Abstract
Objective
The objective of this literature review was to evaluate the existing evidence regarding the cost-effectiveness of treatment options in IBD.
Methods
A systematic review of the literature was conducted to identify economic evaluations of IBD therapy. The literature search was performed using electronic databases MEDLINE and EMBASE. Searches were limited to full economic evaluations published in English or French between 2004 and 2016.
Results
A total of 5,403 potentially relevant studies were identified. After screening titles and abstracts, 48 studies were included, according to the eligibility criteria. A total of 56% and 42% of the studies were assessing treatments of UC or CD, respectively. Treatment options under evaluation included biological agents, mesalamine, immunosuppressants, and surgery. The majority of studies evaluated the cost-effectiveness of biological treatments. Biological therapies were dominant in 23% of the analyses and were cost-effective according to a $CAD50,000/QALY and $CAD100,000/QALY threshold in 41% and 62% of the analyses, respectively.
Conclusion
This literature review provided a comprehensive overview of the economic evaluations for the different treatment options for IBD over the past 12 years and represents a helpful reference for future economic evaluations.
1. Introduction
Inflammatory bowel diseases (IBD) are chronic, progressive, and disabling inflammatory conditions that affect the gastrointestinal (GI) track. Although not fatal, these conditions are associated with many symptoms, which have a major impact on patients' quality of life, including abdominal pain, fever, vomiting, diarrhea, rectal bleeding, anemia, and weight loss [1–3]. Patients with IBD often experience periods of remission alternating with periods of disease activity defined as relapse episodes [4, 5].
IBD consist primarily of Crohn's disease (CD) and ulcerative colitis (UC), which are distinguished by the location and the nature of the inflammation. Specifically, CD occurs most commonly in the ileum and colon although it can affect any part of the digestive system. This condition is associated with deep and transmural mucosal inflammation and is characterized by segmental inflammation along the GI track. Patients diagnosed with CD often suffer from fistulas and perianal impairments. As opposed to CD, UC is mostly associated with continuous and diffused inflammation. Thus, the inflammation is often limited to the inner lining of the colon and rectum area; patients with UC generally present symptoms such as bloody diarrhea as well as mucus or pus in stools.
As opposed to several other chronic or inflammatory diseases, IBD affect a young population, as the first onset is generally seen in early adulthood or even in late adolescence [6]. Several risk factors have been attributed to the onset of IBD, including the environment and Western lifestyle, which is associated with smoking, a diet rich in fat and sugar, excessive consumption of drugs, and high socioeconomic status. Genetic factors play an important role in disease susceptibility, with over 200 genetic loci being associated with CD and UC. Moreover, environmental factors, such as the intestinal flora, play a central role in the initiation and maintenance of disease [7]. In addition, immune factors, appendectomy, and stress may also affect the development of IBD [8].
Worldwide, Canada is among the countries with the highest IBD prevalence and incidence rates [9, 10]. It was estimated that, in 2012, there were 129,000 people living with CD in Canada, while over 5,700 new cases were diagnosed every year. A similar epidemiology pattern is seen in UC. In Canada, it was estimated that, in 2012, there were 104,000 people living with UC, while there were 4,500 new cases diagnosed every year [11]. The total prevalence of IBD in Canada is estimated at 1 in 150 Canadians (0.67% of the population). As opposed to Canada, incidence rates for CD and UC in Europe, between 1991 and 1993, were 7.0 and 11.8 cases per 100,000-person year, respectively [9]. Similarly, in the US, the reported incidence rates, between 1996 and 2002, for CD and UC were 6.3 and 12.0, respectively [10]. In 2004, more than 1.4 million residents in the US and 2.2 million in Europe suffered from IBD [9].
The main goal of current treatments for IBD is not to cure the disease, but rather to improve patients' quality of life and to decrease morbidity by inducing and maintaining remission [12, 13]. More precisely, for CD, conventional therapies are given as first-line treatments and comprise anti-inflammatory drugs such as glucocorticoids and mesalamine (aminosalicylic acid (5-ASA)) and immunosuppressive therapies such as 6-mercaptopurine (6MP), azathioprine (AZA), cyclosporine, or methotrexate (MTX) [14]. If a patient still has symptoms after first-line treatment with conventional therapy or is unable to tolerate conventional therapy, biological treatments could be considered as subsequent-line therapies. Biological therapies include anti-TNFs, such as infliximab (IFX), adalimumab (ADA), golimumab (GOL), and ustekinumab (UTK), and anti-integrin treatments, such as vedolizumab (VED). In the context of UC, 5-ASA is the cornerstone of the treatment of mild to moderate UC [15]. For moderate to severe UC, treatment should be initiated with corticosteroids, followed by 5-ASA, immunosuppressant, or biological agents. If the patient reaches clinical response, the therapy can be maintained. Otherwise, a biological agent in combination with immunosuppressive drugs can be administered [16]. Finally, for both CD and UC, surgeries, including colectomy (removal of the large intestine and rectum) and ileostomy (connecting the small bowel to an exterior bag), represent other treatment options [17].
The economic burden of IBD is substantial considering the prevalence of the disease and the high cost of treatment options. In 2012, Rocchi et al. conducted a literature review to establish the economic and epidemiological profile of IBD in Canada [18]. The authors evaluated the total annual cost to $CAD2.8 billion in 2012, which corresponds to approximately $CAD12,000 per patient affected by UC or CD. They estimated that the direct medical costs associated with IBD exceeded $CAD1.2 billion a year and were mainly attributable to drug costs ($CAD521 million), hospitalizations ($CAD345 million), and medical visits ($CAD132 million). Indirect costs totalized $CAD1.6 billion and were attributable mainly to the long-term productivity losses ($CAD979 million).
2. Objective
As the economic burden of IBD is significant, numerous economic evaluations assessing the cost-effectiveness of treatment options in IBD have been performed during the past years. The objective of this literature review was to explore the existing evidence regarding the cost-effectiveness of these treatments.
3. Methods
3.1. Literature Search
A systematic review of the literature was conducted to identify complete economic evaluations of IBD therapy. The review question was established using the PICO method [67]: population consisted of patients with IBD; interventions and comparators were standard therapies for IBD (drugs or surgery); outcomes of interest were results of cost-utility analyses (CUA), cost-effectiveness analyses (CEA), cost-minimization analyses (CMA), cost-consequence analyses (CCA), or cost-benefit analyses (CBA). CUA were expressed in terms of cost per QALY whereas CEA were expressed in terms of cost per remission, cost per response, cost per life year gained (LYG), cost per mucosal healing (MH), or cost per days without symptoms or steroids (DWSS).
A structured literature search was performed using electronic databases MEDLINE and EMBASE, in addition to a manual search of health technology reports and NICE technology appraisals that were not published in a peer-reviewed journal. PubMed was also searched to ensure that more recent studies (June 18th 2015 to June 18th 2016) not yet indexed in MEDLINE were identified. The keywords used for search were “crohn disease”, “crohn's disease”, “ulcerative colitis”, “inflammatory bowel disease”, and “IBD”, combined with the National Health Service Economic Evaluation Database (NHS EED) filters for economic evaluations. The search was limited to studies that were published in English or French, between 2004 and 2016 (June 18th). Furthermore, a cross-reference search was performed to identify additional publications.
3.2. Study Selection
Studies were initially selected based on titles and abstracts. Full-text articles of studies deemed eligible according to the abstract were then reviewed using a predefined eligibility form. Only full economic evaluations of IBD therapy available as full-text articles were included in this review. Studies were excluded if they were not full economic evaluations such as cost of illness, costs studies, or systematic review. All eligibility criteria were defined a priori. Study selection was performed by two independent reviewers for validation purposes. Disagreement between the reviewers was discussed and resolved by consensus.
3.3. Data Extraction
For each economic evaluation selected for inclusion, the following characteristics and parameters were extracted using a self-developed data extraction form: first author, journal, year of publication, title, type of funding, country, type of evaluation, time horizon, perspective, population, treatments of interest, comparators, type of model, cost description, outcome measures, utility values (including health states, source of utility values, methodology, and number of patients) and study results. Two reviewers independently extracted data to ensure appropriate validation.
3.4. Data Analysis
In order to provide a comprehensive overview of the economic evaluations of treatments for IBD, study characteristics were first summarized using descriptive statistics. In addition, characteristics, parameters, and results of economic evaluations for both UC and CD and for the different treatment options were assessed and compared. For comparison purposes, all the costs in this study have been transposed to 2016 Canadian dollars ($CAD) using the health and personal care component of the Canadian Consumer Price Index [68].
4. Results
4.1. Literature Search
A flowchart describing the selection of studies included in this systematic review is presented in Figure 1. A total of 5,403 potentially relevant studies were identified by the literature search. After the screening of titles and abstracts, 78 full-text articles were assessed according to the eligibility criteria. Of these studies, 48 articles meeting inclusion criteria were included.
Figure 1.
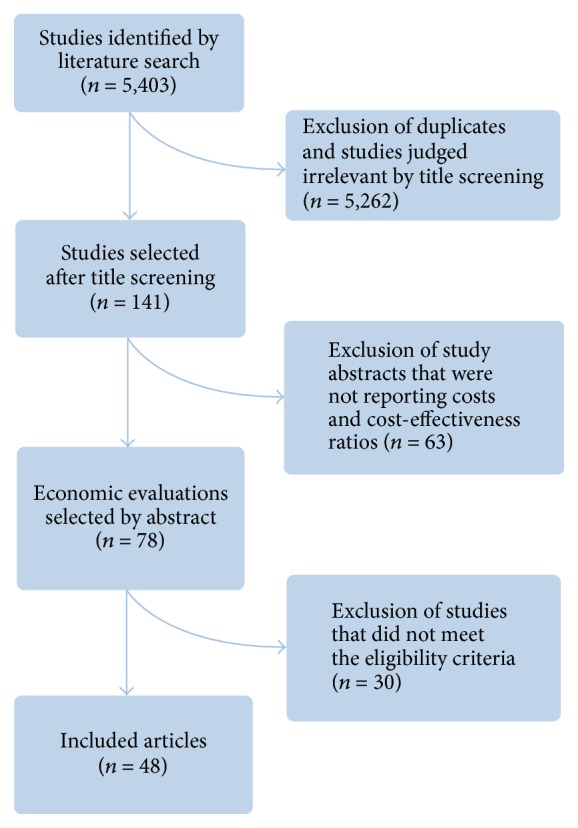
Study flowchart.
4.2. Overview of Included Studies
More than half of the included studies (56%) were assessing treatments of UC, 42% were evaluating treatments of CD, and only 1 study analyzed treatment for both CD and UC patients (Table 1). Different treatment options were evaluated such as IFX, ADA, other biological treatments (golimumab, natalizumab, or VED), 5-ASA, immunosuppressants (AZA, 6MP, MTX, or cyclosporine), surgery (colectomy or Ileal Pouch-Anal Anastomosis (IPAA)), and granulocyte-monocyte aphaeresis (GMA). Overall, most studies were CUA that assessed the cost-effectiveness of a new biological therapy from a healthcare system perspective over a time horizon of 1 year or less. Moreover, nearly half of the studies used standard of care as a comparator. Furthermore, Canadian, American, and European studies accounted for 13%, 29%, and 52% of all the studies, respectively.
Table 1.
Study Characteristics.
| Number of studies n (%) | |
|---|---|
| Type of analysis | n (%) |
| CUA | 40 (83) |
| CEA | 5 (10) |
| CMA | 3 (6) |
| Study population | |
| UC | 27 (56) |
| CD | 20 (42) |
| Both CD and UC | 1 (2) |
| Type of treatments under investigation | |
| Biologic treatments | 33 (69) |
| 5-ASA | 11 (23) |
| Immunosuppressant | 5 (10) |
| Surgery | 2 (4) |
| GMA | 1 (2) |
| Study perspective | |
| Healthcare system perspective | 47 (98) |
| Societal | 1 (2) |
| Time horizon | |
| ≤1 year | 24 (50) |
| 2–5 years | 11 (23) |
| 6–10 years | 4 (8) |
| 30 years | 2 (4) |
| Lifetime | 4 (8) |
| Not reported | 3 (6) |
| Model structure | |
| Markov model | 24 (50) |
| Decision tree | 16 (33) |
| Markov and decision tree | 2 (4) |
| No model | 4 (8) |
| Not reported | 2 (4) |
CUA: cost-utility analysis; CEA: cost-effectiveness analyses; CMA: cost-minimisation analysis; GMA: granulocyte-monocyte aphaeresis; UC: ulcerative colitis; CD: Crohn's disease; 5-ASA: 5-aminosalicylic acid.
4.3. Modelling Approach and Health State Definition
Half of the included studies used Markov modelling while one-third used decisions tree models. As for the remaining studies, some had no specific models employed [29, 30, 34, 65] or reported [33, 45]. The economic evaluations (EE) using a nonmodelling approach derived mainly from prospective data analysis or randomized clinical trials, whereas the studies not reporting their modelling approach were EE reports from the Canadian Agency of Drug Technology in Health (CADTH). Various criteria and scales, often based on previous clinical trials' remission and response definitions, defined models' health states. Among the studies assessing treatments for CD, a high proportion of the studies defined their health states according to Crohn's Disease Activity Index (CDAI), while some studies used the Harvey-Bradshaw Index (HBI). In economic evaluations assessing treatments for UC, most studies defined their health states according to the Mayo score, while others based their health states on symptom recurrence, on the Ulcerative Colitis Disease Activity Index (UCDAI) definition, on the Simple Clinical Colitis Activity Index (SCAI), or on the Physician Global Assessment (PGA).
4.4. Cost Parameters
Several cost parameters were taken into account in the included studies. More specifically, all studies reported drug costs, including curative and supportive treatment costs. Moreover, costs associated with hospitalization and outpatient visits were comprised in 73% and 69% of the studies, respectively, while costs associated with surgical procedures were reported in 67% of the studies. Imaging, lab tests (tuberculin skin, hepatitis B blood tests, and biochemistry testing), and endoscopy costs were included in 42%, 38%, and 17% of the study, respectively. Among studies assessing the cost-effectiveness of a biological treatment, 70% included infusion costs.
4.5. Outcomes
As most included studies were CUA, results were most frequently expressed in terms of cost per QALY. As for the CEAs, results were reported in terms of cost per response or remission, cost per DWSS, cost per LYG, and cost per MH.
4.6. Cost-Effectiveness Results
For comparison purposes, only CUA results for CD and UC were taken into account, which are presented in Tables 2 and 3, respectively.
Table 2.
Summary of economic evaluations in the treatment of CD.
| Study, year of publication | Study treatment | Comparators | CE ratio (Cost/QALY) (Currency year) |
CE ratio ($/QALY) (CAD 2016) |
|---|---|---|---|---|
| IFX | ||||
|
| ||||
| Ananthakrishnan et al., 2011 [19] | IFX (tailored) | Antibiotics | Dominated | Dominated |
| IFX (upfront) | Antibiotics | $2,757,857/QALY (USD 2010) |
$3,121,546/QALY | |
|
| ||||
| Ananthakrishnan et al., 2013 [20] | IFX (MTN or dose escalation) | IFX (dose escalation) | $49,278/QALY (USD 2010) |
$55,776/QALY |
|
| ||||
| Blackhouse et al., 2012 [21] | IFX | Std care |
$222,955/QALY (CAD 2011)∗ |
$229,197/QALY |
|
| ||||
| Bodger et al., 2009 [22] | IFX (1 year tx) | Std care | £19,050/QALY (GBP 2006) |
$49,290/QALY |
| IFX (2 year tx) | Std care | £21,300/QALY (GBP 2006) |
$55,112/QALY | |
|
| ||||
| Doherty et al., 2012 [23] | IFX | AZA/6MP | $1,831,912/QALY (USD 2010) |
$2,073,493/QALY |
|
| ||||
| Dretzke et al., 2011 [24] | IFX IND (severe disease) | Std care | Dominant | Dominant |
| IFX MTN (severe disease) | Std care | £68,315/QALY (GBP 2011)∗ |
$157,706/QALY | |
| IFX MTN (severe disease) | IFX IND (severe disease) | £5,030,000/QALY (GBP 2011)∗ |
$11,611,849/QALY | |
| IFX IND (moderate disease) | Std care | £94,321/QALY (GBP 2011)∗ |
$217,741/QALY | |
| IFX MTN (moderate disease) | Std care | £317,991/QALY (GBP 2011)∗ |
$734,088/QALY | |
| IFX MTN (moderate disease) | IFX IND (moderate disease) | £13,900,000/QALY (GBP 2011)∗ |
$32,088,410/QALY | |
|
| ||||
| Jaisson-Hot et al., 2004 [25] | IFX (retreatment with relapse or no response) | Std care (including surgery) | €63,700.82/QALY (EUR 2004) |
$122,252/QALY |
| IFX MTN | Std care (including surgery) | €784,057.49/QALY (EUR 2004) |
$1,504,736/QALY | |
|
| ||||
| Kaplan et al., 2007 [26] | IFX (increasing dose) | ADA | $332,032/QALY (USD 2006) |
$426,928/QALY |
|
| ||||
| Lindsay et al., 2008 [27] | IFX (luminal CD) | Std care | £26,128/QALY (GBP 2006) |
$60,316/QALY |
| IFX (fistulizing CD) | Std care | £29,752/QALY (GBP 2006) |
$68,683/QALY | |
|
| ||||
| Punekar et al., 2010 [28] | IFX | Std care | £14,607/QALY (GBP 2006) |
$37,794/QALY |
|
| ||||
| Steenholdt et al., 2014 [29] | Individualised therapy (serum IFX and IFX antibody levels using the proposed algorithm) | IFX dose intensification | Dominant | Dominant |
|
| ||||
| Steenholdt et al., 2015 [30] | Individualised therapy (serum IFX and IFX antibody levels using the proposed algorithm) | IFX dose intensification | Dominant | Dominant |
|
| ||||
| Tang et al., 2012 [31] | IFX | ADA | Dominant | Dominant |
| IFX | Certolizumab Pegol | Dominant | Dominant | |
| IFX | NAT | Dominant | Dominant | |
|
| ||||
| Velayos et al., 2013 [32] | Testing-based strategy (IFX) | Dose escalation | Dominant | Dominant |
|
| ||||
| ADA | ||||
|
| ||||
| Blackhouse et al., 2012 [21] | ADA | Std care |
$193,305/QALY (CAD2011)∗ |
$198,717/QALY |
| ADA | IFX |
$451,165/QALY (CAD 2011)∗ |
$463,797/QALY | |
|
| ||||
| Bodger et al., 2009 [22] | ADA (1 year tx) | Std care | £7,190/QALY (GBP 2006) |
$18,603/QALY |
| ADA (2 year tx) | Std care | £10,310/QALY (GBP 2006) |
$26,676/QALY | |
|
| ||||
| CADTH, 2008 [33] | ADA | Std care |
$113,034/QALY (CAD 2008)∗ |
$128,067/QALY |
| ADA | IFX | Dominant | Dominant | |
|
| ||||
| Dretzke et al., 2011 [24] | ADA IND (severe disease) | Std care | Dominant | Dominant |
| ADA MTN (severe disease) | Std care | £7,749/QALY (GBP 2011)∗ |
$17,888/QALY | |
| ADA MTN (severe disease) | ADA MTN | £4,980,000/QALY (GBP 2011)∗ |
$11,496,423/QALY | |
| ADA IND (moderate disease) | Std care | Dominant | Dominant | |
| ADA MTN (moderate disease) | Std care | £160,079/QALY (GBP 2011)∗ |
$369,545/QALY | |
| ADA MTN (moderate disease) | ADA IND (moderate disease) | £13,900,000/QALY (GBP 2011)∗ |
$32,088,410/QALY | |
|
| ||||
| Loftus Jr et al., 2009 [34] | ADA (severe disease) | Std care | £16,064/QALY (GBP 2006) |
$38,195/QALY |
| ADA (moderate-to-severe disease) | Std care | £33,731/QALY (GBP 2006) |
$80,202/QALY | |
|
| ||||
| Yu et al., 2009 [35] | ADA MTN | IFX MTN | Dominant | Dominant |
|
| ||||
| IFX + AZA | ||||
|
| ||||
| Marchetti et al., 2013 [36] | IFX + AZA (top-down strategy) | Steroid (step-up strategy) | Dominant | Dominant |
|
| ||||
| Saito et al., 2013 [37] | IFX + AZA | IFX | £24,917/QALY (GBP 2004) |
$57,051/QALY |
|
| ||||
|
Other biologics (GOL, NAT, VED) |
||||
|
| ||||
| Ananthakrishnan et al., 2012 [38] | NAT | Certolizumab Pegol | $381,678/QALY (USD 2010) |
$432,011/QALY |
|
| ||||
|
Immunosuppressants (AZA, 6MP, cyclosporine) |
||||
|
| ||||
| Ananthakrishnan et al., 2011 [19] | AZA | Antibiotics | Dominated | Dominated |
|
| ||||
| Doherty et al., 2012 [23] | AZA/6MP | No therapy | $299,188/QALY (USD 2010) |
$338,643/QALY |
|
| ||||
| Priest et al., 2006 [39] | AZA | MTX | Dominant | Dominant |
| AZA | No immunosuppressant therapy | Dominant | Dominant | |
ADA: adalimumab; AZA: azathioprine; CAD: Canadian dollar; CADTH: Canadian Agency for Drugs and Technologies in Health; CE: cost-effectiveness; EUR: Euros; GBP: Great British Pound; GMA: granulocyte-monocyte aphaeresis; GOL: golimumab; IFX: infliximab; IND: induction; MTN: maintenance; NAT: natalizumab; QALY: quality adjusted life years; Std: standard; tx: treatment; US: United States; USD: United States Dollar; VED: vedolizumab; 6MP: 6-mercaptopurine. Studies in italic are Canadian studies. A study may appear in more than one table if different treatments were analyzed. ∗Publishing year.
Table 3.
Summary of economic evaluations in the treatment of UC.
| Study, year of publication | Study treatment | Comparators | CE ratio (Cost/QALY) (Currency year) |
CE ratio ($/QALY) (CAD 2016) |
|---|---|---|---|---|
| IFX | ||||
|
| ||||
| Archer et al., 2016 [40] | IFX | Surgery | Dominated | Dominated |
| IFX | ADA | Dominated | Dominated | |
|
| ||||
| Chaudhary and Fan, 2013 [41] | IFX | Cyclosporine | €24,277/QALY (EUR 2010) |
$34,241/QALY |
| IFX | Surgery | €14,639/QALY (EUR 2010) |
$20,647/QALY | |
|
| ||||
| Hyde et al., 2009 [42] | Strategy A (IFX responders who achieved and maintained remission and mild health states) | Std care | £33,866/QALY (GBP 2009)∗ |
$78,180/QALY |
| Strategy B (IFX responders who achieved and maintained remission) | Std care | £25,044/QALY (GBP 2009)∗ |
$57,814/QALY | |
|
| ||||
| Punekar and Hawkins, 2010 [43] | IFX | Cyclosporine | £19,545/QALY (GBP 2006) |
$50,571/QALY |
| IFX | Std care | £18,388/QALY (GBP 2006) |
$47,578/QALY | |
|
| ||||
| Stawowczyk et al., 2016 [44] | IFX + std care | Std care | $106,743/QALY (USD 2015) |
$135,934/QALY |
|
| ||||
| Thorlund et al., 2014 [45] | IFX | Std care |
$65,982/QALY (CAD 2013) |
$68,093/QALY |
| IFX | ADA | Dominant | Dominant | |
|
| ||||
| Toor et al., 2015 [46] | IFX | Std care |
$1,975/Remission (CAD 2015)∗ |
$2,038/Remission |
| IFX | Std care |
$1,311/Response (CAD 2015)∗ |
$1,352/Response | |
| IFX | GOL (100 mg) |
$14,659/Remission (CAD 2015)∗ |
$15,128/Remission | |
| IFX | GOL |
$4,753/Response (CAD 2015)∗ |
$4,905/Response | |
|
| ||||
| Tsai et al., 2008 [47] | IFX MTN (responder strategy) | Std care | £27,424/QALY (GBP 2007) |
$70,958/QALY |
| IFX MTN (remission strategy) | Std care | £19,696/QALY (GBP 2007) |
$50,962/QALY | |
|
| ||||
| Ung et al., 2014 [48] | IFX | Std care |
$79,000/QALY (USD 2013) |
$85,596/QALY |
|
| ||||
| Williams et al., 2016 [49] | IFX | Cyclosporin | Dominated | Dominated |
|
| ||||
| Yokomizo et al., 2016 [50] | IFX (5 mg/kg) | IFX (10 mg/kg) | $1,243,310/MH (USD 2014) |
$1,366,933/MH |
| IFX (5 mg/kg) | ADA | Dominated | Dominated | |
| IFX (5 mg/kg) | VED | Dominated | Dominated | |
|
| ||||
| ADA | ||||
|
| ||||
| Archer et al., 2016 [40] | ADA | Surgery | Dominated | Dominated |
| ADA | Std care | £50,278/QALY (GBP2015)∗ |
$83,804/QALY | |
|
| ||||
| Thorlund et al., 2014 [45] | ADA | Std care |
$68,722/QALY (CAD 2013) |
$70,921/QALY |
|
| ||||
| Toor et al., 2015 [46] | ADA | Std care |
$7,430/Remission (CAD 2015)∗ |
$7,667/Remission |
| ADA | Std care |
$2,361/Response (CAD 2015)∗ |
$2,436/Response | |
| ADA | GOL (100 mg) | Dominated | Dominated | |
| ADA | GOL (100 mg) | Dominated | Dominated | |
|
| ||||
| IFX and ADA | ||||
|
| ||||
| Xie et al., 2009 [51] | IFX (5 mg/kg) then ADA (5 mg/kg) | Std care |
$358,088/QALY (CAD 2008) |
$402,490/QALY |
| IFX (5 mg/kg) then ADA (10 mg/kg) | Std care |
$575,540/QALY (CAD 2008) |
$646,907/QALY | |
|
| ||||
|
Other biologics (GOL, NAT, VED) |
||||
|
| ||||
| Archer et al., 2016 [40] | GOL | Surgery | Dominated | Dominated |
|
| ||||
| Essat et al., 2016 [52] | VED | Std care | £33,297/QALY (GBP 2016)∗ |
$54,283/QALY |
| VED | Surgery | Dominant | Dominant | |
| VED (Anti-TNF naïve pt) | IFX (Anti-TNF naïve pt) | Dominant | Dominant | |
| VED (Anti-TNF naïve pt) | GOL (Anti-TNF naïve pt) | Dominant | Dominant | |
| VED (Anti-TNF naïve pt) | ADA (Anti-TNF naïve pt) | £6,634/QALY (GBP 2016)∗ |
$10,815/QALY | |
| VED (Anti-TNF naïve pt) | Std care (Anti-TNF naïve pt) | £4,862/QALY (GBP 2016)∗ |
$7,926/QALY | |
| VED (Anti-TNF naïve pt) | Surgery (Anti-TNF naïve pt) | Dominant | Dominant | |
| VED (Anti-TNF failure) | Std care (Anti-TNF failure) | £64,999/QALY (GBP 2016)∗ |
$105,966/QALY | |
| VED (Anti-TNF failure) | Surgery (Anti-TNF failure) | Dominant | Dominant | |
|
| ||||
| Thorlund et al., 2014 [45] | GOL (50 mg) | Std care |
$41,591/QALY (CAD 2013) |
$42,921/QALY |
| GOL (100 mg) | Std care |
$42,271/QALY (CAD 2013) |
$43,623/QALY | |
| GOL (50 mg) | IFX | Dominant | Dominant | |
| GOL (100 mg) | IFX | Dominant | Dominant | |
| GOL (50 mg) | ADA | Dominant | Dominant | |
| GOL (100 mg) | ADA | Dominant | Dominant | |
|
| ||||
| Toor et al., 2015 [46] | GOL (100 mg) | Std care |
$935/Remission (CAD 2015)∗ |
$964/Remission |
| GOL (100 mg) | Std care |
$701/Response (CAD 2015)∗ |
$723/Response | |
| GOL (50 mg) | Std care |
$1,048/Remission (CAD 2015)∗ |
$1,081/Remission | |
| GOL (50 mg) | GOL (100 mg) |
$207/Remission (CAD 2015)∗ |
$213/Remission | |
| GOL (50 mg) | Std care |
$770/Response (CAD 2015)∗ |
$794/Response | |
| GOL (50 mg) | GOL (100 mg) |
$224/Response (CAD 2015)∗ |
$231/Response | |
|
| ||||
| 5-ASA | ||||
|
| ||||
| Brereton et al., 2010 [53] | 5-ASA (Mezavant XL, MMX) | 5-ASA (Asacol) | £749/QALY (GBP 2010) |
$2,058/QALY |
|
| ||||
| Buckland and Bodger, 2008 [54] | 5-ASA (High dose, Asacol) | 5-ASA (Std dose, Asacol) | Dominant | Dominant |
|
| ||||
| Connolly et al., 2009 [55] | 5-ASA (Oral + topical) | 5-ASA (Oral) | Dominant | Dominant |
|
| ||||
| Connolly et al., 2009 [56] | 5-ASA (2 g once daily) | 5-ASA (1 g twice daily) | Dominant | Dominant |
|
| ||||
| Connolly et al., 2012 [57] | 5-ASA (Oral + topical) | 5-ASA (Oral) | Dominant | Dominant |
| 5-ASA (2 g once daily) | 5-ASA (1 g twice daily) | Dominant | Dominant | |
|
| ||||
| Connolly et al., 2014 [58] | 5-ASA (2 g once daily) | 5-ASA (1 g twice daily + enema) | Dominant | Dominant |
|
| ||||
| Mackowiak, 2006 [59] | Oral balsalazide capsules | Oral 5-ASA specific formulation | Dominant | Dominant |
|
| ||||
| Nishikawa et al., 2013 [60] | 5-ASA (once daily) | 5-ASA (twice daily) | $86,200/LYG (RD 2011) |
$55,649/LYG |
|
| ||||
| Prenzler et al., 2011 [61] | 5-ASA (Mezavant XL, MMX) | 5-ASA (Asacol) | Dominant | Dominant |
|
| ||||
| Saini et al., 2012 [62] | SYMPT (5-ASA treatment for symptomatic disease flares only) | INFLAM (5-ASA therapy for only patients with a stool sample positive for an inflammatory marker) | $575,894/QALY (USD 2009) |
$715,331/QALY |
| SYMPT (5-ASA treatment for symptomatic disease flares only) | CONT (continuous 5-ASA maintenance) | Dominant | Dominant | |
|
| ||||
| Yen et al., 2008 [63] | MTN 5-ASA (2.4 g/day escalated and maintained at 4.8 g/day after the first flare) | No MTN 5-ASA (5-ASA 4.8 g/day given for flares) | $224,000/QALY (USD 2004) |
$353,545/QALY |
|
| ||||
|
Immunosuppressants (AZA, 6MP, cyclosporine) |
||||
|
| ||||
| Priest et al., 2006 [39] | AZA | MTX | Dominant | Dominant |
| AZA | No immunosuppressant therapy | Dominant | Dominant | |
|
| ||||
| Punekar and Hawkins, 2010 [43] | Cyclosporine | Standard care | Dominant | Dominant |
| Cyclosporine | Surgery | £9,032/QALY (GBP 2006) |
$23,370/QALY | |
|
| ||||
| Surgery | ||||
|
| ||||
| Archer et al., 2015 [40] | Surgery (colectomy) | Std care | Dominant | Dominant |
|
| ||||
| Park et al., 2012 [64] | Surgery (early colectomy + IPAA) | Std care | $1,476,783/QALY (USD 2009) |
$1,834,347/QALY |
|
| ||||
| Swenson et al., 2005 [65] | Two-Stage IPAA | Three-Stage IPAA | Dominant | Dominant |
|
| ||||
| GMA | ||||
|
| ||||
| Panes et al., 2007 [66] | GMA | Std care | €23,898/Remission (EUR 2004) |
$45,864/Remission |
ADA: adalimumab; AZA: azathioprine; CAD: Canadian dollar; CE: cost-effectiveness; EUR: Euros; g: gram; GBP: Great British Pound; GMA: granulocyte-monocyte aphaeresis; GOL: golimumab; IFX: infliximab; IPAA: Ileal Pouch-Anal Anastomosis; kg: kilogram; mg: milligram; LYG: life year gained; MH: mucosal healing; MTN: maintenance; MTX: methotrexate; NAT: natalizumab; pt: patient; QALY: quality adjusted life years; RD: Real Dollar; Std: standard; US: United States; USD: United States Dollar; VED: vedolizumab; 5-ASA: 5-aminosalicylic acid; 6MP: 6-mercaptopurine. Studies in italic are Canadian studies. A study may appear in more than one table if different treatments were analyzed. ∗Publishing year.
4.6.1. Cost-Effectiveness of Biological Therapies
The majority of studies evaluated the cost-effectiveness of biological treatments. More specifically, regardless of the IBD type and the comparative treatment, biological therapies were dominant in 23% of the analyses and were cost-effective according to a $CAD50,000/QALY and $CAD100,000/QALY threshold in 41% and 62% of the analyses, respectively. Biological treatments tended to be more cost-effective when compared with surgery (dominant in 43% and cost-effective according to a $CAD50,000/QALY ratio in 57% of the analyses) and when compared with other biological treatments (dominant in 48% and cost-effective according to a $CAD50,000/QALY ratio in 52% of the analyses), rather than with standard of care (dominant in only 8% of the analyses and cost-effective in 33% of the analyses according to a $CAD50,000/QALY threshold).
In CD, the incremental cost-effectiveness ratio (ICER) of biological treatments ranged from dominant to $CAD32,088,410/QALY when IFX or ADA maintenance treatment was compared to IFX or ADA induction treatment. Moreover, ADA tended to lead to more favourable ICERs than IFX when compared to standard of care, while IFX led to more favourable ICERs than ADA when compared to other biological treatments. Notably, moderate CD treatment regimens encountered greater cost-effectiveness ratios (CERs) compared to severe CD.
In the context of UC, dominance was mostly reported in studies where biological treatments were compared with other biological treatments or surgery. Moreover, all analyses were under a $CAD100,000/QALY threshold when IFX or ADA alone was compared with standard of care.
As for the Canadian setting, all studies in CD comparing biological treatments to standard (STD) of care resulted in an ICER above the $CAD100,000/QALY threshold. The opposite is seen in UC, where all studies resulted in an ICER under the $CAD100,000/QALY threshold. Furthermore, all ICERs resulting from the comparison of GOL to ADA were dominant.
4.6.2. Cost-Effectiveness of Immunosuppressants
Most included studies assessing the cost-effectiveness of an immunosuppressant demonstrated that these treatments are cost-effective. More specifically, in the context of CD, AZA and cyclosporine were dominant alternatives when compared to therapy excluding immunosuppressants, when compared with MTX and when compared to standard of care. Moreover, cyclosporine was cost-effective according to a $CAD50,000/QALY threshold when compared with surgery.
In UC, only 1 economic evaluation was retrieved and indicated that immunosuppressant was a dominant alternative when compared to standard of care and was cost-effective according to a $CAD50,000/QALY willingness to pay threshold when compared with surgery.
4.6.3. Cost-Effectiveness of Mesalamine (5-ASA)
All studies assessing the cost-effectiveness of 5-ASA were performed in the context of UC and compared 5-ASA with different 5-ASA formulations, doses, and treatment regimen. 5-ASA was dominant in 72.7% of the analysis and cost-effective according to a $CAD50,000/QALY willingness to pay (WTP) threshold in 81.9% of the analyses.
4.6.4. Cost-Effectiveness of Surgery
Surgery was evaluated in treatments for UC only and was dominant when colectomy was compared with standard of care. However, when colectomy was performed at an early stage and combined with IPAA, surgery was not cost-effective according to a $CAD100,000/QALY WTP threshold compared to standard of care.
5. Discussion
Recent years have witnessed a rapid growth in IBD treatments. More specifically, the addition of biological treatments in the therapeutic arsenal of IBD has allowed significant clinical benefits, although it is associated with a substantial economic burden. Numerous economic evaluations have been performed in the last years in order to evaluate the cost-effectiveness of IBD treatments. The objective of this literature review was to explore the existing evidence regarding the cost-effectiveness of IBD treatments. This review found that a high proportion of biological therapies were cost-effective according to a $CAD100,000/QALY. Studies evaluating biological treatments in patients with severe disease and inadequate response to conventional therapies were found to be particularly cost-effective. Immunosuppressants and 5-ASA were also cost-effective strategies. On the other hand, the ranged ICER presented for IFX and ADA maintenance therapy versus induction therapy in CD was substantially wide. This variation could be explained by change in treatment regimen costs, despite the similarity between the associated QALY values. For the Canadian studies, the results seemed to differ by type of IBD, where ICERs for CD were much higher than ICERs for UC.
Up to now, other literature reviews on economic evaluations of IBD treatments were performed. Most of these studies assessed the cost-effectiveness of biological treatments only [24, 69–76], while only a few have taken into consideration all treatments for IBD [77, 78]. The present study is considering all IBD treatment options, including biological agents (IFX, ADA, GOL, NAT, and VED), immunosuppressants (AZA, 6MP, and cyclosporine), 5-ASA, GMA, and surgery (colectomy, IPAA). The findings of the present study are in line with the results of the previous literature reviews.
This study provides an exhaustive and complete overview of the economic evaluations performed in the context of IBD during the past years. A rigorous systematic review was conducted according to a predefined protocol, based on best practice guidelines. Even if this was not a specific selection criterion, economic evaluations included in this review were, in general, of good quality. Moreover, a 12-year time period was covered, which allowed the identification and the selection of a large number of relevant cost-effectiveness and cost-utility analyses. Such a long timeframe provided a good overview of the key characteristics of pharmacoeconomic analyses conducted in IBD during the last years. Furthermore, a high proportion of studies were of Canadian and American origin, which is in line with the high prevalence and incidence rates of IBD in theses' respective countries. Among other things, Canada detains one of the highest IBD prevalence and incidence worldwide.
However, this review has some limitations. This review was limited to English and French articles only. In addition, this review did not use a standardized tool for assessing the methodological quality of included studies. Another limitation involves the heterogeneity and variability of the characteristics and parameters of the studies included in this literature review. For instance, the methods used to assess the effectiveness differed from one study to the other. Many studies in UC or in CD used different disease progression index scores for definition of their model health states, including CDAI, HBI, or UCDAI scores. However, the latter scores are not based on the same patient disease characteristics and could therefore explain the variability in effectiveness among the studies. Moreover, different time horizons were chosen among included studies, which could have accounted for variability among studies. As IBD are chronic diseases, a longer time horizon allows better capturing remission and relapsing cycles and complications. However, a low proportion of studies have accounted for such a long time horizon. IBD complications, such as gastrointestinal cancers, are widely acknowledged as a long-term complication, likely as a result of chronic inflammation [79, 80]. Though, only few authors have incorporated colorectal cancer (CRC) risk in their economic model. In addition, the study population varied in terms of patients' age (adults or paediatric patients), previous exposition to treatment options (biological naïve patients, steroid refractory patients, and patients with inadequate response or medical contraindications for conventional therapies), and disease severity (patients with mild disease, patients with moderate to severe disease, patients with active luminal or fistulizing disease, and patients with acute exacerbations of disease).
Furthermore, the majority of selected economic evaluations have focused on a public healthcare system perspective, whereas only one study considered the societal perspective and led to a more favourable ICER than other studies comparing the same treatment options. IBD is a disease diagnosed as early adults, hence leading to a substantial impact on productivity loss and related costs. It has been demonstrated that biological therapies were associated with improved health outcomes, such as reduction in absenteeism [34, 81]. Considering that productivity losses account for a significant portion of the disease burden, the societal perspective is relevant and could have been considered [82]. Nevertheless, despite these limitations, this review adds to the current literature by providing a comprehensive overview of the existing economic evaluations in IBD therapy.
6. Conclusion
This literature review provided a comprehensive overview of the economic evaluations for the different treatment options for IBD over the past 12 years and represents a helpful reference for future economic evaluations.
Acknowledgments
The iGenoMed Consortium would like to acknowledge the financial support of Génome Québec, Genome Canada, the government of Canada, the Ministère de l'Enseignement Supérieur, de la Recherche, de la Science et de la Technologie du Québec, the Canadian Institutes of Health Research (with contributions from the Institutes of Infection and Immunity, of Genetics, of Nutrition, of Metabolism and Diabetes), Genome BC and Crohn's Colitis Canada. The authors would also like to thank Djouher Nait Ladjemil, M.S., Faculty of Pharmacy of University of Montreal, and Marie-Eve Richard, M.S. Candidate, Faculty of Pharmacy of University of Montreal. iGenoMed Consortium members are Ashwin Ananthakrishnan, M.D., Fabiano Armellini, Ph.D., Reiner Banken, M.D., Catherine Beaudry, Ph.D., Alain Bitton, M.D., Brian Bressler, M.D., Justin Côté-Daigneault, M.D., Mark J. Daily, Ph.D., Christine Des Rosiers, Ph.D., Lawrence Joseph, Ph.D., Jean Lachaine, Ph.D., Sylvie Lesage, Ph.D., Yvette Leung, M.D., Megan Levings, Ph.D., Dominique Morin, Ph.D., Pierre Poitras, M.D., Johanne Queeton, Ph.D., John D. Rioux, Ph.D., Sachdev Sidhu, Ph.D., Sophie Veilleux, Ph.D., and Ramnik Xavier, M.D.
Disclosure
Djouher Nait Ladjemil was a student at the Faculty of Pharmacy of University of Montreal at the time of the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Romberg-Camps M. J. L., Bol Y., Dagnelie P. C., et al. Fatigue and health-related quality of life in inflammatory bowel disease: Results from a population-based study in the Netherlands: The IBD-South Limburg cohort. Inflammatory Bowel Diseases. 2010;16(12):2137–2147. doi: 10.1002/ibd.21285. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart D. C., Sandborn W. J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. The Lancet. 2007;369(9573):1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein C. N., Fried M., Krabshuis J. H., et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflammatory Bowel Diseases. 2010;16(1):112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 4.Dudley-Brown S. Prevention of psychological distress in persons with inflammatory bowel disease. Issues in Mental Health Nursing. 2002;23(4):403–422. doi: 10.1080/01612840290052596. [DOI] [PubMed] [Google Scholar]
- 5.Berstein C., et al. Inflammatory Bowel Disease: A Global Perspective. 2009. [Google Scholar]
- 6.Statistics Canada Health Statistics Division. Canadian Community Health Survey (CCHS) Cycle 4.1. Ottawa, Canada: Statistics Canada; 2008. [Google Scholar]
- 7.Wlodarska M., Kostic A., Xavier R. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host & Microbe. 2015;17(5):577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese S., Sans M., Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmunity Reviews. 2004;3(5):394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Loftus E. V., Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 10.Molodecky N. A., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 11.The impact of inflammatory bowel disease in Canada 2012 - Final Report and Recommendations. 2012. http://www.isupportibd.ca/pdf/ccfc-ibd-impact-report-2012.pdf. [Google Scholar]
- 12.Drugs for inflammatory bowel disease. Treatment Guidelines from the Medical Letter. 2012;10(115):19–28. [PubMed] [Google Scholar]
- 13.Crohn's Colitis Foundation of America, Inflammatory Bowel Diseases (IBD) Treatment Options Medications. 2015. [Google Scholar]
- 14.Panaccione R., Fedorak R. N., Aumais G., et al. Canadian association of gastroenterology clinical practice guidelines: The use of infliximab in Crohn's disease. Canadian Journal of Gastroenterology & Hepatology. 2004;18(8):503–508. doi: 10.1155/2004/670161. [DOI] [PubMed] [Google Scholar]
- 15.Bressler B., et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148(5):1035–1058. doi: 10.1053/j.gastro.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Iskandar H. N., Dhere T., Farraye F. A. Ulcerative colitis: update on medical management. Current Fungal Infection Reports. 2015;17(11, article no. 44) doi: 10.1007/s11894-015-0466-9. [DOI] [PubMed] [Google Scholar]
- 17.Bitton A., Buie D., Enns R., et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. American Journal of Gastroenterology. 2012;107(2):179–194. doi: 10.1038/ajg.2011.386. [DOI] [PubMed] [Google Scholar]
- 18.Rocchi A., Benchimol E. I., Bernstein C. N., et al. Inflammatory bowel disease: a Canadian burden of illness review. Canadian Journal of Gastroenterology & Hepatology. 2012;26(11):811–817. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthakrishnan A. N., Hur C., Juillerat P., Korzenik J. R. Strategies for the prevention of postoperative recurrence in crohn's disease: Results of a decision analysis. American Journal of Gastroenterology. 2011;106(11):2009–2017. doi: 10.1038/ajg.2011.237. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan A. N., Korzenik J. R., Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn's disease? A decision analysis. Inflammatory Bowel Diseases. 2013;19(1):37–44. doi: 10.1002/ibd.22951. [DOI] [PubMed] [Google Scholar]
- 21.Blackhouse G., et al. Canadian cost-utility analysis of initiation and maintenance treatment with anti-TNF-alpha drugs for refractory Crohn's disease. Journal of Crohn's & Colitis. 2012;6(1):77–85. doi: 10.1016/j.crohns.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Bodger K., Kikuchi T., Hughes D. Cost-effectiveness of biological therapy for Crohn's disease: Markov cohort analyses incorporating United Kingdom patient-level cost data. Alimentary Pharmacology & Therapeutics. 2009;30(3):265–274. doi: 10.1111/j.1365-2036.2009.04033.x. [DOI] [PubMed] [Google Scholar]
- 23.Doherty G. A., Miksad R. A., Cheifetz A. S., Moss A. C. Comparative cost-effectiveness of strategies to prevent postoperative clinical recurrence of Crohn's disease. Inflammatory Bowel Diseases. 2012;18(9):1608–1616. doi: 10.1002/ibd.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dretzke J., Edlin R., Round J., et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for crohn's disease. Health Technology Assessment. 2011;15(6):1–244. doi: 10.3310/hta15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaisson-Hot I., Flourié B., Descos L., Colin C. Management for severe Crohn's disease: A lifetime cost-utility analysis. International Journal of Technology Assessment in Health Care. 2004;20(3):274–279. doi: 10.1017/S0266462304001084. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan G. G., Hur C., Korzenik J., Sands B. E. Infliximab dose escalation vs. initiation of adalimumab for loss of response in Crohn's disease: A cost-effectiveness analysis. Alimentary Pharmacology & Therapeutics. 2007;26(11-12):1509–1520. doi: 10.1111/j.1365-2036.2007.03548.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay J., Punekar Y. S., Morris J., Chung-Faye G. Health-economic analysis: Cost-effectiveness of scheduled maintenance treatment with infliximab for Crohn's disease - Modelling outcomes in active luminal and fistulizing disease in adults. Alimentary Pharmacology & Therapeutics. 2008;28(1):76–87. doi: 10.1111/j.1365-2036.2008.03709.x. [DOI] [PubMed] [Google Scholar]
- 28.Punekar Y. S., Sunderland T., Hawkins N., Lindsay J. Cost-effectiveness of scheduled maintenance treatment with infliximab for pediatric Crohn's disease. Value in Health. 2010;13(2):188–195. doi: 10.1111/j.1524-4733.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 29.Steenholdt C., Brynskov J., Thomsen O. Ø., et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63(6):919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 30.Steenholdt C., Brynskov J., Thomsen O. Ø., et al. Individualized Therapy Is a Long-Term Cost-Effective Method Compared to Dose Intensification in Crohn’s Disease Patients Failing Infliximab. Digestive Diseases and Sciences. 2015;60(9):2762–2770. doi: 10.1007/s10620-015-3581-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang D. H., Armstrong E. P., Lee J. K. Cost-utility analysis of biologic treatments for moderate-to-severe Crohn's disease. Pharmacotherapy:The Journal of Human Pharmacology & Drug Therapy. 2012;32(6):515–26. doi: 10.1002/j.1875-9114.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 32.Velayos F. S., Kahn J. G., Sandborn W. J., Feagan B. G. A Test-based strategy is more cost effective than empiric dose escalation for patients with crohn's disease who lose responsiveness to infliximab. Clinical Gastroenterology and Hepatology. 2013;11(6):654–666. doi: 10.1016/j.cgh.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 33.CADTH. Overview of CDR Clinical and Pharmacoeconomic Reports Adalimumab. Canadian Agency for Drugs and Technologies in Health; 2008. [Google Scholar]
- 34.Loftus Jr E. V., et al. Cost-effectiveness of adalimumab for the maintenance of remission in patients with Crohn's disease. European Journal of Gastroenterology & Hepatology. 2009;21(11):1302–1309. doi: 10.1097/MEG.0b013e32832a8d71. [DOI] [PubMed] [Google Scholar]
- 35.Yu A. P., Johnson S., Wang S.-T., et al. Cost utility of adalimumab versus infliximab maintenance therapies in the United States for moderately to severely active crohns disease. PharmacoEconomics. 2009;27(7):609–621. doi: 10.2165/11312710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti M., Liberato N. L., Di Sabatino A., Corazza G. R. Cost-effectiveness analysis of top-down versus step-up strategies in patients with newly diagnosed active luminal Crohn's disease. European Journal of Health Economics. 2013;14(6):853–861. doi: 10.1007/s10198-012-0430-7. [DOI] [PubMed] [Google Scholar]
- 37.Saito S., Shimizu U., Nan Z., et al. Economic impact of combination therapy with infliximab plus azathioprine for drug-refractory Crohn's disease: A cost-effectiveness analysis. Journal of Crohn's and Colitis. 2013;7(2):167–174. doi: 10.1016/j.crohns.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Ananthakrishnan A. N., Hur C., Korzenik J. R. Certolizumab pegol compared to natalizumab in patients with moderate to severe crohn's disease: Results of a decision analysis. Digestive Diseases and Sciences. 2012;57(2):472–480. doi: 10.1007/s10620-011-1896-3. [DOI] [PubMed] [Google Scholar]
- 39.Priest V. L., Begg E. J., Gardiner S. J., et al. Pharmacoeconomic analyses of azathioprine, methotrexate and prospective pharmacogenetic testing for the management of inflammatory bowel disease. PharmacoEconomics. 2006;24(8):767–781. doi: 10.2165/00019053-200624080-00004. [DOI] [PubMed] [Google Scholar]
- 40.Archer R., Tappenden P., Ren S., et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (Including a review of TA140 and TA262): Clinical effectiveness systematic review and economic model. Health Technology Assessment. 2016;20(39) doi: 10.3310/hta20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhary M. A., Fan T. Cost-Effectiveness of Infliximab for the Treatment of Acute Exacerbations of Ulcerative Colitis in the Netherlands. Biologics in Therapy. 2013;3(1):45–60. doi: 10.1007/s13554-012-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyde C., Bryan S., Juarez-Garcia A., Andronis L., Fry-Smith A. Infliximab for the treatment of ulcerative colitis. Health Technology Assessment. 2009;13(Suppl 3) doi: 10.3310/hta13suppl3/02. [DOI] [PubMed] [Google Scholar]
- 43.Punekar Y. S., Hawkins N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. European Journal of Health Economics. 2010;11(1):67–76. doi: 10.1007/s10198-009-0199-5. [DOI] [PubMed] [Google Scholar]
- 44.Stawowczyk E., Kawalec P., Pilc A. Cost-Utility Analysis of Infliximab with Standard Care versus Standard Care Alone for Induction and Maintenance Treatment of Patients with Ulcerative Colitis in Poland. Pharmacotherapy. 2016;36(5):472–481. doi: 10.1002/phar.1742. [DOI] [PubMed] [Google Scholar]
- 45.Thorlund K., Druyts E., Mills E. Golimumab (Simponi) (Subcutaneous Injection): Adult Patients with Moderately to Severely Active Ulcerative Colitis Who Have Had an Inadequate Response to, or Have Medical Contraindications for, Conventional Therapies. Ottawa, Canada: 2014. [PubMed] [Google Scholar]
- 46.Toor K., Druyts E., Jansen J. P., Thorlund K. Cost per remission and cost per response with infliximab, adalimumab, and golimumab for the treatment of moderately-to-severely active ulcerative colitis. Journal of Medical Economics. 2015;18(6):437–446. doi: 10.3111/13696998.2015.1012513. [DOI] [PubMed] [Google Scholar]
- 47.Tsai H. H., Punekar Y. S., Morris J., Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2008;28(10):1230–1239. doi: 10.1111/j.1365-2036.2008.03839.x. [DOI] [PubMed] [Google Scholar]
- 48.Ung V., et al. Real-life treatment paradigms show infliximab is cost-effective for management of ulcerative colitis. Clinical Gastroenterology & Hepatology. 2014;12(11, p. 1871-8.e8) doi: 10.1016/j.cgh.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Williams J. G., Alam M. F., Alrubaiy L., et al. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: Pragmatic randomised trial and economic evaluation (CONSTRUCT) Health Technology Assessment. 2016;20(44):1–322. doi: 10.3310/hta20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokomizo L., Limketkai B., Park K. T. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterology. 2016;3(1):p. e000093. doi: 10.1136/bmjgast-2016-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie F., Blackhouse G., Assasi N., Gaebel K., Robertson D., Goeree R. Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Effectiveness and Resource Allocation. 2009;7, article no. 20 doi: 10.1186/1478-7547-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essat M., Tappenden P., Ren S., et al. Vedolizumab for the Treatment of Adults with Moderate-to-Severe Active Ulcerative Colitis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics. 2016;34(3):245–257. doi: 10.1007/s40273-015-0334-3. [DOI] [PubMed] [Google Scholar]
- 53.Brereton N., Bodger K., Kamm M. A., Hodgkins P., Yan S., Akehurst R. A cost-effectiveness analysis of MMX mesalazine compared with mesalazine in the treatment of mild-to-moderate ulcerative colitis from a UK perspective. Journal of Medical Economics. 2010;13(1):148–161. doi: 10.3111/13696990903562861. [DOI] [PubMed] [Google Scholar]
- 54.Buckland A., Bodger K. The cost-utility of high dose oral mesalazine for moderately active ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2008;28(11-12):1287–1296. doi: 10.1111/j.1365-2036.2008.03856.x. [DOI] [PubMed] [Google Scholar]
- 55.Connolly M. P., Nielsen S. K., Currie C. J., Marteau P., Probert C. S. J., Travis S. P. L. An economic evaluation comparing concomitant oral and topical mesalazine versus oral mesalazine alone in mild-to-moderately active ulcerative colitis based on results from randomised controlled trial. Journal of Crohn's and Colitis. 2009;3(3):168–174. doi: 10.1016/j.crohns.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Connolly M. P., Nielsen S. K., Currie C. J., Poole C. D., Travis S. P. L. An economic evaluation comparing once daily with twice daily mesalazine for maintaining remission based on results from a randomised controlled clinical trial. Journal of Crohn's and Colitis. 2009;3(1):32–37. doi: 10.1016/j.crohns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Connolly M. P., Boersma C., Oldenburg B. The economics of mesalazine in active ulcerative colitis and maintenance in the Netherlands. The Netherlands Journal of Medicine. 2012;70(6):272–277. [PubMed] [Google Scholar]
- 58.Connolly M. P., Kuyvenhoven J. P., Postma M. J., Nielsen S. K. Cost and quality-adjusted life year differences in the treatment of active ulcerative colitis using once-daily 4g or twice-daily 2g mesalazine dosing. Journal of Crohn's and Colitis. 2014;8(5):357–362. doi: 10.1016/j.crohns.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Mackowiak J. I. A two-stage decision analysis to assess the cost of 5-aminosalicylic acid failure and the economics of balsalazide versus mesalamine in the treatment of ulcerative colitis. Managed Care Interface. 2006;19(10):39–56. [PubMed] [Google Scholar]
- 60.Nishikawa A. M., Paladini L., Delfini R., Kotze P. G., Clark O. Decision tree construction and cost-effectiveness analysis of treatment of ulcerative colitis with pentasa ® mesalazine 2 g sachet. Arquivos de Gastroenterologia. 2013;50(4):297–303. doi: 10.1590/S0004-28032013000400011. [DOI] [PubMed] [Google Scholar]
- 61.Prenzler A., Yen L., Mittendorf T., Von Der Schulenburg J.-M. Cost effectiveness of ulcerative colitis treatment in Germany: A comparison of two oral formulations of mesalazine. BMC Health Services Research. 2011;11, article no. 157 doi: 10.1186/1472-6963-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saini S. D., Waljee A. K., Higgins P. D. R. Cost Utility of Inflammation-Targeted Therapy for Patients With Ulcerative Colitis. Clinical Gastroenterology and Hepatology. 2012;10(10):1143–1151. doi: 10.1016/j.cgh.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yen E. F., Kane S. V., Ladabaum U. Cost-effectiveness of 5-aminosalicylic acid therapy for maintenance of remission in ulcerative colitis. American Journal of Gastroenterology. 2008;103(12):3094–3105. doi: 10.1111/j.1572-0241.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 64.Park K. T., Tsai R., Perez F., Cipriano L. E., Bass D., Garber A. M. Cost-effectiveness of early colectomy with ileal pouch-anal anastamosis versus standard medical therapy in severe ulcerative colitis. Annals of Surgery. 2012;256(1):117–124. doi: 10.1097/SLA.0b013e3182445321. [DOI] [PubMed] [Google Scholar]
- 65.Swenson B. R., Hollenbeak C. S., Poritz L. S., Koltun W. A. Modified two-stage ileal pouch-anal anastomosis: Equivalent outcomes with less resource utilization. Diseases of the Colon & Rectum. 2005;48(2):256–261. doi: 10.1007/s10350-004-0848-9. [DOI] [PubMed] [Google Scholar]
- 66.Panes J., et al. Treatment cost of ulcerative colitis is apheresis with Adacolumn cost-effective? Digestive & Liver Disease. 2007;39(7):617–25. doi: 10.1016/j.dld.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Higgings J. P. T., Green S., editors. The Cochrane Collaboration. Chapter 5 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [Google Scholar]
- 68.Statistics Canada. Consulmer Price Index, Health Care: CANSIM Table 326-0020. 2013.
- 69.Di Sabatino A., Liberato L., Marchetti M., Biancheri P., Corazza G. R. Optimal use and cost-effectiveness of biologic therapies in inflammatory bowel disease. Internal and Emergency Medicine. 2011;6(S1):17–27. doi: 10.1007/s11739-011-0673-9. [DOI] [PubMed] [Google Scholar]
- 70.Fleurence R., Spackman E. Cost-effectiveness of biologic agents for treatment of autoimmune disorders: Structured review of the literature. The Journal of Rheumatology. 2006;33(11):2124–2131. [PubMed] [Google Scholar]
- 71.Huoponen S., Blom M. A systematic review of the cost-effectiveness of biologics for the treatment of inflammatory bowel diseases. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145087.e0145087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchetti M., Liberato N. L. Biological therapies in Crohn's disease: Are they cost-effective? A critical appraisal of model-based analyses. Expert Review of Pharmacoeconomics & Outcomes Research. 2014;14(6):815–824. doi: 10.1586/14737167.2014.957682. [DOI] [PubMed] [Google Scholar]
- 73.Martelli L., Olivera P., Roblin X., Attar A., Peyrin-Biroulet L. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. Journal of Gastroenterology. 2016;52(1):19–25. doi: 10.1007/s00535-016-1266-1. [DOI] [PubMed] [Google Scholar]
- 74.Smart C., Selinger C. P. The cost-effectiveness of infliximab in Crohn's disease. Expert Review of Pharmacoeconomics & Outcomes Research. 2014;14(5):589–598. doi: 10.1586/14737167.2014.950235. [DOI] [PubMed] [Google Scholar]
- 75.Tang D. H., Harrington A. R., Lee J. K., Lin M., Armstrong E. P. A systematic review of economic studies on biological agents used to treat Crohn's disease. Inflammatory Bowel Diseases. 2013;19(12):2673–2694. doi: 10.1097/MIB.0b013e3182916046. [DOI] [PubMed] [Google Scholar]
- 76.Assasi N., et al. Anti-TNF-α drugs for refractory inflammatory bowel disease: Clinical- and cost-effectiveness analyses [Technology report number 120] Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 77.Bodger K. Cost effectiveness of treatments for inflammatory bowel disease. PharmacoEconomics. 2011;29(5):387–401. doi: 10.2165/11584820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.Park K. T., Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflammatory Bowel Diseases. 2011;17(7):1603–1609. doi: 10.1002/ibd.21488. [DOI] [PubMed] [Google Scholar]
- 79.Beaugerie L., Itzkowitz S. H. Cancers Complicating Inflammatory Bowel Disease. The New England Journal of Medicine. 2015;373(2):p. 195. doi: 10.1056/NEJMc1505689. [DOI] [PubMed] [Google Scholar]
- 80.Kappelman M. D., Farkas D. K., Long M. D., et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clinical Gastroenterology and Hepatology. 2014;12(2):265.e1–273.e1. doi: 10.1016/j.cgh.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Binion D. G., Louis E., Oldenburg B., et al. Effect of adalimumab on work productivity and indirect costs in moderate to severe Crohn's disease: A meta-analysis. Canadian Journal of Gastroenterology & Hepatology. 2011;25(9):492–496. doi: 10.1155/2011/938194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Valk M. E., Mangen M. J., Siersema P. D., van Oijen M. G., Oldenburg B. Sa1283 Productivity Losses in Inflammatory Bowel Disease: A Systematic Review. Gastroenterology. 2012;142(5):p. S-262. doi: 10.1016/S0016-5085(12)60990-1. [DOI] [Google Scholar]


