To the Editor:
The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire that quantifies daytime sleepiness, with higher scores indicating increased daytime hypersomnolence (1). Although it is frequently used as an endpoint in intervention trials of patients with obstructive sleep apnea syndrome (2, 3), the minimum clinically important difference (MCID) of the ESS has not been established.
In a prospective service evaluation of 125 consecutive patients with obstructive sleep apnea syndrome (apnea–hypopnea index [4], or ≥4% oxygen desaturation index, >7.5 events/h, and symptoms of daytime tiredness and hypersomnolence) offered continuous positive airway pressure (CPAP), ESS was measured at baseline and follow-up (3-months post-CPAP initiation). At follow-up, patients were asked, “Compared with your last visit (before treatment), how would you describe the change in your daytime sleepiness?” Responses were recorded using a seven-point Likert global rating of change in sleepiness questionnaire (“1: Much less sleepy” to “7: Much more sleepy,” with “4: No change”) (5).
Distribution- and anchor-based methods were used to estimate the MCID of the ESS. For distribution-based methods, we calculated half the SD (0.5SD) (6) and the SE of measurement (7), using the equation: . Based on previous data, we assumed the test–retest reliability of the ESS to be 0.82 (8). For anchor-based methods, we estimated the MCID to be the mean change in ESS with CPAP for those reporting feeling “3: Little less sleepy.” Receiver operating characteristic curves were plotted to determine the ESS change cutoff that best discriminated between those who did or did not report at least a little improvement in sleepiness (global rating of change in sleepiness questionnaire responses 1–3 vs. 4–7), with equal weighting given to sensitivity and specificity (9).
Ninety-nine of 125 patients receiving CPAP returned for follow-up. Baseline characteristics were as follows: 66 men (67%); age [mean (SD)], 55 (12) years; body mass index, 33.8 (7.5) kg/m2; neck circumference, 43 (12) cm; ESS, 12.7 (5.3); apnea–hypopnea index, 28.9 (23.4); oxygen desaturation index, 28.1 (22.3); and median Mallampati score, 3 (interquartile range, 2–3).
With CPAP, mean change in ESS was −4.5 (95% confidence interval, −5.6 to −3.5), with a mean (SD) self-reported compliance of 4.5 (2.8) hours. Of the participants, 39% reported feeling “much less sleepy,” 14% “moderately less sleepy,” 13% “little less sleepy,” 31% “no change,” and 2% “little more sleepy.” No patients reported feeling “moderately more sleepy” or “much more sleepy.” There was a significant correlation between self-reported CPAP compliance and change in ESS (Spearman rank rho, −0.46; P < 0.0001).
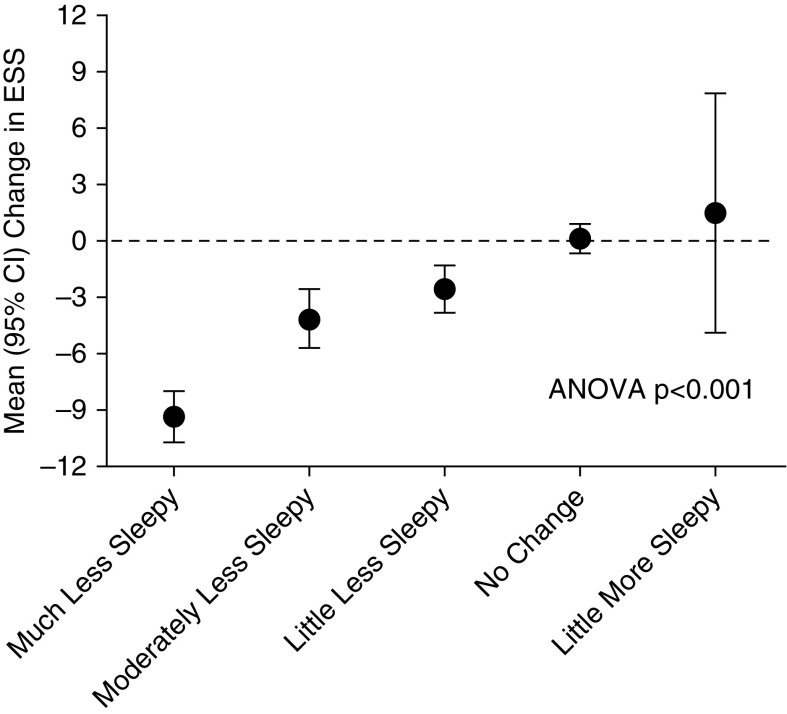
Using distribution-based methods, the MCID of the ESS was estimated as −2.65, using 0.5 × SD, and −2.21, using the SE of measurement assuming a test–retest reliability of 0.82. For the anchor-based methods, the mean (SD) change in ESS for those reporting “little less sleepy” was −2.5 (2.1) (Figure 1).
Figure 1.
Mean (95% confidence intervals [CIs]) change in Epworth Sleepiness Scale (ESS) according to response to the global rating of change in Sleepiness questionnaire. No patient reported feeling moderately or much more sleepy.
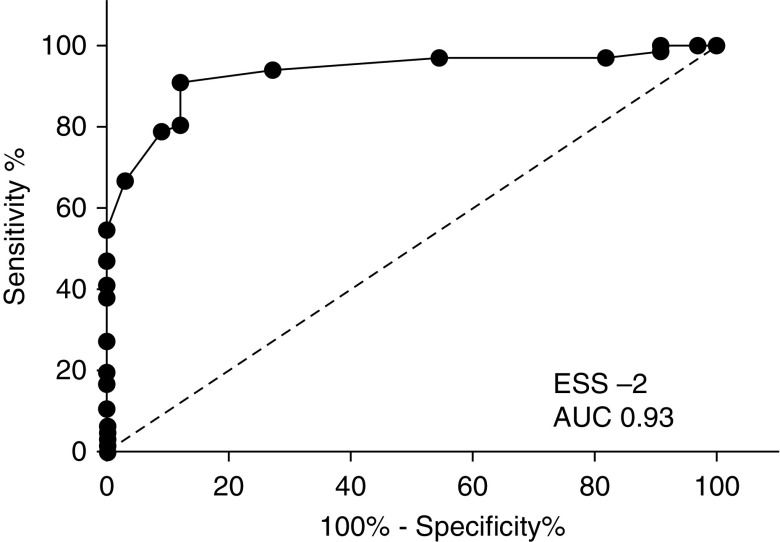
An ESS change of −2 had an area under curve of 0.93 (sensitivity, 91%; specificity, 88%) in identifying those who scored at least feeling “little less sleepy” (Figure 2). An ESS change of −3 had a sensitivity of 80% and specificity of 88%, and an ESS change of −1 had a sensitivity of 94% and specificity of 74%. Assuming the MCID lies somewhere between −2 and −3, responder analysis showed that 58–65% of our cohort noticed clinical improvements in daytime sleepiness after 3 months of CPAP treatment. This is in line with the results of recent randomized controlled trials of CPAP therapy (3).
Figure 2.
A receiver operating characteristic plot with sensitivity (y-axis) plotted against 100% − specificity% (x-axis), demonstrating the predictive value of the Epworth Sleepiness Scale (ESS) change in identifying patients who reported at least a little improvement in sleepiness. An ESS change of −2 had an area under the curve (AUC) of 0.93, sensitivity of 81%, and specificity of 88%.
The MCID represents the smallest change considered beneficial or detrimental, and it is useful in interpreting an outcome measure, as it is recognized that not all statistically significant changes are clinically important. Furthermore, the MCID is useful in determining sample size for clinical trials. The determination of the MCID remains controversial with no consensus on methodology (9). Our study used both distribution- and anchor-based methods, with consistent estimates of the MCID irrespective of methodology, providing a degree of reassurance about the validity.
Limitations of our study are that we did not use a validated subjective or objective measure of daytime sleepiness or quality of life as an anchor, as these were not used routinely in our unit’s clinical practice. However, we used a global rating of change questionnaire, which is considered an acceptable anchor for determining the MCID of questionnaires in obstructive sleep apnea syndrome and other disorders (5, 9–11). Second, few patients reported deterioration in their daytime sleepiness; hence, our data estimate the minimum clinically important improvement rather than the true MCID of the ESS. Further studies are required to corroborate our data and assess whether patients perceive size of deterioration different to size of improvement in the ESS.
In summary, using distribution- and anchor-based methods, we estimate the minimum clinically important improvement of the ESS to lie between −2 and −3.
Footnotes
This work was supported by the National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London, United Kingdom, who fund or part fund S.P., R.E.B., and A.K.S. C.M.N. is supported by an NIHR Doctoral Fellowship. W.D.‐C.M. was supported by an NIHR Clinician Scientist Award, Medical Research Council (UK) New Investigator Research Grant, NIHR Clinical Trials Fellowship, and the NIHR Collaboration for Leadership in Applied Health Research and Care Northwest London (CLAHRC) for Northwest London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Originally Published in Press as DOI: 10.1164/rccm.201704-0672LE on September 29, 2017.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 2.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 3.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. A multicentre randomised controlled trial and economic evaluation of continuous positive airway pressure for the treatment of obstructive sleep apnoea syndrome in older people: PREDICT. Health Technol Assess. 2015;19:1–188. doi: 10.3310/hta19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;282:1157–1162. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- 6.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 7.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 8.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev. 2014;18:321–331. doi: 10.1016/j.smrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 10.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Lacasse Y, Godbout C, Sériès F. Independent validation of the Sleep Apnoea Quality of Life Index. Thorax. 2002;57:483–488. doi: 10.1136/thorax.57.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]