Abstract
Background:
The use of neuraxial anesthesia has dramatically increased. Acute postoperative pain is an undesirable outcome that can delay functional recovery for patients undergoing surgery. Nausea and vomiting in the postoperative period occurs in 20%–30% of the patients and together are the second-most common complaint reported (pain is the most common). Efficacy of glucocorticoids for reducing pain and inflammation after surgery is being explored. Glucocorticoids are strong anti-inflammatory agents, which can be used for a short-time postoperative pain control in various surgeries. Dexamethasone is a glucocorticoid with little mineralocorticoid effect commonly used perioperatively to reduce postoperative nausea and vomiting (PONV) and has a beneficial role in postoperative analgesia. Dexamethasone has also an antiemetic effect, in addition to its anti-inflammatory and analgesic effects.
Aim:
The main purpose of this study is to evaluate the effect of administration of single-dose intravenous (i.v.) dexamethasone on postoperative pain and PONV in patients undergoing surgery under spinal anesthesia.
Settings and Design:
A double-blind randomized clinical study was performed in our institute between November 2014 and October 2015 after obtaining clearance from the ethical committee.
Materials and Methods:
A double-blind randomized clinical study was performed on 60 patients posted for surgery under spinal anesthesia. Patients were randomly assigned into two groups: A (study: 2 ml [8 mg] dexamethasone) and B (control: 2 ml saline). In both the groups, variables such as mean arterial blood pressure (MAP), heart rate (HR), respiratory rate, severity of pain (based on visual analog scale), and other symptoms such as nausea and vomiting were recorded at different time points during the first 24 h after surgery. Statistical methods using Student t-test (two-tailed, independent) and Fischer's exact test were used for analyzing the data.
Results:
Between-group comparisons indicated significant differences in terms of severity of postoperative pain and PONV (P < 0.001), MAP (P = 0.063), and HR (P = 0.071), which in the study group were lower than the control group.
Conclusion:
i.v. dexamethasone is efficient in reducing postoperative pain, requirement of rescue analgesia on the first postoperative day, and incidence of PONV with no significant changes in vital signs.
Keywords: Dexamethasone, pain, postoperative nausea vomiting, spinal anesthesia
INTRODUCTION
The use of neuraxial anesthesia has dramatically increased. Spinal anesthesia has been shown to blunt the “stress response” to surgery, to decrease intraoperative blood loss, to lower the incidence of postoperative thromboembolic events, and to decrease morbidity and mortality in high-risk surgical patients.[1]
Neuraxial anesthesia techniques also have the advantage of decreased risk of failed intubation and aspiration of gastric contents, avoidance of depressant agents, and the ability of the patient to remain awake.[1] Hyperbaric bupivacaine is the most commonly used agent for spinal anesthesia.
Any surgery, due to tissue damage, causes the release of chemical mediators such as substance P, hydroxytryptophan, serotonin, bradykinin, and prostaglandins, which stimulate the A (delta) and C nerve fibers and therefore cause pain perception.[2] This can stimulate autonomic nervous system and increase heart rate (HR) and cardiac output.[3]
Emergence from anesthesia and surgery may be accompanied by a number of physiologic disturbances that affect multiple-organ systems, most commonly postoperative nausea and vomiting (PONV), hypoxia, hypothermia, shivering, and cardiovascular instability.[1]
Acute postoperative pain is an undesirable outcome that can delay functional recovery for patients undergoing surgery.[4]
Nausea and vomiting in the postoperative period occurs in 20%–30% of the patients and together are the second-most common complaint reported (pain is the most common).[5]
Efficacy of glucocorticoids for reducing pain and inflammation after surgery is being explored. Glucocorticoids are strong anti-inflammatory agents, which can be used for a short-time postoperative pain control in various surgeries. Dexamethasone has also an antiemetic effect, in addition to its anti-inflammatory and analgesic effects.[6]
The effects of corticosteroids are numerous and widespread. They endow the organism with the capacity to resist stressful circumstances such as noxious stimuli and environmental changes.[2]
Glucocorticoids are strong anti-inflammatory agents, which can be used for a short-time postoperative pain control in various surgeries. Dexamethasone has also an antiemetic effect, in addition to its anti-inflammatory and analgesic effects.[3] Mechanism of action of glucocorticoids is not fully understood; however, the suggested theories include inhibition of production of inflammatory mediators (prostaglandin and bradykinin), preventing reduction of “pain threshold” which occurs in surgeries, and reducing tissue swelling because of anti-inflammatory effects and therefore inhibit nerve compression by inflammatory tissue.[7]
Dexamethasone is a glucocorticoid with little mineralocorticoid effect commonly used perioperatively to reduce PONV and has a beneficial role in postoperative analgesia.[8]
A single dose of glucocorticoid, even a large one, is virtually without harmful effects, and a short course of therapy (up to 1 week) in the absence of specific contraindications is unlikely to be harmful.[2]
By reducing the requirement of postoperative analgesics (opioids and nonsteroidal anti-inflammatory drugs), the adverse effects such as sedation, mental clouding, lethargy, respiratory depression, and gastric irritation can be reduced.[2]
MATERIALS AND METHODS
A double-blind prospective randomized clinical study was planned on patients scheduled for surgery under spinal anesthesia after obtaining approval from the Ethics Committee of the Institute between November 2014 and October 2016. A total of 60 patients scheduled for surgery under spinal anesthesia with American Society of Anesthesiologists Physical Status I and II with height 145–175 cm and weight 40–80 kg were recruited in this study. Written informed consents were obtained from all participants after describing all aspects of the study. Patients were randomly allocated to either study group (Group A) who received 2 ml (8 mg) i.v. dexamethasone or control group (Group B) who received 2 ml normal saline (0.9%) based on simple randomization process. Neither the participants nor the investigators responsible for following the participants, collecting data, and assessing the outcomes were aware of the intervention assignments.
Patients were excluded if they had any conditions such as sensitivity or allergy to glucocorticoids, present or history of peptic ulcer disease, glaucoma, diabetes mellitus Type 1or 2, heart failure, fungal or viral systemic infection, hypertension, or any other poorly controlled disease.
Both groups received the same hydration therapy and local anesthesia method. Spinal anesthesia (subarachnoid block) in the L2–L3 or L3–L4 interspace was provided by 3 ml bupivacaine 0.5% in sitting or lateral position.
All participants in both groups received 8 mg i.v. dexamethasone or 2 ml normal saline 0.9% at the same period of time (immediately after spinal anesthesia).
During the follow-up period (up to 24 h after surgery), averages of mean arterial blood pressure (MAP), HR, respiratory rate (RR), and severity of pain and vomiting were monitored and recorded at 0, 15, 30, 60, 90, 120, 150, and 180 min (in the recovery room) and 4, 5, 6, 12, and 24 h (in the postoperative ward) after surgery.
Severity of pain and vomiting based on visual analog scale (VAS) was measured using a 10-cm ruler according to self-reporting by patients. In this method, patient was asked to indicate zero in case of having no symptoms and ten if he/she had the most severe symptoms. For both the pain and vomiting, a score ≤4 was considered as mild, 5–7 as moderate, and 8–10 as severe.
All patients received a dose of 75 mg diclofenac intramuscularly on demand and then if necessary (VAS >4) for pain relief; however, caution was taken that the diclofenac administration intervals should not be <8 h. Rescue antiemetic (metoclopramide 10 mg) was given i.v. if vomiting occurred.
The incidence of side effects such as agitation and rise of blood pressure during 24 h and patients' satisfaction was evaluated.
The primary outcome measure was control of postoperative pain and PONV.
The secondary outcome measures were duration of action and patient satisfaction.
Statistical methods
Sample size
Keeping power of study at 90%, confidence interval of 95%, to detect a 20% difference in VAS score, the sample size of 27 was required in each group; however, 30 patients were included in each group.
Student t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (intergroup analysis) on metric parameters. Levene's test for homogeneity of variance has been performed to assess the homogeneity of variance.
Chi-square and Fisher's exact tests have been used to find the significance of study parameters on categorical scale between two or more groups.
Significant figures
+Suggestive significance (P value: 0.05 < P < 0.10)
*Moderately significant (P value: 0.01 < P ≤ 0.05)
**Strongly significant (P value: P ≤ 0.01).
Statistical software
The statistical software, namely, SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver. 2.11.1, were used for the analysis of the data, and Microsoft Word and Excel have been used to generate graphs, tables, etc.
RESULTS
There were no significant differences among two groups in terms of age (mean age was 37.73 ± 15.09 in the study group and 41.07 ± 10.96 in the control group). The results suggest that the levels of severity of pain and PONV, MAP, and HR made across the study and control groups, studied at specific time points, are dependent on the type of treatment groups – who have received dexamethasone or normal saline. Between-group comparisons indicate significant differences in terms of severity of postoperative pain (P < 0.001), PONV (P < 0.001), MAP (P = 0.063), and HR (P = 0.071), while nonsignificant difference for RR (P = 0.408) [Tables 1–4 and Figures 1–3].
Table 1.
Comparison of study variables in two groups of patients studied
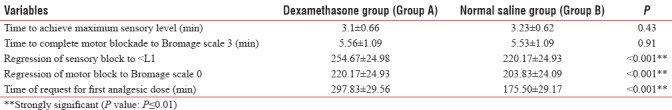
Table 4.
Others findings in two groups of patients studied
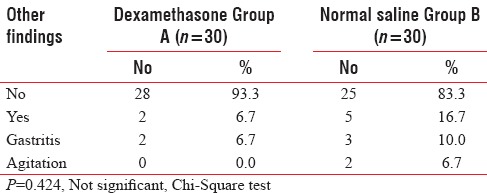
Figure 1.
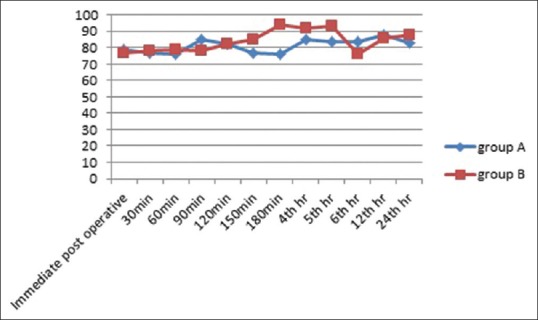
Graph depicting comparison of heart rate between the two groups in the postoperative period
Figure 3.
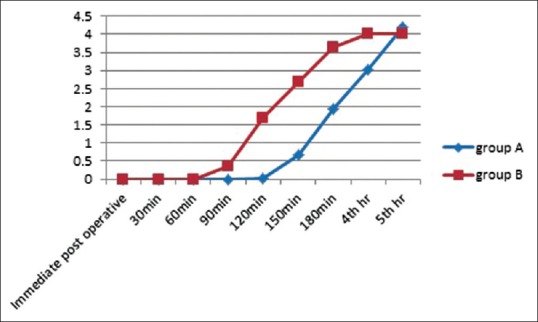
Graph depicting comparison of visual analog scale scores between the two groups
Table 2.
Comparison of VAS score in two groups of patients studied
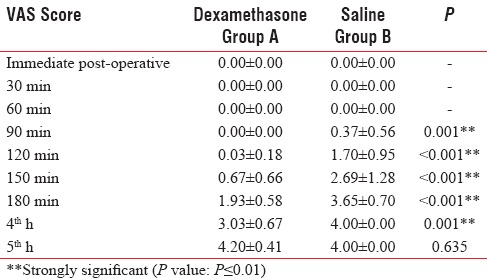
Table 3.
Side effects in two groups of patients studied
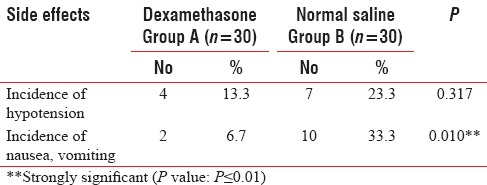
Figure 2.
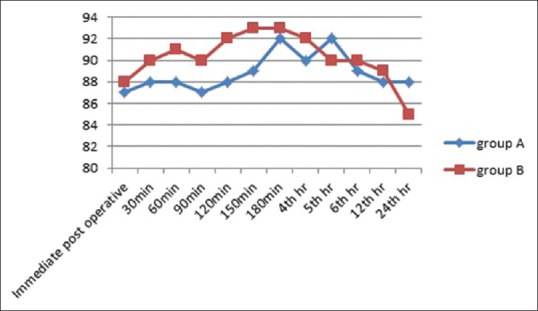
Graph depicting comparison of mean arterial blood pressure between the two groups in the postoperative period
There was also difference in terms of total analgesic (diclofenac) use between treatment (average of 160 mg) and control (average of 217 mg) groups (P < 0.001).
Limitations
A single dose of 8 mg dexamethasone was used for all participants in the study; however, only patients weighing between 40 and 80 kg were included in the study.
DISCUSSION
The main endpoint of our study, i.e., level of postoperative pain and PONV among patients who received i.v. dexamethasone, was compared with the control group (normal saline 0.9%). The obtained result in the current study was in line with the majority of the other studies that have confirmed the effectiveness of analgesic effect of dexamethasone after different surgeries.[3,7,8,9,10,11,12,13]
The results of this study are similar to the studies of Jokela et al. and Hongs et al. that showed that the postcesarean administration of dexamethasone led to reduce the need for morphine and other analgesic consumption.[9]
Dexamethasone has an antiemetic effect by inhibition of releasing prostaglandins and serotonin in the gastrointestinal tract and endorphin in the nervous system.[14] The effect of different dosages of glucocorticoids on reduction of PONV has been documented in numerous studies,[7,13,14,15,16] though the minimum dose of dexamethasone has been reported from 2.5 mg for gynecologic surgeries to 5 mg for thyroidectomy.[17,18,19]
A study conducted by Cardoso et al. in 2013 concluded that dexamethasone reduced the cumulative incidence of nausea and vomiting after cesarean section under spinal anesthesia with morphine and lowered pain scores on the first postoperative day.[13]
Shahraki et al. in 2013 in their study concluded that dexamethasone could efficiently reduce postoperative pain severity and the need for analgesic consumption and improve vital signs after cesarean section.[20]
A study conducted by Mohtadi et al. in 2014 concluded that single dose of i.v. dexamethasone led to less pain intensity and amounts of meperidine consumption, in comparison with placebo.[6]
A study conducted by Kadur et al. in 2016 concluded that administration of i.v. dexamethasone (0.1 mg/kg) just before subarachnoid block is an effective mode of enhancing postoperative analgesia and also reduces incidence of PONV.[21]
Multiple doses of corticosteroid therapy (>1 week) may cause side effects such as increased risk of infection, glucose intolerance, delayed wound healing, superficial ulceration of gastric mucosa, avascular necrosis of the femoral head, and adrenal suppression; however, these side effects were not found after a single dose of dexamethasone therapy.
Although another side effect which can be found by a single-dose therapy is agitation,[3] in our study, no early-onset side effects such as agitation or rise of blood pressure were seen in the dexamethasone group.
There was a significant difference between two groups in terms of total analgesic dose; the amounts of administered diclofenac were higher in the control group compared with the treatment one.
CONCLUSION
Administration of i.v. dexamethasone after spinal anesthesia is efficient in reducing postoperative pain, the requirement of rescue analgesia on the first postoperative day, and the incidence of PONV with no significant changes in vital signs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nicholau D. The postanesthesia care unit, Postoperative nausea and vomiting. In: Aphel CC, editor. Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2015. pp. 2924–73. [Google Scholar]
- 2.Bernard P, Schimmer Keith L. 12th ed. Pennsylvania: McGraw-Hill Education/Medical; 2011. Adrenocortical steroids and their synthetic analogs Parker in the Pharmacological Basics of Therapeutics - Goodman & Gillman; pp. 1649–76. [Google Scholar]
- 3.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–25. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- 4.De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–88. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 5.Bernard CM. Barash Clinical Anaesthesia. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. Spinal and Epidural anesthesia; pp. 905–36. [Google Scholar]
- 6.Mohtadi A, Neisoonpur S, Salari A, Akhondzadeh R, et al. The effect of single dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014;4:e17872. doi: 10.5812/aapm.17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Hsu CC, Chia YY. Effect of dexamethasone on postoperative emesis and pain. Br J Anaesth. 1998;80:85–6. doi: 10.1093/bja/80.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Kaan MN, Odabasi O, Gezer E, Daldal A. The effect of preoperative dexamethasone on early oral intake, vomiting and pain after tonsillectomy. Int J Pediatr Otorhinolaryngol. 2006;70:73–9. doi: 10.1016/j.ijporl.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Jokela RM, Ahonen JV, Tallgren MK, Marjakangas PC, Korttila KT. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109:607–15. doi: 10.1213/ane.0b013e3181ac0f5c. [DOI] [PubMed] [Google Scholar]
- 10.Kardash K, Sarrazin F, Tressler M, Velly A. Single dose dexamethasone reduces dynamic pain after total hip arthroplasty. Pain Med. 2008;4:1253–7. doi: 10.1213/ANE.0b013e318164f319. [DOI] [PubMed] [Google Scholar]
- 11.Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine (Phila Pa 1976) 2006;31:2415–7. doi: 10.1097/01.brs.0000238668.49035.19. [DOI] [PubMed] [Google Scholar]
- 12.Jaafarpour M, Khani A, Dyrekvandmoghadam A, Khajavikhan J, Saaidpour KH. The effect of dexamethasone on nausea, vomiting and pain in parturients undergoing caesarean delivery. J Clin Diagn Res. 2008;3:854–8. [Google Scholar]
- 13.Cardoso MM, Leite AO, Santos EA, Gozzani JL, Mathias LA. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: A randomised, placebo-controlled, double-blind trial. Eur J Anaesthesiol. 2013;30:102–5. doi: 10.1097/EJA.0b013e328356676b. [DOI] [PubMed] [Google Scholar]
- 14.Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: A randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:651–60. doi: 10.1097/01.sla.0000094390.82352.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzeng JI, Wang JJ, Ho ST, Tang CS, Liu YC, Lee SC, et al. Dexamethasone for prophylaxis of nausea and vomiting after epidural morphine for post-caesarean section analgesia: Comparison of droperidol and saline. Br J Anaesth. 2000;85:865–8. doi: 10.1093/bja/85.6.865. [DOI] [PubMed] [Google Scholar]
- 16.Splinter W, Roberts DJ. Prophylaxis for vomiting by children after tonsillectomy: Dexamethasone versus perphenazine. Anesth Analg. 1997;85:534–7. doi: 10.1097/00000539-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Wang JJ, Ho ST, Lee SC, Liu YC, Ho CM. The use of dexamethasone for preventing postoperative nausea and vomiting in females undergoing thyroidectomy: A dose-ranging study. Anesth Analg. 2000;91:1404–7. doi: 10.1097/00000539-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 18.López-Olaondo L, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Sáez A, et al. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76:835–40. doi: 10.1093/bja/76.6.835. [DOI] [PubMed] [Google Scholar]
- 19.Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Granisetron/dexamethasone combination for reducing nausea and vomiting during and after spinal anesthesia for cesarean section. Anesth Analg. 1999;88:1346–50. doi: 10.1097/00000539-199906000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Shahraki AD, Feizi A, Jabalameli M, Nouri S. The effect of intravenous dexamethasone on post-cesarean section pain and vital signs: A double-blind randomized clinical trial. J Res Pharm Pract. 2013;2:99–104. doi: 10.4103/2279-042X.122370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadur SN, Ahmed F, Purohit A, Khandelwal M, Mistry T. The effect of intravenous dexamethasone on postoperative pain, nausea vomiting after intrathecalpethidine and bupivacaine in lower limb orthopaedic surgeries. Anaesth Pain Intensive Care. 2016;19:254–9. [Google Scholar]


