Abstract
Context:
Different trials have shown that multimodal analgesia through different techniques is associated with superior pain relief. Opioids as epidural adjunct to local anesthetics have been in use for long and α2 agonists are being increasingly used for same. The present study aims at comparing the hemodynamic, sedative, and analgesic effects of epidurally administered fentanyl and dexmedetomidine when combined with bupivacaine.
Aims:
The aim of this study was to compare the efficacy of epidural dexmedetomidine with bupivacaine versus epidural fentanyl with bupivacaine for postoperative pain relief.
Subjects and Methods:
In this ongoing randomized double-blind study, 70 patients with ASA physical status classes I and II of either sex between 20 and 60 years scheduled for lower limb orthopedic surgeries under epidural block were randomly divided into two Groups (n = 35). After epidural block with 15 ml of 0.5% bupivacaine, Group I received 1 μg/kg of fentanyl and Group II received 1 μg/kg of dexmedetomidine. Onset and duration of sensory block, motor block, and time to request for the first postoperative analgesia were recorded.
Statistical Analysis Used:
The statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) Version 15.0 Statistical Analysis Software, Mann–Whitney U-test and Chi-square test.
Results:
The time to achieve T10 sensory block was early in Group I (dexmedetomidine) (8.10 + 1.03 min) as compared to Group II (15.03 + 1.67 min). Onset of motor was earlier in Group I (15.10 + 1.49 min) as compared to Group II (22.77 + 1.41 min). In Group I (dexmedetomidine), the majority of patients required 2–3 rescue doses, while in Group II (fentanyl), the majority of patients required 3–4 rescue doses.
Conclusions:
Dexmedetomidine seems to be a better alternative to fentanyl as an epidural adjuvant due to early onset of sensory anesthesia, prolonged postoperative analgesia, and lower consumption of rescue analgesia.
Keywords: Bupivacaine, dexmedetomidine, epidural, fentanyl, postoperative pain relief
INTRODUCTION
Evidence suggests that less than half of patients who undergo surgery report adequate postoperative pain relief.[1] Inadequately controlled pain negatively affects quality of life, function, and functional recovery, the risk of postsurgical complications, and the risk of persistent postsurgical pain. Randomized trials[2,3] have shown that multimodal analgesia through different techniques is associated with superior pain relief and decreased opioid consumption compared with the use of a single medication administered through one technique. Use of epidural analgesic technique for major surgery should provide effective pain relief with minimal side effects and high levels of patient satisfaction. It should also obtund central sensitization and pain-induced organ dysfunction, leading to improved outcome.[4]
Keeping the benefits of epidural adjunct to bupivacaine in consideration, our study is designed to compare the efficacy of dexmedetomidine versus fentanyl in combination with bupivacaine for postoperative analgesia in orthopedic surgery.
SUBJECTS AND METHODS
The present study was carried out at the department of anesthesiology in a tertiary medical college, with an aim to compare the efficacy of epidural dexmedetomidine with bupivacaine versus epidural fentanyl with bupivacaine for postoperative pain relief.
This was a prospective, randomized study. The study was conducted at the Department of Anesthesiology, Era's Lucknow Medical College. The duration of the study was 18 months.
Inclusion criteria
Patients with ASA physical status Classes I and II
Scheduled for elective lower limb orthopedic surgery.
Adult patients of age group 20–50 years.
Exclusion criteria
Patient refusal
Patients with significant cardiovascular disease, renal failure, hepatic dysfunction, chronic pulmonary disease, and diabetes mellitus
Neuromuscular disorder
Infection
Bleeding disorder
Obesity (BMI >30 kg/m2)
History of allergy or sensitivity to any of the study drugs in previous surgeries.
Sixty adult patients with ASA physical status Classes I and II and between 20 and 50 years of age were allocated randomly into two groups (30 patients each).
Group I: Epidural bupivacaine with dexmedetomidine
Group II: Epidural bupivacaine with fentanyl.
The patients visited before surgery for preanesthetic check, and standard institutional preoperative advices were given. Written and informed consent was taken from the patients. The patients were asked nil per oral for solid food for 8 h and clear liquids for 2 h before surgery. On the day of surgery, the patients were wheeled in operation theater, and noninvasive monitors such as pulse oximeter, noninvasive blood pressure (NIBP), and ECG were attached, and baseline parameters were recorded. Intravenous (IV) access was secured and IV fluid was started.
The patients were made to sit and under strict aseptic precautions, 18G Tuohy's needle was inserted into L2–L3 interspinous epidural space. Epidural space was confi rmed by loss of resistance method and epidural catheter was threaded 3–4 cm inside the epidural space and fixed, after institution of test dose (3 ml injection lidocaine 0.2% with adrenaline). Patients were randomly divided into two groups using computer-generated randomization tool.
Group I received 15 ml of 0.5% bupivacaine with 1 μg/kg injection dexmedetomidine epidurally
Group II received 15 ml of 0.5% bupivacaine with 1 μg/kg of injection fentanyl epidurally.
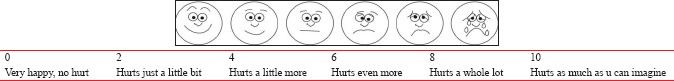
Hemodynamic parameters were recorded at baseline (T0), immediately after the study drug is given (T1), every 5 min thereafter till 15 min, and then every 15 min thereafter till end of surgery and postoperatively till demand of first rescue analgesic. Patients with block failure and having surgeries lasting longer than 2 h were excluded from the study. After completion of surgery, the patients were shifted to the postoperative ward; hemodynamic parameters and duration of analgesia were recorded in postoperative period at every 30 min interval. Pain was assessed using 10-point visual analog scale (VAS) in which a score of “0” indicated “no pain” and a score of “10” indicated “worst pain imaginable.” Duration of analgesia was recorded as the first complaint of pain (VAS >4) in the postoperative period and rescue analgesic was administered. Rescue analgesic as 10 ml 0.25% bupivacaine was administered at the onset of pain (VAS >4) in postoperative period and at each incidence of complaint of pain (VAS >4) in next 24 h. The number of rescue analgesic epidural doses was recorded during the first 24 h.
VAS vomiting, shivering, sedation, hypotension, pruritus, and urinary retention were documented and managed.
Adverse effects such as nausea, accordingly
Hypotension (defined by decrease in MAP below 20% of baseline or systolic blood pressure (SBP) (<90 mmHg)) was treated by injection mephentermine 6 mg boluses
Bradycardia (heart rate [HR] <50 bpm) was treated by atropine 0.6 mg IV
Respiratory depression (relative risk <8 breaths per min or SpO2 < 95%) was treated by oxygen supplementation and respiratory support if required
Nausea and vomiting were treated with injection ondansetron 0.1 mg/kg IV.
Statistical tools employed
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 Statistical Analysis Software. The values were represented in number (%) and mean ± standard deviation. To test the significance of two means, the student “t”-test was used, Mann–Whitney U-test was performed for nonparametric data, and Chi-square test was used for categorical outcome. The confidence level of the study was 95% and P < 0.05 indicated statistically significant association.
RESULTS
The present study was conducted at the Department of Anesthesiology, Era's Luckow Medical College, Lucknow, to study of efficacy of epidural dexmedetomidine with bupivacaine versus epidural fentanyl with bupivacaine for postoperative pain relief in lower limb orthopedic surgery. A total of 60 patients of lower limb orthopedic surgery fulfilling the inclusion criteria were included in the study. These patients were randomly allocated to two groups [Table 1].
Table 1.
Group-wise distribution of the study population

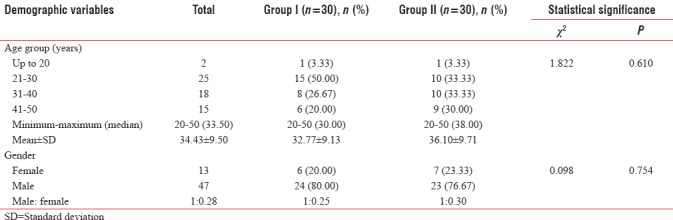
Difference in age of both the groups was not found to be statistically significant (P = 0.610). Out of 60 patients recruited in the study, only 13 (21.67%) were females and rest 47 (78.33%) were males. Difference in gender of patients in both the groups was not found to be statistically significant (P = 0.754) [Table 2].
Table 2.
Between-group comparison of demographic profile of study population
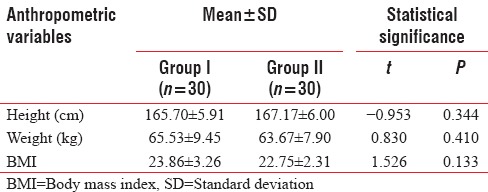
Between-group differences for above anthropometric variables were not found to be statistically significant (P > 0.05) [Table 3].
Table 3.
Between-group comparison of anthropometric variables
At baseline (immediately after epidural block), difference in none of the above hemodynamic variables of above two groups was found to be statistically significant (P > 0.05) [Table 4].
Table 4.
Between-group comparison of baseline hemodynamic variables
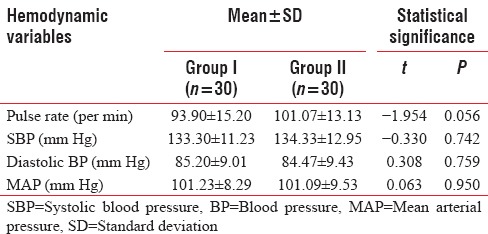
At baseline (immediately after epidural block), pulse rate of Group II (101.07 + 13.13 per min) was found to be higher than that of Group I (93.90 + 15.20 per min), but this difference was not found to be statistically significant. Between-group difference in pulse rate was not found to be statistically significant at any of the periods of observation [Table 5].
Table 5.
Between-group comparison of pulse rate at different time intervals
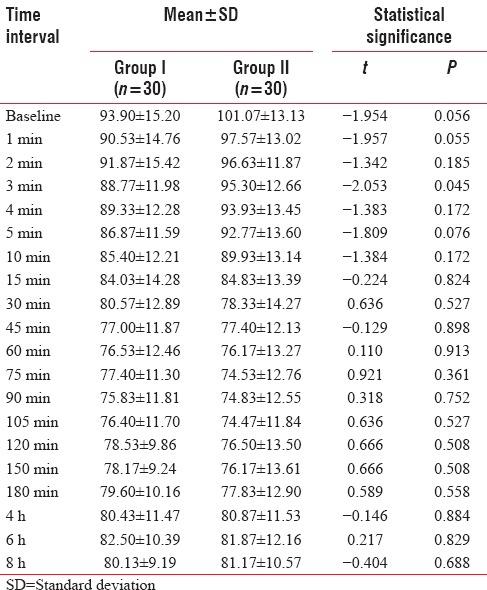
At baseline, SBP of Group II (134.33 + 12.95 mm Hg) was higher than that of Group I (133.30 + 11.23 mm Hg). Thereafter at 1 min to 10 min and at 75 min, SBP of Group II was found to be higher than that of Group I, and at rest of the periods of observation, SBP of Group I was found to be higher than that of Group II. Between-group difference of SBP was not found to be statistically significant at any of the periods of observation except at 120 min and 180 min [Table 6].
Table 6.
Between-group comparison of systolic blood pressure at different time intervals
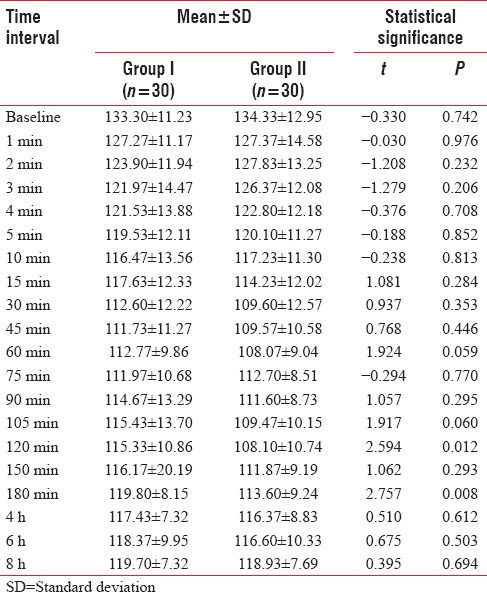
At baseline, diastolic blood pressure of Group I (85.20 + 9.01 mm Hg) was found to be higher than that of Group II (84.47 + 9.43 mm Hg). At all time periods of observation except at 2 min, 3 min, 4 min, 75 min, 90 min, and 4 h, diastolic blood pressure of Group I was found to be higher than that of Group II. Difference in diastolic blood pressure of above two groups was not found to be statistically significant at any of the periods of observation [Table 7].
Table 7.
Between-group comparison of diastolic blood pressure at different time intervals
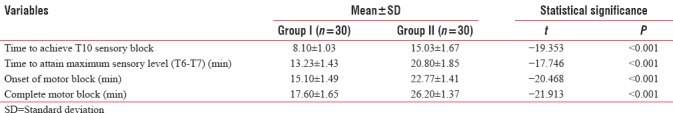
Mean duration to achieve T10 sensory block in Group I (8.10 + 1.03 min) was early than that in Group II (15.03 + 1.67 min); difference in mean duration of achieving T10 sensory block was found to be statistically highly significant (P < 0.001). Time to attain T6/T7 (maximum sensory level) in Group I (13.23 + 1.43 min) was statistically significantly earlier (P < 0.001) than that of Group II (20.80 + 1.85 min).
Mean duration of onset of motor block in Group I (15.10 + 1.49 min) was statistically significantly earlier (P < 0.001) than that of Group II (22.77 + 1.41 min). Complete motor block was achieved statistically significantly earlier (P < 0.001) in Group I (17.60 + 1.65 min) as compared to Group II (26.20 + 1.37 min) [Table 8].
Table 8.
Between-group comparison of analgesic characteristics
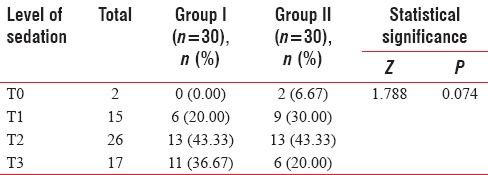
Higher levels of sedation (T0-T1) were achieved by higher proportion of patients of Group II as compared to Group I [Table 9].
Table 9.
Between-group comparison of maximum sedation achieved by study population (Mann-Whitney U-test)
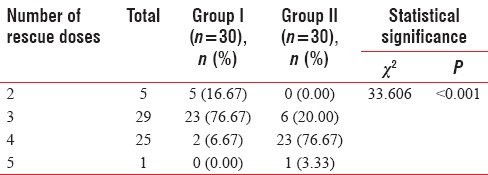
Majority of patients of Group I required 2–3 rescue doses (93.33%) during the surgery while majority of patients of Group II required 3–4 rescue doses (80.0%). Difference in requirement of rescue doses by Group I and Group II was found to be statistically significant (P < 0.001) [Table 10].
Table 10.
Between-group comparison of number of rescue doses required by study population
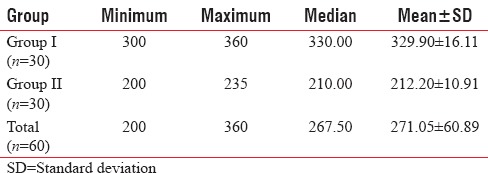
The first analgesic requirement in Group II was earlier (212.20 + 10.91 min) as compared to Group I (329.90 + 16.11 min). Difference in time of first analgesic requirement between the above two groups was found to be statistically significant (P < 0.001) [Table 11].
Table 11.
Between-group comparison of time of first dose of analgesic agent (min)
DISCUSSION
Lower abdominal and lower limb surgeries may be performed under local, regional (spinal or epidural), or general anesthesia, but neuraxial blockade is the preferred mode of anesthesia.
In recent years, use of adjuvants during epidural anesthesia has gained popularity with the aim of prolonging the duration of block, better success rate, patient satisfaction, decreased resource utilization compared with general anesthesia, and faster recovery.
The common problem during infraumblical and lower abdominal surgeries under regional anesthesia is visceral pain, nausea, and vomiting.[5] The addition of fentanyl to hyperbaric bupivacaine improves the quality of intraoperative and early postoperative block.[6] The addition of opioid provides a dose-sparing effect of local anesthetic and superior analgesia, but there is always a possibility of an increased incidence of pruritus, urinary retention, nausea, vomiting, and respiratory depression.[7,8]
Dexmedetomidine is a new addition to the class of α2 agonist which has got numerous beneficial effects when used through epidural route.[9] It acts on both pre- and post-synaptic sympathetic nerve terminal and central nervous system, thereby decreasing the sympathetic outflow and norepinephrine release causing sedative, antianxiety, analgesic, sympatholytic, and hemodynamic effects.[10,11] Dexmedetomidine causes a manageable hypotension and bradycardia, but the striking feature of this drug is the lack of opioid-related side effects such as respiratory depression, pruritus, nausea, and vomiting.[12,13] Both dexmedetomidine (α2 agonist) and fentanyl (opioid) are being used as adjunct with bupivacaine to increase duration of regional anesthesia.
Multimodal analgesia is a pharmacologic method of pain management which combines various groups of medications for pain relief such as local anesthetics, opioids, NSAIDs, and α2 agonists. It has been seen that dexmedetomidine has been used successfully as part of a multimodal analgesic plan and can be an alternative choice for opioid-tolerant patients.[14]
With this background, the present study was carried out with an aim to evaluate the efficacy of epidural dexmedetomidine with bupivacaine versus epidural fentanyl with bupivacaine for postoperative pain relief.
For this purpose, a randomized controlled trial was carried out in which a total of 60 patients with ASA physical status classes I and II scheduled to undergo elective lower limb orthopedic surgery aged 20–50 years were enrolled in the study and were randomly allocated to one of the two study groups – Group I (n = 30) received 15 ml of 0.5% bupivacaine with 1 μg/kg injection dexmedetomidine epidurally and Group II (n = 30) received 15 ml of 0.5% bupivacaine with 1 μg/kg of injection fentanyl epidurally.
In the present study, age of patients ranged from 20 to 50 years. Maximum number of patients presented in 21–30 years of age (41.7%). Mean age of patients was 34.43 ± 9.50 years. Lower limb injuries are generally caused by trauma, fall from height, combat, road traffic accidents, and sporting injuries – mainly involving active outdoor life which is reflective in most active years of life.
As such age and gender profile of two groups was matched and did not show a significant difference between two groups, thus indicating that the two groups were matched.
At baseline, the hemodynamic parameters were within normal limits and matched statistically between the two groups. Thus, the two study groups showed a statistical matching for all the demographic, clinical, and hemodynamic parameters and did not show the presence of any confounding factor.
The two groups were found to have no hemodynamic difference throughout the study except for pulse rate at 3 min interval when mean value in fentanyl group was higher as compared to that in dexmedetomidine group. Mean % change from baseline ranged from 2.17% (2 min) to 19.24% (90 min) in dexmedetomidine group and from 3.46% (1 min) to 26.32% (105 min) in fentanyl group. Thus, change in pulse rate was higher in fentanyl group as compared to that in dexmedetomidine group. Compared to the present study, Prakash et al.[15] also found significant difference in HR among placebo, fentanyl, and dexmedetomidine group with dexmedetomidine group having minimum mean values as compared to other two groups. The difference between two studies could be owing to difference in drug interactions owing to different combinations of drugs being used. In the present study, 0.5% bupivacaine was used as the primary drug with 1 μg/kg dexmedetomidine or 1 μg/kg fentanyl, respectively, whereas Prakash et al.[15] used 0.25% bupivacaine as the principal drug with same dose of dexmedetomidine or fentanyl as the adjuvants.
Group I (n = 30) received 15 ml of 0.5% bupivacaine with 1 μg/kg injection dexmedetomidine epidurally, while Group II (n = 30) received 15 ml of 0.5% bupivacaine with 1 μg/kg of injection fentanyl epidurally. Similar to the present study, Prakash et al.[15] in their study also found mean HR to be lower in fentanyl group as compared to that in fentanyl group throughout the study period yet did not find a statistically significant difference between two groups at any of the follow-up evaluations.
In the present study, SBP was found to be matched statistically between two groups, except at 120 and 180 min follow-up intervals when mean value was significantly lower in fentanyl as compared to dexmedetomidine group. During the entire study period, dexmedetomidine group showed a reduction ranging from 4.53% (1 min) to 16.18% (45 min) whereas fentanyl group showed a reduction ranging from 4.84% (2 min) to 19.55% (60 min). For diastolic blood pressure, the difference between two groups was not found to be significant statistically at any of the time intervals. At all the follow-up intervals, mean values were lower as compared to baseline. Mean % reduction ranged from 3.95% (1 min) to 15.92% (120 min) in dexmedetomidine group whereas the same ranged from 3.99% (1 min) to 16.46% (60 min). Similar observations were observed for mean arterial pressure. At none of the time intervals, the reduction in blood pressure exceeded 20% cutoff level to be classified as hypotension. In the present study, hypotension was noticed as an adverse hemodynamic outcome in only 1 (3.33%) of fentanyl group patient whereas in dexmedetomidine group no hemodynamic side effect was noticed at the given dose combination. Hemodynamic events such as hypotension or bradycardia have rarely been reported in different studies reviewed by us. In a study by Akin et al.,[16] mean HR, respiratory depression, hypotension, oversedation, hypoxia, and hypercapnia decreased significantly in the dexmedetomidine group.
The findings in the present study in general are in accordance with the findings in literature. Gupta et al.[17] in their study found dexmedetomidine to offer a better hemodynamic stability as compared to fentanyl in their study which is similar to the findings made in present study. Hanoura et al.[18] in their study also indicated statistically no significant difference between fentanyl and dexmedetomidine groups with respect to hemodynamic stability. Mahendru et al.[19] also found that adjuvant dexmedetomidine provided a better hemodynamic stability as compared to fentanyl when used as adjuvant to epidural bupivacaine. In the study of Gupta et al.,[20] who used 0.5% levobupivacaine in combination with 25 μg dexmedetomidine as compared to 0.5% levobupivacaine in combination with 50 μg fentanyl found both the groups comparable for hemodynamics. Kaur et al.[21] also found both fentanyl as well dexmedetomidine to be comparable when used in combination with 0.75% ropivacaine. Prakash et al.,[15] Dilesh et al.,[22] Karhade et al.,[23] and Soliman and Eltaweel[24] also made similar observations. As such none of the studies has reported any hemodynamic side effect resulting into any hemodynamic emergency.
In the present study, duration of surgery ranged from 90 to 120 min. The mean duration was 107.55 ± 8.29 min. There was no statistically significant difference between two groups with respect to duration of surgery.
In the present study, onset of sensory block was considered to be at T10. The sensory block was achieved in 8.10 ± 1.03 min in dexmedetomidine as compared to 15.03 ± 1.67 min in fentanyl group, thus showing that onset of sensory block was earlier in dexmedetomidine group as compared to fentanyl group. The sensory onset time for adjuvant use of dexmedetomidine and fentanyl in combination with bupivacaine has been shown to vary in different studies [Table 12].
Table 12.
Duration of sensory block onset as seen in different series evaluating adjuvant use of dexmedetomidine and fentanyl (min)
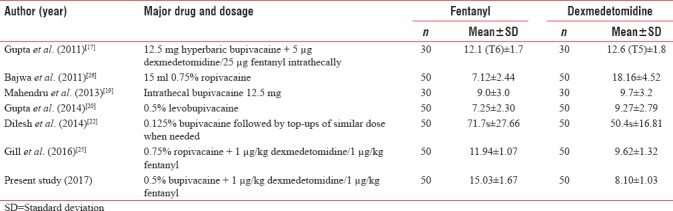
Table 12 shows a variable onset time for different dose/route combinations. In studies using bupivacaine as the major drug, the onset time has been reported to be comparable by Gupta et al.[17] and Mahendru et al.[19] In another study using a low concentration startup dose of bupivacaine, Dilesh et al.[22] also found the fentanyl to consume shorter onset time as compared to dexmedetomidine. In the present study using dexmedetomidine and fentanyl in combination with 0.5% bupivacaine, the onset time was higher in fentanyl group (15.03 ± 1.67 min) as compared to dexmedetomidine group (8.10 ± 1.03 min).
In the present study, in both the groups, maximum block level achieved was T6-T7. Mean time taken to achieve maximum block level was 13.23 ± 1.43 min in dexmedetomidine group and 20.80 ± 1.85 min in fentanyl group. This difference was statistically significant. In different studies, this time interval has been shown to vary substantially. In the series of Gupta et al.,[17] maximum block level achieved was higher in dexmedetomidine group (T5) as compared to fentanyl group (T6) and time taken to achieve the maximum block level was 12.6 min in dexmedetomidine group and 12.1 min in fentanyl group. Mahendru et al.[19] in their study reported time taken to achieve maximum block level as 9.6 min in fentanyl and 10.3 min in dexmedetomidine group and did not report a significant difference between two groups. Dilesh et al.[22] reported 246.6 s in dexmedetomidine group and 160.8 s in Fetanyl group. Gill et al. (2016)[25] in their study, similar to our study, also reported mean time taken to achieve maximum sensory block level to be shorter in dexmedetomidine group (15.04 min) as compared to that in fentanyl group (16.68 min) and thus showing a significant difference between two groups. Gupta et al.[20] in their study reported this time to be 21.37 min in dexmedetomidine and 27.7 min in fentanyl group. The differences in direction and magnitude of time taken to achieve maximum sensory block level in different studies seem to be dependent on various drug-dosage interactions.
In the present study with respect to motor block too, time of onset and time taken to achieve complete motor block were shorter in dexmedetomidine group as compared to that in fentanyl group, and this difference was significant statistically too. Table 13 shows a comparative assessment of time of onset and time taken to achieve complete motor block level in different contemporary series comparing adjuvant use of dexmedetomidine and fentanyl [Table 13].
Table 13.
Motor block onset time and time taken to achieve complete block as seen in different series evaluating adjuvant use of dexmedetomidine and fentanyl (min)

With respect to onset time, Gupta et al.[17] and Mahendru et al.[19] reported the onset time to be shorter in fentanyl group; however, Gill et al.[25] reported it to be shorter in dexmedetomidine group as compared to fentanyl group. In the present study, the difference in onset time of motor block was quite distinct between two groups showing a difference of 7.67 min between two groups with an earlier block in dexmedetomidine as compared to fentanyl group. With respect to time taken to achieve complete/maximum motor block, all the three studies whose records on this aspect were available[20,25,26] reported it to be shorter in dexmedetomidine group as compared to fentanyl group. The findings of the present study endorse these observations. The probable mechanism may be the intrinsic ability to block conduction in C and A δ fibers by stimulation of α2 receptors will increase the intensity of conduction block of local anesthetic agents.[27]
In the present study, no significant difference between two groups was observed with respect to maximum sedation level achieved; however, proportion of patients showing T3 score was higher in dexmedetomidine group (33.3%) as compared to fentanyl group (20%).
In the present study, rescue analgesic need was lower in dexmedetomidine group where only 6.7% patients required more than 3 rescue dosages in fentanyl group where 80% of patients required more than 3 rescue analgesic dosages. Mean time taken for first rescue analgesic dose was also longer in dexmedetomidine as compared to fentanyl group. Bajwa et al.[26] and Gupta et al.[20] did not record total number of analgesic dosages as the outcome yet they reported the time for first analgesic need to be longer in dexmedetomidine group as compared to fentanyl group. In another study, Gill et al. (2016)[25] also recorded lower 24 h analgesic need in dexmedetomidine as compared to fentanyl group. Similar observations were also made by Gupta et al.[17] Thus, as far as analgesic effect is concerned, the findings in the present study are in accordance with the empirical observations.
The findings in the present study showed that dexmedetomidine is a safe, complication-free, and hemodynamically stable drug that can be used as adjuvant in epidural anesthesia with similar safety profile as for fentanyl but with a relatively better analgesic profile. The findings showed that with the drug-dose schedule used in the present study, the side effects were well under control, and hemodynamic stability was maintained for both the adjuvants. Further comparative studies with other opioids and β2-adrenergic drugs are also recommended to find out the optimum drug-dose combination with a better and safe analgesic profile.
CONCLUSIONS
The present study was conducted to study the efficacy of epidural dexmedetomidine with bupivacaine versus epidural fentanyl with bupivacaine for postoperative pain relief in lower limb orthopedic surgery. We have seen that the time to achieve T10 sensory block was early in Group I (dexmedetomidine) (8.10 + 1.03 min) as compared to Group II (15.03 + 1.67 min). Onset of motor was earlier in Group I (15.10 + 1.49 min) as compared to Group II (22.77 + 1.41 min), and complete motor block too was earlier in Group I (17.60 + 1.65 min) as compared to Group II (26.20 + 1.37 min). In Group I (dexmedetomidine), majority of patients required 2–3 rescue doses only; while in Group II (fentanyl), majority of patients required 3–4 rescue doses. Difference in requirement of rescue doses among patients of both the groups was found to be statistically significant. Considering these findings, we can say that dexmedetomidine has much more efficacy than fentanyl when given epidurally for postoperative pain relief.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 2.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103:1296–304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- 3.McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N, et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: A systematic review. Health Technol Assess. 2010;14:1. doi: 10.3310/hta14170. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87:47–61. doi: 10.1093/bja/87.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Alahuhta S, Kangas-Saarela T, Hollmén AI, Edström HH. Visceral pain during caesarean section under spinal and epidural anaesthesia with bupivacaine. Acta Anaesthesiol Scand. 1990;34:95–8. doi: 10.1111/j.1399-6576.1990.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535–40. doi: 10.1097/00000542-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Salomäki TE, Laitinen JO, Nuutinen LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after thoracotomy. Anesthesiology. 1991;75:790–5. doi: 10.1097/00000542-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzini C, Moreira LB, Ferreira MB. Efficacy of ropivacaine compared with ropivacaine plus sufentanil for postoperative analgesia after major knee surgery. Anaesthesia. 2002;57:424–8. doi: 10.1046/j.0003-2409.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- 9.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 11.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the Intensive Care Unit. Crit Care Clin. 2001;17:881–97. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 12.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloor BC, Abdul-Rasool I, Temp J, Jenkins S, Valcke C, Ward DS, et al. The effects of medetomidine, an alpha 2-adrenergic agonist, on ventilatory drive in the dog. Acta Vet Scand Suppl. 1989;85:65–70. [PubMed] [Google Scholar]
- 14.Patch Iii RK, Eldrige JS, Moeschler SM, Pingree MJ. Dexmedetomidine as part of a multimodal analgesic treatment regimen for opioid induced hyperalgesia in a patient with significant opioid tolerance. Case Rep Anesthesiol. 2017;2017:9876306. doi: 10.1155/2017/9876306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash R, Kushwaha BB, Shashi B, Bhatia VK, Chandra G, Singh BP. A comparative study of bupivacaine 0.25% alone and with fentanyl or dexmedetomidine for percutaneous nephrolithotomy (pcnl) under epidural anaesthesia. Indian J Sci Res. 2014;5:39–46. [Google Scholar]
- 16.Akin S, Aribogan A, Arslan G. Dexmedetomidine as an adjunct to epidural analgesia after abdominal surgery in elderly intensive care patients: A prospective, double-blind, clinical trial. Curr Ther Res Clin Exp. 2008;69:16–28. doi: 10.1016/j.curtheres.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK, et al. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanoura SE, Hassanin R, Singh R. Intraoperative conditions and quality of postoperative analgesia after adding dexmedetomidine to epidural bupivacaine and fentanyl in elective cesarean section using combined spinal-epidural anesthesia. Anesth Essays Res. 2013;7:168–72. doi: 10.4103/0259-1162.118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R, et al. Acomparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: A double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013;29:496–502. doi: 10.4103/0970-9185.119151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta K, Rastogi B, Gupta PK, Jain M, Gupta S, Mangla D. Epidural 0.5% levobupivacaine with dexmedetomidine versus fentanyl for vaginal hysterectomy: A prospective study. Indian J Pain. 2014;28:149–54. [Google Scholar]
- 21.Kaur S, Attri JP, Kaur G, Singh TP. Comparative evaluation of ropivacaine versus dexmedetomidine and ropivacaine in epidural anesthesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2014;8:463–9. doi: 10.4103/1658-354X.140838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilesh PK, Eapen S, Kiran S, Chopra V. A comparison of intrathecal dexmedetomidine verses intrathecal fentanyl with epidural bupivacaine for combined spinal epidural labor analgesia. J Obstet Anaesth Crit Care. 2014;4:69–74. [Google Scholar]
- 23.Karhade SS, Acharya SA, Harnagale K. Comparative analysis of epidural bupivacaine versus bupivacaine with dexmedetomidine for vaginal hysterectomy. Anesth Essays Res. 2015;9:310–3. doi: 10.4103/0259-1162.158007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman R, Eltaweel M. Comparative study of dexmedetomidine and fentanyl as an adjuvant to epidural bupivacaine for postoperative pain relief in adult patients undergoing total knee replacement: A randomized study. J Anesthesiol Clin Sci. 2016;5:1–7. [Google Scholar]
- 25.Gill RS, Acharya G, Rana A, Arora KK, Kumar D, Sonkaria LK. Comparative evaluation of addition of fentanyl and dexmedetomidine to ropivacaine for epidural anaesthesia and analgesia in lower abdominal and lower limb orthopedic surgeries. EJPMR. 2016;3:200–5. [Google Scholar]
- 26.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5:365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joy R, Pujari VS, Chadalawada MV, Cheruvathoor AV, Bevinguddaiah Y, Sheshagiri N, et al. Epidural ropivacaine with dexmedetomidine reduces propofol requirement based on bispectral index in patients undergoing lower extremity and abdominal surgeries. Anesth Essays Res. 2016;10:45–9. doi: 10.4103/0259-1162.164676. [DOI] [PMC free article] [PubMed] [Google Scholar]


