Abstract
AF in patients with heart failure and reduced ejection fraction (HFrEF) is common and is associated with an increased risk of stroke, heart failure hospitalisation and all-cause mortality. Rhythm control of AF in this population has been traditionally limited to the use of antiarrhythmic drugs. Clinical trials assessing superiority of pharmacological rhythm control over rate control have been largely disappointing. Catheter ablation has emerged as a viable alternative to pharmacological rhythm control in symptomatic AF and has enjoyed significant technological advancements over the past decade. Recent clinical trials have suggested that catheter ablation is superior to pharmacological interventions in patients with co-existing AF and HFrEF. In this article, we will review the therapeutic options for AF in patients with HFrEF in the context of the latest clinical trials beyond the current established guidelines.
Keywords: Catheter ablation, atrial fibrillation, heart failure, reduced ejection fraction, rhythm control
AF is the most common rhythm disorder. It is estimated AF will affect 6–12 million Americans by 2050 and 17.9 million Europeans by 2060.[1–4] AF is responsible for significant morbidity, mortality and healthcare costs.[5–7] Heart failure with reduced ejection fraction (HFrEF) is also a rising epidemic that will afflict over 8 million Americans by 2030.[8] AF is common in patients with HFrEF[9,10] and leads to increased risk of stroke, heart failure hospitalisation and death.[11–13]
Current Recommendations for Management of AF in Patients with HFrEF
The current American and European guidelines for AF management do not make specific recommendations for patients with co-existing AF and HFrEF (Figure 1). Rather, they suggest a strategy similar to that in AF patients with structurally normal hearts, combined with heart failure specific therapies.[14–16] Rate control and rhythm control are considered to be equally effective and aggressive rhythm control is recommended only in highly symptomatic patients despite rate control. For sinus rhythm maintenance, pharmacologic rhythm control is preferred over catheter ablation. Amiodarone is recommended as the only pharmacologic rhythm-control agent in the European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines, while either amiodarone or dofetilide are recommended in the American Heart Association/American College of Cardiology/Heart Rhythm Society (HRS) guidelines.[14,15] Catheter ablation is recommended as a second line therapy unless it is the patient’s initial preference. In the 2017 HRS/European Heart Rhythm Association/European Cardiac Arrhythmia Society/Asia Pacific Heart Rhythm Society/Latin American Society of Electrophysiology and Cardiac Stimulation AF ablation guidelines, use of ablation for treatment of AF ablation in patients with HFrEF was again given the same recommendation as in patients with structurally normal hearts.[16] In this updated guideline, ablation has been given class I indication in symptomatic paroxysmal AF when used as second-line therapy (failed medical management) and class IIa recommendation as first-line therapy.
Figure 1: Current Guideline Recommendation for Management of AF in Patients with HFrEF[14,15].
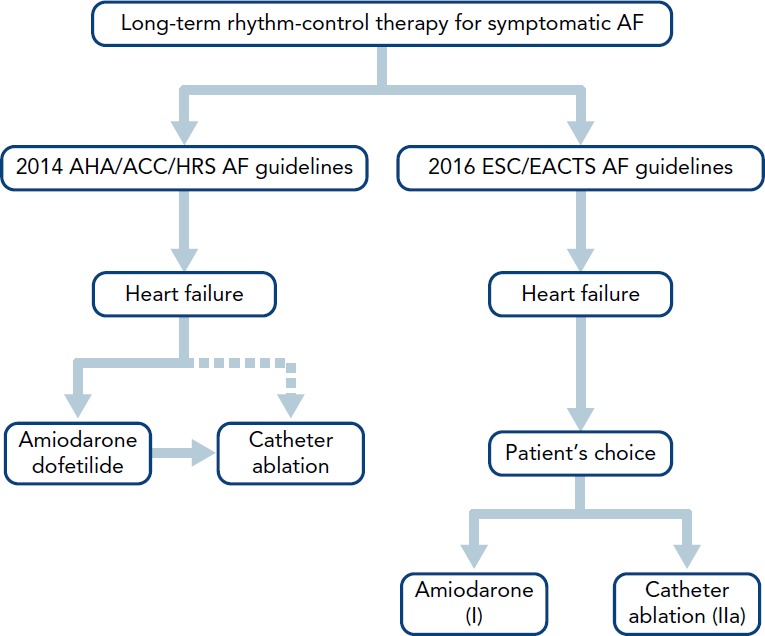
ACC = American College of Cardiology; AHA = American Heart Association;
EACTS = European Association for Cardio-Thoracic Surgery; ESC = European Society of Cardiology; HRS = Heart Rhythm Society.
Current Evidence for Rhythm Control in Patients with Co-existing AF and HFrEF
Pharmacological Rhythm Control
Two landmark studies have assessed the efficacy of pharmacological rhythm control in patients with concomitant AF and HFrEF (Table 1). In the Danish Investigators of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure (DIAMOND-CHF) trial,[17] 1,518 patients were randomised to receive either dofetilide (n=762) or placebo (n=758). At the conclusion of the trial, 65 % of patients in the dofetilide arm were in sinus rhythm versus 30 % of patients in the placebo arm. There was no difference in overall mortality between the dofetilide and placebo groups. Among the patients who were in AF at baseline, those in the dofetilide arm had lower rates of heart failure hospitalisation compared with those in placebo.
Table 1: Landmark Trials for Pharmacological Rhythm Control of Atrial Fibrillation in Patients with Heart Failure and Reduced Ejection Fraction.
| Study | Publication Year | Sample Size | Treatment Arm (n) | Comparator Arm (n) | Follow-up (months) | Primary Endpoint | Results |
|---|---|---|---|---|---|---|---|
| DIAMOND-CHF[17] | 1999 | 1,518 | Dofetilide (762) | Placebo (756) | 36 | Death from any cause | No difference in mortality |
| AF-CHF[18] | 2008 | 1,376 | Rhythm control with mainly amiodarone (682) | Rate control (694) | 60 | Death from cardiovascular causes | No difference for cardiovascular death |
In the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial,[18] 1,376 patients with HFrEF (mean left ventricular ejection fraction 27 %) were assigned to either aggressive rhythm control (n=682) or rate control (n=694). The rhythm-control strategy mainly involved cardioversions and antiarrhythmics (if needed) for sinus rhythm maintenance. Overall, 82 % of patients in the rhythm control arm were placed on amiodarone versus 7 % in the rate control arm. After a median of 47 months, 73 % of patients in the rhythm control arm were in sinus rhythm compared to 35 % in the rate control arm. There was no difference in cardiovascular mortality between the two groups.
Pharmacological rhythm control for patients with co-existing HFrEF and AF is currently limited to amiodarone and dofetilide, with dofetilide mostly unavailable outside of the US. Class I antiarrhythmics, along with sotalol and dronedarone, are contraindicated in patients with reduced ejection fraction.[19–23] Although amiodarone and dofetilide are safe to be used in HFrEF, dofetilide initiation requires initial hospitalisation. Amiodarone use is associated with a high discontinuation rate and is suggested to be associated with increased non-cardiovascular death.[24]
Catheter Ablation
The DIAMOND-CHF and AF-CHF trials raised valid scepticism on the benefit of sinus rhythm maintenance over rate control in patients with co-existing AF and HFrEF. The reason for such a lack of clinical benefit of pharmacologic rhythm control may be in part due to the significant toxicity that is associated with antiarrhythmic drugs. Catheter ablation is an alternative means for sinus rhythm maintenance while sparing the patient from potential side effects of antiarrhythmic drugs.
Several randomised controlled trials have assessed the efficacy of catheter ablation for rhythm control in patients with concomitant AF and HFrEF (Table 2).[25–31] The Pulmonary Vein Antrum Isolation Versus Atrioventricular Node Ablation with Biventricular Pacing for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure (PABA-CHF) trial compared pulmonary vein isolation (n=41) to atrioventricular (AV) node ablation plus biventricular pacing (n=40) for the composite of ejection fraction, 6-minute walk distance and Minnesota Living with Heart Failure (MLWHF) questionnaire.[25] The study showed pulmonary vein isolation to be superior to AV node ablation and biventricular pacing with respect to the primary endpoint.
Table 2: Landmark Trials for Catheter Ablation of Atrial Fibrillation in Patients with Heart Failure and Reduced Ejection Fraction.
| Study | Publication Year | Sample Size | Catheter Ablation Arm (n) | Comparator Arm (n) | Follow-up (months) | Primary Endpoint | Results |
|---|---|---|---|---|---|---|---|
| PABA-CHF[25] | 2008 | 81 | PVI (41) | AV node ablation with biventricular pacing (40) | 6 | Composite of ejection fraction, 6-minute walk distance and MLWHF score | Catheter ablation was superior to AV nodal ablation and biventricular pacing |
| MacDonald et al., 2001[31] | 2011 | 41 | PVI ± linear ablations ± CFAE ablation (22) | Rate control (19) | 6 | Cardiac MRI ejection fraction | No significant difference between groups |
| ARC-HF[26] | 2013 | 52 | PVI ± linear ablations ± CFAE ablation (26) | Rate control (26) | 12 | Peak VO2 | Improvement in peak VO2 in the catheter ablation group compared with rate control |
| CAMTAF[27] | 2014 | 50 | PVI ± linear ablations ± CFAE ablation (26) | Rate control (24) | 12 | Left ventricular ejection fraction at 6 months | Improvement in left ventricular ejection fraction at 6 months in catheter ablation group |
| AATAC[28] | 2016 | 203 | PVI ± posterior wall isolation ± CFAE ablation (102) | Amiodarone (101) | 36 | Freedom from AF | Significant improvement in freedom from AF in the catheter ablation group |
| CAMERA-MRI[29] | 2017 | 68 | PVI + posterior wall isolation (34) | Rate control (34) | 6 | Left ventricular ejection fraction | Significant improvement in ejection fraction in catheter ablation group |
| CASTLE-AF[30] | 2018 | 363 | PVI ± linear ablations ± CFAE ablation (179) | Medical rate or rhythm control (184) | 60 | Death or heart failure hospitalisation | Significant improvement in composite endpoint of death and heart failure hospitalisation in catheter ablation group |
AV = atrioventricular; CFAE = complex fractionated atrial electrograms; MLWHF = Minnesota Living with Heart Failure; PVI = pulmonary vein isolation; VO2 = maximum rate of oxygen consumption.
The Randomised Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Chronic Heart Failure (ARC-HF) trial compared catheter ablation (n=26) with rate control (n=26) in patients with HFrEF (ejection fraction <35 %) and persistent AF Patients who underwent catheter ablation had a significant improvement in the primary endpoint of peak oxygen consumption at 12 months.[26] The patients who underwent catheter ablation also had significant improvements in their B-type natriuretic peptide and MLWHF scores, as well as a trend towards improving their ejection fraction. Eighty-eight per cent of patients who underwent catheter ablation were able to maintain sinus rhythm at 1 year with a single procedure success rate of 68 %.
In the Catheter Ablation Versus Medical Treatment of Atrial Fibrillation (CAMTAF) trial, patients with persistent AF and symptomatic heart failure (ejection fraction <50 %) were randomised to either catheter ablation (n=26) or rate control (n=24).[27] The primary endpoint was improvement in ejection fraction at 6 months. Patients who underwent catheter ablation had a significant improvement in their ejection fraction as well as improvement in their peak oxygen consumption and MLWHF scores compared with patients who underwent rate control.
In the Ablation Versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRT Defibrillator (AATAC) trial, 203 patients with persistent AF and HFrEF (ejection fraction <40 %) and an ICD or CRT defibrillator were randomised to either catheter ablation (n=102) or amiodarone (n=101) for rhythm control.[28] The primary endpoint of the study was freedom from AF or atrial flutter during the follow-up period of 2 years. The patients in the catheter ablation group had significantly higher rates of freedom from AF (70 %) compared with the amiodarone (34 %) group. The secondary endpoints of the AATAC trial were unplanned hospitalisation and death; the catheter ablation group showed significant improvement in both. The number needed to treat (NNT) to avoid one unplanned hospitalisation was 3.8 patients and NNT to avoid one death was 10 patients for catheter ablation versus amiodarone. Patients who underwent catheter ablation also had significantly higher improvements in their ejection fraction, 6-minute walk distance and MLWHF scores.
Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Heart Failure: An MRI Guided Multicentre Randomised Controlled Trial (CAMERA-MRI) randomised 68 patients with persistent AF and idiopathic cardiomyopathy (ejection fraction <45 %) to receive either catheter ablation (n=34) or medical rate control (n=34).[29] All patients received a cardiac MRI prior to randomisation. Patients in the catheter ablation arm had a significantly higher improvement in their left ventricular ejection fraction at 6 months. In patients who underwent catheter ablation, absence of left ventricular late gadolinium enhancement at baseline was a predictor for greater improvement in the ejection fraction at 6 months.
Most recently, the efficacy of catheter ablation in patients with AF and HFrEF was studied in the Catheter Ablation Versus Standard Conventional Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) trial.[30] In this study, 363 patients with either paroxysmal or persistent AF, HFrEF (ejection fraction <35 %), New York Heart Association class >II and an ICD or CRT defibrillator were randomised to undergo catheter ablation (n=179) or medical therapy with either rate or rhythm control (n=184). This was the first ablation study to report hard primary endpoint of the composite of all-cause mortality or hospitalisation for worsening heart failure. The trial showed a significantly improved primary endpoint in the catheter ablation arm compared with the medical therapy arm (Figure 2). Catheter ablation resulted in an 18 % absolute risk reduction and the NNT to prevent one primary endpoint event was six patients. The catheter ablation group also showed significant improvements in endpoints of death, heart failure hospitalisation, cardiovascular death and cardiovascular hospitalisation compared with the medical therapy group. At 60 months, left ventricular ejection fraction in the catheter ablation group had increased by 9 % compared with none in the medical therapy arm.
Figure 2: Kaplan–Meier Curves Comparing Survival Free of the Primary Endpoint (Death From Any Cause) or Admission for Worsening Heart Failure) and its Two Components in the Catheter Ablation and Medical Treatment Groups in CASTLE-AF.
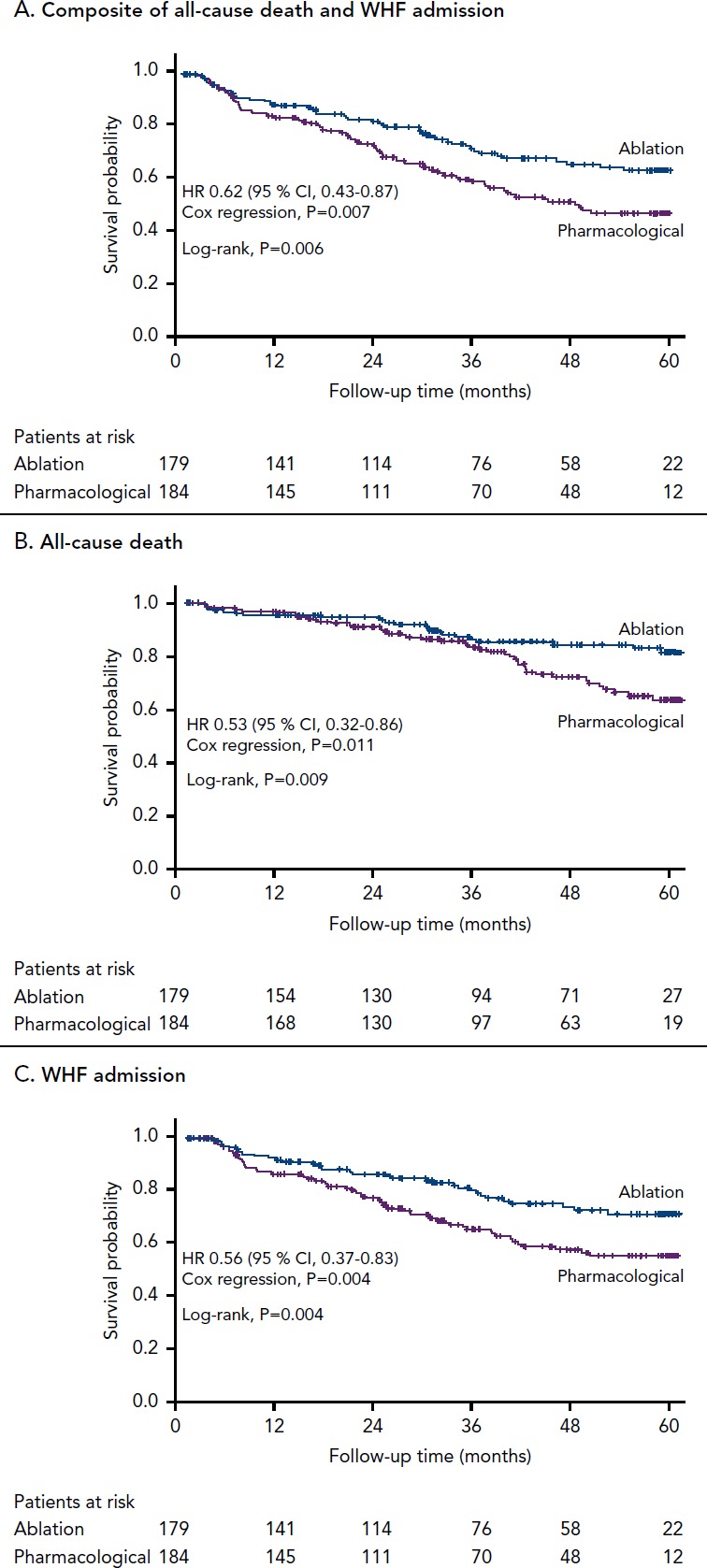
WHF = worsening heart failure. Source: From N Engl J Med, Marrouche NF, Brachmann J, Andresen D, et al., Catheter ablation for atrial fibrillation with heart failure, 378, 417-27, © 2018, Massachusetts Medical Society Reprinted with permission.[30]
CASTLE-AF is distinct from previous randomised controlled trials as it is the first trial to include hard primary endpoints of death and worsening heart failure hospitalisation. It is also the largest study for catheter ablation in AF and HFrEF population and has the longest follow-up period (60 months), which included patients with both paroxysmal and persistent AF. Moreover, the comparison arm included both rate and rhythm control as the target strategy.
Some limitations of CASTLE-AF are prolonged enrolment period, lack of blinding with respect to randomisation and treatment. In addition, all patients in the study had an ICD or a CRT defibrillator, which may have affected the overall mortality.
Should Catheter Ablation be the First Line of Therapy?
The current American and European guidelines for management of patients with AF and HFrEF relied heavily on the DIAMOND-CHF and AF-CHF trials, which found no mortality benefit in pharmacological rhythm control over rate control. At the time of these publications, randomised controlled trials to assess the efficacy of catheter ablation were small and none had assessed mortality as a primary endpoint. The CASTLE-AF trial, on the other hand, evaluated the hard endpoints of death and heart failure hospitalisations and showed catheter ablation to be superior to conventional medical treatment of either rate or rhythm control. Given this information, the CASTLE-AF trial argues the current guidelines endorse catheter ablation as first-line therapy for the treatment of AF in patients with HFrEF regardless of AF type.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–51. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh SS, Havmoeller R, Narayanan K et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colilla S, Crow A, Petkun W et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Andersson T, Magnuson A, Bryngelsson IL et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide longterm case-control study. Eur Heart J. 2013;34:1061–7. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980-1998. Am J Epidemiol. 2002;155:819–26. doi: 10.1093/aje/155.9.819. [DOI] [PubMed] [Google Scholar]
- 7.Kim MH, Johnston SS, Chu BC et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. doi: 10.1161/CIRCOUTCOMES110.958165. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics 2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 9.Santhanakrishnan R, Wang N, Larson MG et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–92. doi: 10.1161/CIRCULATIONAHA.116.022835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 11.Dries DL, Exner DV, Gersh BJ et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/S0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 13.Mamas MA, Caldwell JC, Chacko S et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–83. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 14.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j-jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–78. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:157–208. doi: 10.1093/europace/eux275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torp-Pedersen C, Möller M, Bloch-Thomsen PE et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish investigations of arrhythmia and mortality on dofetilide study group. N Engl J Med. 1999;341:857–65. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 18.Roy D, Talajic M, Nattel S et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 19.Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;3:CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 21.Flaker GC, Blackshear JL, McBride R et al. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The stroke prevention in atrial fibrillation investigators. J Am Coll Cardiol. 1992;20:527–32. doi: 10.1016/0735-1097(92)90003-6. [DOI] [PubMed] [Google Scholar]
- 22.Waldo AL, Camm AJ, deRuyter H et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD investigators survival with oral d-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/S0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 23.Kober L, Torp-Pedersen C, McMurray JJ et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg JS, Sadaniantz A, Kron J et al. Analysis of cause-specific mortality in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation. 2004;109:1973–80. doi: 10.1161/01.CIR.0000118472.77237.FA. [DOI] [PubMed] [Google Scholar]
- 25.Khan MN, Jais P, Cummings J et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 26.Jones DG, Haldar SK, Hussain W et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–903. doi: 10.1016/j-jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 27.Hunter RJ, Berriman TJ, Diab I et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–8. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 28.Di Biase L, Mohanty P, Mohanty S et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–44. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 29.Prabhu S, Taylor AJ, Costello BT et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–61. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 30.Marrouche NF, Brachmann J, Andresen D et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald MR, Connelly DT, Hawkins NM et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–7. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]


