.022. https://www-ncbi-nlm-nih-gov.archer.luhs.org/pubmed/clipboard. [DOI] [PubMed] [Google Scholar]
- 26.Solti F, Vecsey T, Kékesi V, Juhász-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. [Accessed Aug 9, 2017];Cardiovasc Res. 1989 23(10):882–886. doi: 10.1093/cvr/23.10.882. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 27.Sideris DA, Toumanidis ST, Tselepatiotis E, et al. Atrial pressure and experimental atrial fibrillation. [Accessed Aug 9, 2017];Pacing Clin Electrophysiol. 1995 18(9 Pt 1):1679–1685. doi: 10.1111/j.1540-8159.1995.tb06989.x. https://www-ncbi-nlm-nih-gov.archer.luhs.org/ [DOI] [PubMed] [Google Scholar]
- 28.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among medicare beneficiaries hospitalized with sepsis: Incidence and risk factors. [Accessed Aug 9, 2017];Am Heart J. 2013 165(6):955.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]