Abstract
Background and Aims:
Ambulatory surgery is continually evolving specialty in the majority of surgical procedures. Dexmedetomidine and midazolam are newer adjuvants for sedation and reducing the dose of anesthetic agents. The aim of this study was to compare the sedative and propofol-sparing effect of dexmedetomidine and midazolam in minor gynecological day care surgeries. Observer's Assessment of Activity and Sedation, dose of additional propofol, Aldrete and street fitness score were studied as primary outcomes. Hemodynamic parameters and side effects were evaluated as secondary outcomes.
Materials and Methods:
A prospective randomized placebo-controlled study was conducted on 150 American Society of Anesthesiologists ASA physical status Classes I and II gynecological patients between 18 and 50 years and were allocated into three groups of fifty each. Group A received intravenous (i.v.) dexmedetomidine 0.1 μg/kg, Group B received i.v. midazolam 0.04 mg/kg, and Group C received normal saline 10 min before induction.
Results:
Sedation score was statistically highly significant between Group A and B (P < 0.001). Between Group A and C, it was statistically significant (P < 0.05); however, score was nonsignificant between Groups B and C (P > 0.05). During recovery at 120 min after surgery, score 5 was achieved equally by all three groups which was found to be statistically insignificant (P > 0.05). Mean dose of additional propofol used was less in Group A (14 ± 9.25) than B (25 ± 5.40) and C (53 ± 10.96). On intergroup comparison between all three groups, it was found to be statistically highly significant (P < 0.001). Comparison of bispectral index (BIS) values between Groups A and C and Groups B and C were highly significant (P < 0.001). However, it was statistically significant between Groups A and B (P < 0.05). Aldrete scoring and street fitness scores were highly significant between Groups A and B, B and C, and also between Groups A and C (P < 0.001). No significant hemodynamic derangements and side effects were noted in any of three groups.
Conclusion:
Dexmedetomidine had good sedation and better recovery characteristics than midazolam. BIS monitoring was helpful in maintaining the depth of anesthesia.
Keywords: Day care surgery, dexmedetomidine, midazolam, propofol
INTRODUCTION
International Association of Ambulatory Surgery defines day care surgery as an operation or procedure, in office or outpatient, when the patient is discharged on the same working day.[1] Concept of balanced anesthesia is based on the concurrent administration of combination of several anesthetic drugs together to produce desired effect.[2]
Due to pharmacological properties and speedy recovery profile, propofol is universally used induction agent. Despite its favorable profile, higher doses may be required which can cause adverse cardiorespiratory effects.[3] With addition of adjuvants, requirement of propofol can be reduced.[4] The a2 adrenoceptor agonists, such as dexmedetomidine, are known to possess amnesic, analgesic, sympatholytic, and antinociceptive properties.[5] Midazolam is also drug of choice and well-known for its anxiolytic and sedative properties.[6]
Bispectral index (BIS) monitoring is measure of anesthetic depth through analysis of electrocortical activity.[7] It integrates the frequency-domain, time-domain, and bispectral analysis of raw electroencephalography (EEG) signals into a numerical value, ranging from 0 (isoelectric EEG) to 100 (fully awake).[8] BIS value of 40–60 is preferred for surgical procedures.[9] In the present study, we have employed BIS monitoring to compare sedation and propofol-sparing effects.
MATERIALS AND METHODS
The study was conducted after informed written consent from patients and after being approved by institute ethical committee. 150 patients American Society of Anesthesiologists (ASA) physical status Classes I and II, aged 18–50 years undergoing gynecological day care surgeries were enrolled in the study and were divided into three groups of 50 each randomly by labeled sealed envelope approach. Keeping precision of estimates of outcome statistics as 95% confidence limits, and on the basis of previously published studies, the sample size was taken as 50 per group. Patients with hepatic, renal or cardiovascular dysfunction, epilepsy, pregnancy, drug allergies, patients on sedatives and on α adrenergic receptor blockers were excluded from the study.
Preoperative assessment including general, systemic examination, and routine laboratory investigations were done. In operating room, standard monitoring was established and preoperative basal heart rate (HR), mean arterial pressure (MAP), and SpO2 were recorded. B was taken as baseline time value. Antecubital venous access was secured with 20 G intravenous (i.v.) cannula and normal saline started at 8 mL/kg/h. O2 supplementation was done by face mask at rate of 4 L/min. BIS electrodes were placed on the forehead. Study drug was prepared.
Group A (Dexmedetomidine group) received dexmedetomidine 1 μg/kg (50 μg/mL) plus normal saline made to 10 ml as i.v. bolus slowly.
Group B (Midazolam group) received midazolam 0.04 mg/kg plus normal saline made to 10 ml as i.v. bolus slowly.
Group C (Normal saline group) received i.v. normal saline bolus 10 ml.
At 7 min after giving study drug, Observer's Assessment of Activity and Sedation (OAA/S) was noted [Table 1].
Table 1.
Observer's Assessment of Activity and Sedation Scale (OAA/S)

After assessing level of sedation, then injection glycopyrrolate 0.02 mg/kg and tramadol 2 mg/kg body weight were given to all patients and propofol was given in variable boluses starting 2 mg/kg body weight and then titrated till abolition of response to verbal commands, and BIS score of 40–60 is reached. O2 was supplemented by face mask at rate of 4 L/min. During the procedure, if BIS score >60, then further top up increment dose of 10 mg propofol was given. Total dose of additional propofol used was recorded.
During procedure, any complications such as apnea, O2 desaturation, bradycardia, hypotension, nausea, and vomiting were noted and thus managed accordingly. Patients were assessed for complete recovery with end points being BIS score >90, eye opening on command, ability to handle secretions, obeying commands, sustained head lift for 5 s, hemodynamic stability, and maintaining room air saturation >95%. Recovery characteristics were noted using Aldrete Score, Street Fitness Score, and OAA/S. In recovery, parameters were taken first for every 5 min for 30 min and then for every 30 min for 2 h. On achieving Aldrete score of >9, street fitness score was assessed. Postoperative analgesia was given i/m diclofenac sodium 75 mg when VAS score >4. When street fitness score >8 was achieved, patients were shifted to ward.
Aldrete score: Total score is 14. Grade of recovery – Good >10, Fair 6–10, Bad <6
Street fitness: Grading used at time of recovery to ward. Total score was 10. Grading: Good 8–10, Fair 5–7, and bad <5. In recovery, patients were kept in propped up position with O2 supplementation, maintenance i.v. fluids at 2 ml/kg/h was given. Patients were observed for any adverse effects. In case of bradycardia (HR <50 bpm), injection atropine 0.6 mg i.v. was given and hypotension (MAP <20% of preinduction value) was managed with injection mephentermine 6 mg bolus, decrease in SpO2 below 92% was treated with supplemental O2, nausea and vomiting with ondansetron 4 mg i.v.
Statistical analysis was done with SSPS 17.0 software (SPSS Inc., 233 South Wacker Drive, Chicago, United States Of America). Chi-square test was applied for nonparametric data and ANOVA with post hoc Tukey HSD tests for parametric numerical data. Results were expressed as mean ± standard deviation (SD). P < 0.05 was considered significant and <0.001 as highly significant. Power of our study was 100% for dose of propofol with an α error −0.05.
RESULTS
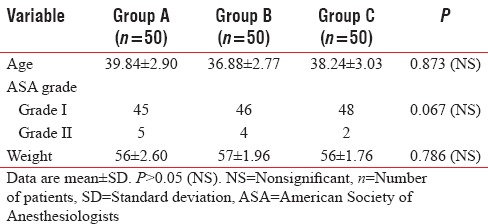
Demographic data including age, weight, and ASA grading were comparable among all three groups (P > 0.05) [Table 2].
Table 2.
Demographic data
Sedation using OAA/S score was same at baseline. After giving study drug at 7 min, score 2 was achieved earliest by Group A than B which was statistically highly significant (P < 0.001). Between Groups A and C, it was statistically significant (P < 0.05); however, score was comparable between Groups B and C (P > 0.05) [Figure 1]. Fall in BIS values after giving study drug was more in Group A in comparison to Groups B and C, which was statistically significant (P < 0.001). Groups A and B had significantly (P < 0.001) lower BIS values compared to Group C till 25 min and nonsignificant thereafter [Figure 2]. During recovery, score 5 achieved equally by all three groups which was found to be statistically insignificant (P > 0.05) [Figure 3].
Figure 1.
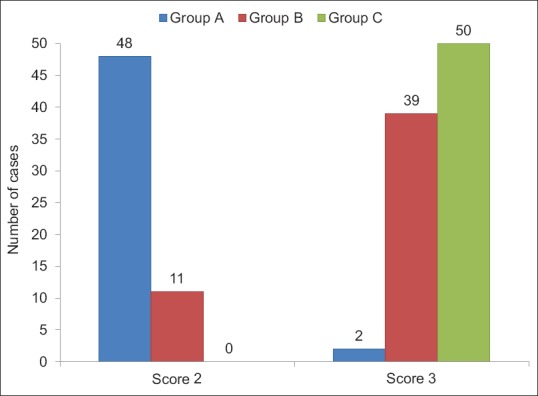
Distribution of cases according to Observer's Assessment of Activity and Sedation score at 7 min
Figure 2.
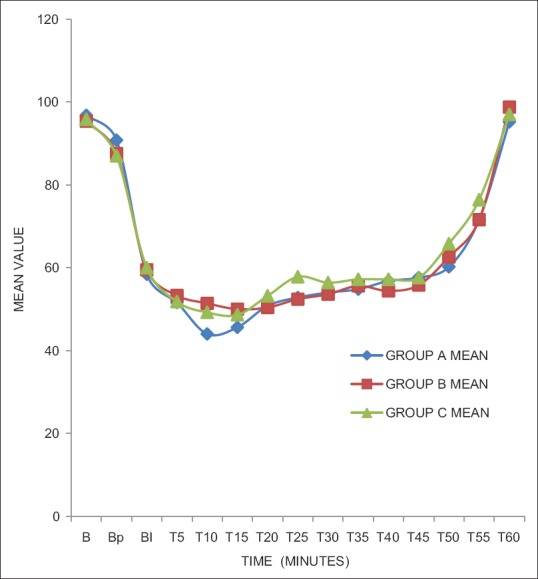
Bispectral index at different time intervals
Figure 3.
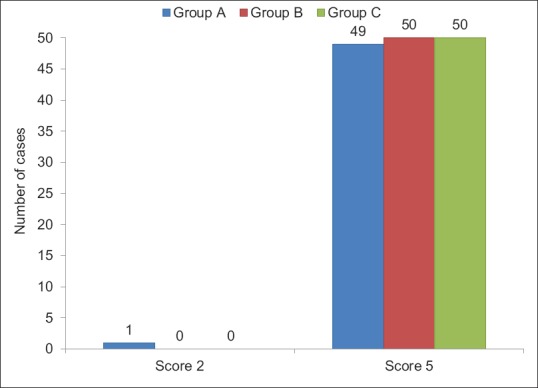
Distribution of cases according to Observer's Assessment of Activity and Sedation score at recovery
Mean dose of additional propofol (mg) used was less in Group A (14 ± 9.25) than B (25 ± 5.40) and C (53 ± 10.96). On intergroup comparison between all three groups, it was found to be statistically highly significant (P < 0.001) [Figure 4 and Table 3].
Figure 4.
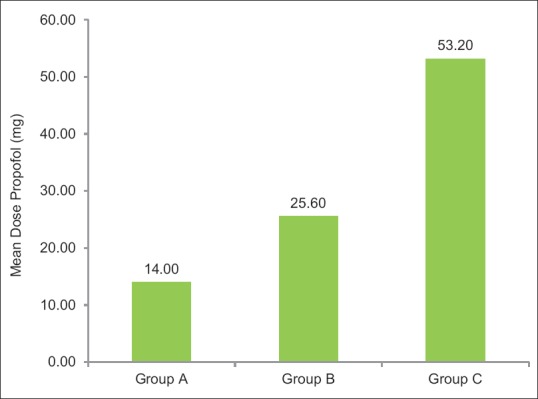
Mean additional propofol (mg) in three groups
Table 3.
Perioperative outcome
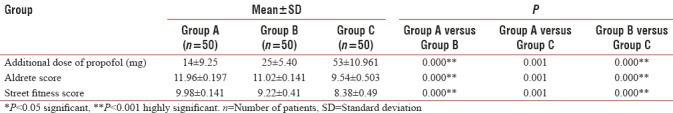
Aldrete score was achieved highest in Group A (11.96 ± 0.197) than Group B (11.02 ± 0.141) and Group C (9.54 ± 0.503). Comparison of Aldrete score was highly significant between Groups A and B, B and C, and also between Groups A and C (P < 0.001) [Table 3].
Street fitness score achieved highest in Group A (9.98 ± 0.141) than B (9.22 ± 0.41) and C (8.38 ± 0.49). However, on comparison between Group A with B, Group B and C, and also between A and C, it was statistically highly significant showing fastest recovery with dexmedetomidine (P < 0.001) [Table 3].
Mean baseline hemodynamic parameters were similar in three groups. Intraoperatively, there was decrease in HR, MAP more in Group A than Groups B and C which was found to be statistically significant (P < 0.05) but clinically insignificant. No ECG changes (arrhythmias) or SpO2 fluctuations were seen among any of three groups. Bradycardia was seen in total 12 patients out of which 5 patients were managed with injection atropine 0.5 mg, hypotension in 11 patients, out of which 9 patients were responsive to i.v. fluids and 2 patients were given injection mephentermine 6 mg and sedation was present in 10 patients. Nausea (12 patients) and vomiting (9 patients) were managed by injection ondansetron 4 mg.
DISCUSSION
Day care surgery represents high-quality patient care with excellent patient satisfaction. Patients endorse day care surgery, with small waiting times, less chances of cancellation, lower rates of infection, and preference of their own surroundings to convalesce.[10]
Essential goals are to provide adequate depth of anesthesia, to maintain hemodynamic stability, and to prevent awareness intraoperatively as well as early recovery. BIS monitor was used, and our goal was to titrate dose of propofol to achieve BIS range 40–60. By maintaining BIS values between 40 and 60, dose requirement of propofol was reduced. We incorporated the use of adjuvants as premedicants with BIS monitoring to reduce bolus dose of propofol in the present study. By maintaining BIS values between 40 and 60, dose requirement of hypnotic agents was reduced by 11%–27%.[11] Many studies have been done using adjuvants with continuous infusion of propofol, realizing need to reduce its dosage.[12]
Dexmedetomidine is useful and safe adjunct. Current uses include sedation in Intensive Care Unit, regional and general anesthesia, neurosurgery, sedation for pediatric procedures, awake fiberoptic intubation, and bariatric surgery. It attenuates but does not completely abolish stress-induced sympathoadrenal responses.[13,14]
Midazolam had rapid onset of action and high metabolic clearance. It produces reliable hypnosis, amnesia, and antianxiety effects. Uses for midazolam in perioperative period include premedication, anesthesia induction and maintenance, and sedation for diagnostic and therapeutic procedures.[15]
In our study, there is reduction in additional dose of propofol more in dexmedetomidine group (14 ± 9.25 mg) than midazolam (25 ± 5.4 mg) and placebo group (53 ± 10.9 mg). Similar results were observed in study conducted by Ong et al. in patients undergoing ERCP procedures.[16] Similar results seen by Ghodki et al.'s study in which there was reduction in additional dose of propofol during maintenance when dexmedetomidine was given along in laparoscopic surgeries.[17] Reduction in propofol requirement by dexmedetomidine is due to decreased neuronal activity and enhancement of vagal activity by activation of a2 receptors located in postsynaptic terminals in the central nervous system.[18,19] Midazolam exhibits propofol-sparing effect by its action on a1 and a5 subunits of gamma-aminobutyric acid A receptors.[20,21]
In our study, OAA/S (score 2) was achieved earliest by dexmedetomidine than midazolam group. Our results are in concordance with Arain and Ebert's study in which dexmedetomidine infusion resulted in more sedation than propofol alone.[22] However, the degree of sedation (OAA/S ≤3) was comparable in midazolam plus fentanyl and dexmedetomidine group along propofol infusion in Tomar et al.'s study.[23] Dexmedetomidine provides sedation without respiratory depression; producing sleep-like phenomena in EEG is by its action on locus coeruleus.[18]
BIS values were lowered in dexmedetomidine group compared to other two groups in our study. BIS-guided general anesthesia reduces exposure time and doses, causing reduction in neurotoxicity and expedites early recovery. Equivalent results were seen by Yongxin et al.'s study in gynecological surgery under epidural anesthesia.[24]
In the present study, recovery characteristics were seen using Aldrete score, and street fitness score was highest in dexmedetomidine (11.96 ± 0.19, 9.98 ± 0.1) compared to midazolam (11.02 ± 0.1, 9.22 ± 0.41) and placebo (9.54 ± 0.50, 8.38 ± 0.4), respectively. Characteristic arousable sedation action of dexmedetomidine is due to its action on a2 adrenoreceptors in locus coeruleus in brainstem where it decreases sympathetic and increases parasympathetic outflow. Tomar et al.'s study also showed similar results as our present study.[23]
In our study, there was significant reduction in HR, MAP in dexmedetomidine group (60/min and 70 mmHg) compared to midazolam (70/min and 90 mmHg). Hypotension and bradycardia could be explained by its action on central a2A receptors which decreased release of noradrenaline from sympathetic nervous system.[25] Midazolam causes reduction in systemic vascular resistance leading to reduction in hemodynamic parameters.[26] Alhashemi and Arain and Ebert's studies also supported the same, more in dexmedetomidine group.[22,27]
Side effects such as hypotension and bradycardia were managed accordingly. There was no additional requirement of analgesics in postoperative period. No case of apnea and O2 desaturation were observed in any of the patients among all three groups.
CONCLUSION
Dexmedetomidine and midazolam are useful anesthetic adjuvants that can be safely coadministrated with propofol to reduce its dosage using BIS monitoring. Dexmedetomidine provides more intense sedation along with sparing of propofol dose compared to midazolam. Recovery characteristics were excellent with dexmedetomidine than midazolam.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Clark JA, Mishler EG. Attending to patients' stories: Reframing the clinical task. Sociol Health Illn. 1992;14:344–72. [Google Scholar]
- 2.Lundy JS. Balanced anesthesia. Minn Med. 1926;9:394–9. [Google Scholar]
- 3.Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbri LP, Nucera M, Marsili M, Al Malyan M, Becchi C. Ketamine, propofol and low dose remifentanil versus propofol and remifentanil for ERCP outside the operating room: Is ketamine not only a “rescue drug”? Med Sci Monit. 2012;18:CR575–80. doi: 10.12659/MSM.883354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH. The effect of dexmedetomidine on adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol. 2012;62:113–8. doi: 10.4097/kjae.2012.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–24. [PubMed] [Google Scholar]
- 7.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Chan MT, Gin T. What does bispectral EEG index monitor? Eur J Anaesthesiol. 2000;17:146–8. doi: 10.1046/j.1365-2346.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 9.Ekman A, Lindholm ML, Lennmarken C, Sandin R. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand. 2004;48:20–6. doi: 10.1111/j.1399-6576.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 10.Daniel J. Day surgery development and practice. Br J Anaesth. 2014;14:256–61. [Google Scholar]
- 11.Liu SS. Effects of bispectral index monitoring on ambulatory anesthesia: A meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311–5. doi: 10.1097/00000542-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006;20:81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the Intensive Care Unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 14.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 15.Griffin CE, 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214–23. [PMC free article] [PubMed] [Google Scholar]
- 16.Ong WC, Santosh D, Lakhtakia S, Reddy DN. A randomized controlled trial on use of propofol alone versus propofol with midazolam, ketamine, and pentazocine “sedato-analgesic cocktail” for sedation during ERCP. Endoscopy. 2007;39:807–12. doi: 10.1055/s-2007-966725. [DOI] [PubMed] [Google Scholar]
- 17.Ghodki PS, Thombre SK, Sardesai SP, Harnagle KD. Dexmedetomidine as an anesthetic adjuvant in laparoscopic surgery: An observational study using entropy monitoring. J Anaesthesiol Clin Pharmacol. 2012;28:334–8. doi: 10.4103/0970-9185.98329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–5. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 20.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 21.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA (A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–92. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 22.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 23.Tomar GS, Singh F, Ganguly S, Gaur N. Is dexmedetomidine better than propofol and fentanyl combination in minor day care procedures? A prospective randomised double-blind study. Indian J Anaesth. 2015;59:359–64. doi: 10.4103/0019-5049.158740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yongxin L, Miaoning G, Shiduan W, Haichen C. A comparison of dexmedetomidine and midazolam for sedation in gynaecologic surgery under epidural anesthesia. J Curr Surg. 2011;1:12–8. [Google Scholar]
- 25.Afsani N. Clinical application of dexmedetomidine. S Afr J Anaesthesiol Analg. 2010;16:50–6. [Google Scholar]
- 26.Adams P, Gelman S, Reves JG, Greenblatt DJ, Alvis JM, Bradley E, et al. Midazolam pharmacodynamics and pharmacokinetics during acute hypovolemia. Anesthesiology. 1985;63:140–6. doi: 10.1097/00000542-198508000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Alhashemi JA. Dexmedetomidine vs. midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]


