Abstract
Introduction:
Ropivacaine is preferred over racemic bupivacaine for postoperative analgesia as it is less cardiotoxic and has high selectivity for sensory fibers. We aim to compare postoperative epidural analgesia using 0.2% bupivacaine and 0.2% ropivacaine in major lower limb orthopedic surgery.
Materials and Methods:
In a prospective, randomized, double-blind study, 100 patients, aged 18–70 years, undergoing elective major lower limb orthopedic surgery under spinal anesthesia, were randomly allocated to receive either 7 ml ropivacaine 0.2% (Group R) or 7 ml bupivacaine 0.2% (Group B) for postoperative analgesia through a lumbar epidural catheter. The onset and duration of epidural analgesia, total epidural dose requirement, mean number of epidural topup, rescue analgesia, incidence of motor blockade, and adverse effects were recorded.
Results:
No differences were noted in demographic data and hemodynamic variables in either group. The onset time of epidural analgesia was 10.46 min ± 0.68 (Group B) and 10.52 min ± 0.71 (Group R). The duration of analgesia was 253.10 ± 17.46 min (Group B) and 251.80 ± 15.77 min (Group R). The total analgesic dose requirement was 78.40 mg ± 6.93 in Group B while in Group R, it was 78.96 mg ± 6.79. Epidural topup requirement and the need for rescue analgesia were similar in both the groups. Motor blockade, hypotension, and nausea were noted more in Group B compared to Group R.
Conclusion:
In patients undergoing major lower limb orthopedic surgery under subarachnoid block, epidural ropivacaine 0.2% produces effective postoperative analgesia similar to bupivacaine 0.2% with a distinct sensory-motor dissociation resulting in analgesia without motor blockade.
Keywords: Bupivacaine, epidural analgesia, postoperative pain, ropivacaine
INTRODUCTION
International Association for the Study of Pain defines PAIN as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”[1] The most important concern of a patient preoperatively is “postoperative pain.” It remains grossly underrated and untreated leading to high degree of patient dissatisfaction.
Postoperative pain, if not controlled, leads to detrimental acute and chronic effects. The nociceptive input to the central nervous system leads to activation of neuroendocrine stress response mainly involving hypothalamic–pituitary–adrenocortical and sympathoadrenal interactions. Sympathetic stimulation causes tachycardia, hypertension, and increased myocardial oxygen consumption. It decreases myocardial oxygen supply through coronary vasoconstriction leading to myocardial ischemia and infarction in vulnerable patients. There is increase in catabolic hormones and a decrease in the levels of anabolic hormones leading to sodium and water retention, increased blood glucose, free fatty acids, ketone bodies, and lactate levels. Hyperglycemia leads to poor wound healing and immunosuppression. Inadequate pain relief causes spinal reflex inhibition of phrenic nerve activity. Patients cannot breathe adequately, particularly with upper abdominal surgeries and are unable to cough out secretions. There is decrease in vital capacity, tidal volume, and functional residual capacity leading to pulmonary complications. Nausea and vomiting occur due to nociceptive impulses from viscera and somatic structures. Hypomotility of gastrointestinal tract and bladder leads to paralytic ileus and urinary retention. There is risk of deep vein thrombosis. Persistent noxious input can lead to rapid neuronal sensitization progressing to chronic pain states.
Among the many options available for the control of postoperative pain, analgesia delivered through an indwelling epidural catheter is a safe and effective method for the management of acute postoperative pain. It can provide analgesia superior to systemic opioids.[2] Apart from providing adequate pain relief, epidural local anesthetics promote reconvalescence by blunting autonomic and somatic reflexes to pain.
Ropivacaine is an amide type of local anesthetic. It differs from bupivacaine in that it is prepared as a pure S-enantiomer and it provides more differential sensory-motor block and has less central nervous system and cardiovascular toxicity. Based on this profile, it is beneficial in situ ations where motor block is undesirable and where there is a potential for high plasma concentrations of local anesthetics such as postoperative analgesia.
We propose to study postoperative pain relief with epidural ropivacaine in comparison with epidural bupivacaine in patients undergoing lower limb orthopedic surgery. This study would be a prospective randomized trial in patients who will be given postoperative epidural analgesia with 0.2% bupivacaine as control arm and 0.2% ropivacaine as the study arm. We aim to compare the onset and duration of analgesia, total epidural dose requirement in 24 h postoperatively, the incidence of motor blockade, and other adverse effects of intermittent epidural topup of 0.2% ropivacaine and 0.2% bupivacaine.
MATERIALS AND METHODS
After obtaining the hospital ethics committee approval, we studied 100 patients of either sex, aged 18–70 years, scheduled for elective major lower limb orthopedic surgery under spinal anesthesia. All patients, who belong to the American Society of Anesthesiologists (ASA) physical status Classes I and II, were enrolled for this prospective randomized, double-blinded study.
Patients were not included if they refused to participate in the study, a history of allergy to the study medications, a history of clotting or bleeding disorders, and infection at the site of lumbar puncture. Also excluded were pregnant patients, patients with preexisting neurological deficit, ASA physical status Classes III and IV, a history of psychiatric illness, and those with body mass index >35 kg/m2.
After obtaining written informed consent, all patients were examined and investigated a day before surgery. All patients were kept fasting 6 h before surgery. They were advised to take tablet alprazolam 0.5 mg and tablet ranitidine 150 mg the night before surgery. On the day of surgery, intravenous access was secured. The patients were preloaded with Ringer lactate solution 15 ml/kg half an hour before the procedure. The patients were shifted to operation theater, connected to multiparameter monitor. Patients were randomly allocated using sealed envelope technique into two groups:
Group R (study arm): Patients were given 7 ml of 0.2% ropivacaine for postoperative analgesia
Group B (control arm): Patients were given 7 ml of 0.2% bupivacaine for postoperative analgesia.
Fifty milliliters of 0.2% bupivacaine or 0.2% ropivacaine were prepared in identical bottles under all aseptic precautions by a senior anesthesiologist, who was not involved in the patient care or data collection. Neither the patient nor the anesthesiologist providing postoperative epidural analgesia knew the contents of the epidural solutions.
A pilot study was conducted with 5 patients in each group. They were given 5 ml of either 0.2% ropivacaine or 0.2% bupivacaine. We found that pain relief after 5 ml of epidural drug was not adequate. Addition of 2 ml of control or study drug resulted in effective pain relief. We conducted this study with 7 ml of epidural drug. The patients in the pilot study were not included in the study.
Under all aseptic precautions with the patient in sitting position, the skin over the 3rd or 2nd lumbar interspace was infiltrated with 1% lignocaine and the extradural space located with 16-gauge Tuohy needle using midline loss of resistance to saline injection. 16-gauge epidural catheter was inserted 3 cm in cephalad direction and then taped to the skin. After negative aspiration of blood and CSF, a test dose of 3 ml of 2% lignocaine with adrenaline (1:200000) was injected. Subarachnoid block was performed at L3–4 level with 25-gauge Quincke spinal needle with 3cc of 0.5% bupivacaine heavy. The patient was made to lie down supine immediately after the block. The anesthesiologist who is performing the block will record the intraoperative data (pulse rate, blood pressure, and oxygen saturation). Oxygen through nasal prongs at 2 L/min was kept for all the patients.
Postoperative recordings and pain assessment
Preoperatively, all patients were educated about grading of pain using a visual analog scale (VAS) pain score (0 cm – no pain and 10 cm – worst imaginable pain) and to request supplementary analgesics if needed. Patients who complained of pain with VAS score >3 were given 7 ml of either study or control drug according to randomization. If patients complained of pain after 15 min of epidural dose, 100 ml of paracetamol was given as rescue analgesic. Hemodynamic parameters such as pulse rate, mean arterial pressure, and oxygen saturation were monitored. The onset of analgesia was the time taken for the relief of pain with VAS score to become <2. The duration of postoperative analgesia was measured from the time of epidural drug injection to the next complaint of pain or VAS >3. Total epidural dose requirement and rescue analgesia required in 24 h was noted. The incidence of motor blockade was recorded after each epidural topup in nonoperated leg using a modified Bromage score.
Bromage 0: The patient can move the hip, knee, and ankle
Bromage 1: The patient is unable to move the hip but able to move the knee and ankle
Bromage 2: The patient is unable to move hip and knee but able to move the ankle
Bromage 3: The patient is unable to move hip, knee, and ankle.
Any adverse effects such as nausea, vomiting, bradycardia (heart rate <60 beats/min), respiratory depression (defined as respiratory rate <8 breath/min), hypotension (defined as fall in mean arterial pressure >20% of base line value), and other postoperative complications were recorded and treated accordingly.
Statistical analysis
The statistical analysis of the data was done using IBM Statistical Package for Social Sciences statistics software version 20.0 (IBM Corp., Chicago, USA). The data were expressed as either mean or standard deviation for quantitative data variables and as frequency and percentages for qualitative data variables. Unpaired t-test was used to compare the two groups for quantitative data variables, and Chi-square test was used for qualitative data variables. All statistical tests used at 5% level of confidence. P value less than 0.05 was considered statistically significant.
RESULTS
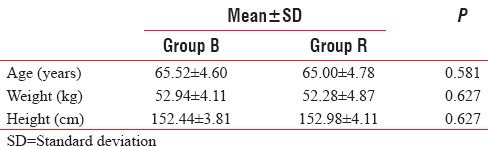
Patient demographic characteristics were comparable among the groups with respect to age, sex, weight, and height. There was no statistically significant difference (P > 0.05) [Table 1].
Table 1.
Demography
The heart rate and mean arterial pressure were measured at various intervals and were comparable among the groups [Tables 2 and 3].
Table 2.
Comparison of mean heart rate before and 30 min after each epidural topup in Group B and Group R
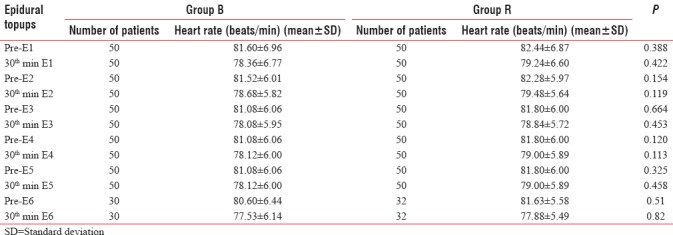
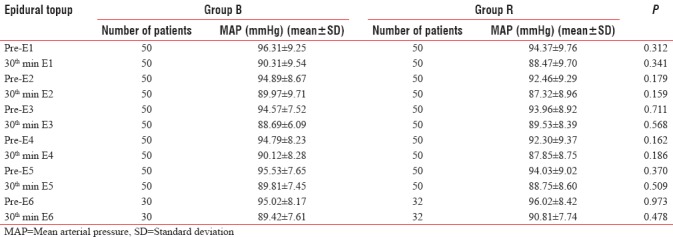
Table 3.
Comparison of mean arterial pressure before and 30 minutes after each epidural topup in Group B and Group R
Mean heart rate before and 30 min after epidural topup is comparable in both the groups. There was no statistically significant difference (P > 0.05) [Table 2 and Figure 1].
Figure 1.
Mean heart rate
Mean arterial pressure before and 30 min after epidural analgesia was comparable in both the groups. There was no statistically significant difference (P > 0.05) [Table 3 and Figure 2].
Figure 2.
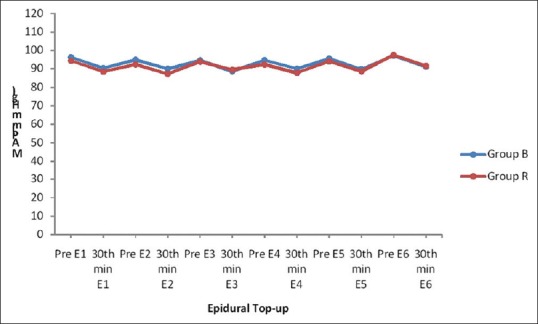
Mean arterial pressure
The mean onset time of epidural analgesia in Group B was 10.46 ± 0.68 min and in Group R was 10.52 ± 0.71 min, which was comparable in both the groups and was statistically not significant (P = 0.665). The duration of epidural analgesia was comparable (P = 0.697) in Group B and Group R. The duration of epidural analgesia was 253.10 ± 17.46 min in Group B and 251.80 ± 15.77 min in Group R [Table 4].
Table 4.
Comparison of mean onset time of epidural analgesia and duration of action of epidural analgesic dose in Group B and Group R
There was no statistically significant difference (P = 0.684) in the total epidural dose requirement and the mean number of epidural topups required for epidural analgesia in 24 h between Group B and Group R [Table 5].
Table 5.
Total epidural dose requirement and mean number of epidural topup in 24 h between Group B and Group R

Fifteen patients (30%) in Group B and 16 patients (32%) in Group R required rescue analgesia. There was no statistically significant difference (P = 0.829). This indicates comparable postoperative analgesia between both the groups [Figure 3].
Figure 3.
Requirement of rescue analgesia in Group B and Group R
Bromage 1 motor blockade was seen in 7 patients (14%) in Group B. No patients in Group R showed motor blockade. This is statistically significant (P = 0.012), indicating that bupivacaine resulted in significant motor blockade compared to ropivacaine when used for postoperative epidural analgesia [Table 6].
Table 6.
Comparison of motor blockade in Group B and Group R

In our study, 5 patients (10%) in bupivacaine group and 3 patients (6%) in ropivacaine group suffered from nausea. Hypotension was seen in 8 patients (16%) in bupivacaine group and 6 patients (12%) in ropivacaine group. No other adverse effects were observed. There was no statistically significant difference in the incidence of side effects between both groups [Figure 4].
Figure 4.
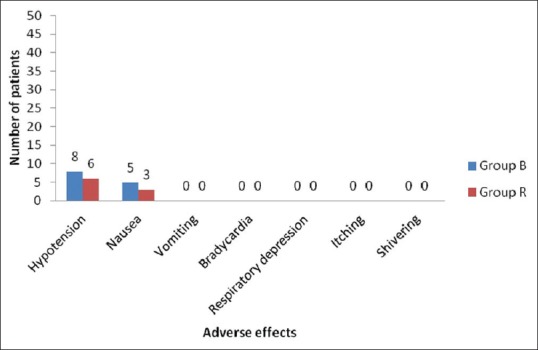
Comparison of adverse effects in Group B and Group R
DISCUSSION
The aim of postoperative analgesia is to provide subjective comfort with minimum side effects, to blunt autonomic and somatic reflex responses to pain, to allow early ambulation and restoration of function.
Among the aminoamide group, bupivacaine is the most commonly used local anesthetic drug. It has high protein binding and high lipid solubility. The high lipid solubility confers benefit by reducing absorption from the intended site of action. On the other hand, this property is not relevant if this site is bypassed and the drug is injected directly into the circulation. Local anesthetics exert their direct toxic effect on the heart by blocking sodium influx through sodium channels. This causes depression of the maximal rate of increase (Vmax) of the cardiac action potential and results in delayed conduction. When bupivacaine is bound to cardiac muscle, recovery from the block is slow. The sodium channels are blocked in a “fast-in, slow-out” manner, this makes resuscitation of patient difficult when ventricular fibrillation occurs. Ropivacaine has a favorable cardiotoxic profile as it depresses Vmax less than bupivacaine and recovery is quicker after ropivacaine.[3]
In this prospective, randomized, double-blind study, in patients scheduled for elective major lower limb orthopedic surgery, we have compared postoperative epidural analgesia between 0.2% bupivacaine and 0.2% ropivacaine. We have studied onset time, duration of action of epidural analgesia, total analgesic dose requirement, epidural topup requirement, postoperative rescue analgesia, motor blockade, hemodynamic response, and associated adverse effects such as hypotension, nausea, and vomiting.
The demographic profile in both the groups was comparable and statistically insignificant [Table 1]. The cardiovascular changes, i.e., mean heart rate and mean arterial pressure recorded before and 30 min after the epidural topup were comparable in both the groups [Tables 2 and 3]. Mean heart rate and mean arterial pressure after 30 min of epidural topup are lower than that at starting of epidural topup suggesting adequate analgesia [Figures 1 and 2].
The mean onset time of epidural analgesia in Group B was 10.46 ± 0.68 min and 10.52 ± 0.71 min in Group R, which is statistically insignificant (P = 0.665) [Table 4]. Our results can be compared with McCrae et al.[4] and Brockway et al.[5] Brockway et al. compared different concentrations of extradural ropivacaine and bupivacaine. When the same concentration of each drug was administered, there were statistically insignificant differences in the onset and duration of sensory block.
The mean duration of action of epidural analgesia in Group B was 253.10 ± 17.46 min and 251.80 ± 15.77 min in Group R [Table 4]. The mean duration of epidural analgesia varied between 240 and 300 min. There was no statistically significant difference between both the groups (P = 0.697). Korula et al.[6] compared the clinical efficacy of the equipotent doses of ropivacaine 0.75% and bupivacaine 0.125% for epidural anesthesia and ropivacaine 0.2% and bupivacaine 0.125% for postoperative analgesia in patients undergoing bilateral mesh hernioplasty. They found no significant variation in the sensory block profile. Our results can be compared to this study and to Brockway et al.[5] which shows comparable duration of epidural analgesia when used at equal concentration and dosage.
Our study is comparable to Meister et al.[7] with respect to the total epidural dose requirement and rescue analgesia. They concluded that by using patient-controlled epidural analgesia (PCEA) technique, the mean total volume of ropivacaine with fentanyl administered was similar to that of bupivacaine with fentanyl during labor. PCEA demands and delivered doses were similar between the groups. In our study, the total epidural drug requirement in 24 h in Group B was 78.40 ± 6.93 and in Group R was 78.96 ± 6.79, which was statistically not significant (P = 0.684). The mean number of epidural topups required for epidural analgesia in 24 h in Group B was 5.60 ± 0.49 and 5.64 ± 0.48 in Group R and was statistically not significant (P = 0.684). 15 (30%) patients in Group B required rescue analgesia compared to 16 (32%) patients in Group R, which was statistically not significant (P = 0.829).
Small unmyelinated C fibers and small myelinated A fibers (Aδ) are responsible for pain transmission whereas large A fibers (Aβ) transmit motor impulses. Wildsmith et al.[8] found that ropivacaine blocked C fibers faster than A fibers and was a potent producer of frequency (or use-)-dependent block, that is block which only occurs when the fiber is stimulated. High pKa and low lipid solubility favors block of C fibers before A fibers. Frequency-dependent block is related to lipid solubility and the molecular weight of the drug. Ropivacaine is less lipid soluble than bupivacaine which retards its penetration of myelin sheaths. Clinically, these physiochemical properties of ropivacaine compared to bupivacaine offer an advantage in providing analgesia with minimal motor block. In our study, 7 (14%) patients in Group B had Bromage 1 motor blockade while no patients in Group R had motor blockade (P = 0.012) which was statistically significant. Our results are comparable with Muldoon et al.[9] and Korula et al.;[6] they have also noticed that bupivacaine produces significantly more frequent motor blockade in similar concentration and dose compared to ropivacaine. Equal volumes and concentration of ropivacaine and bupivacaine produce a similar pattern of sensory block but motor block is slower in onset, less in intensity, and shorter in duration with ropivacaine.[5] This has been confirmed in studies of lumbar extradural block in humans.
In our study, hypotension was seen in 8 (16%) patients in Group B compared to 6 (12%) patients in Group R. Episodes of hypotension were treated with intravenous fluid administration. We observed nausea in 5 (10%) patients in Group B and 3 (6%) patients in Group R. There was no episode of vomiting in both the groups. The incidence of adverse effects was statistically insignificant. Our study is comparable with McCrae et al.,[4] H. Jørgensen et al.,[10] and Owen et al.[11]
CONCLUSION
In our study of postoperative epidural analgesia for major lower limb orthopedic surgery, we conclude that 0.2% ropivacaine and 0.2% bupivacaine in equal doses were comparable in regard to the onset and duration of analgesia, 24-h total epidural dose requirement, requirement of rescue analgesics, and adverse effects. In the dosage used by us, 0.2% ropivacaine showed a distinct sensory-motor dissociation resulting in analgesia without motor blockade which improves ambulation and patient satisfaction compared to 0.2% bupivacaine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosenquist RW, Vrooman BW. Chronic pain management. In: Morgan GE, Mikhail MS, Murray MJ, editors. Clinical Anesthesiology. 5th ed. New York: Tata McGraw Hill; 2013. p. 1025. [Google Scholar]
- 2.Hurley RW, Murphy JD, Wu CL. Acute postoperative pain. In: Miller RD, editor. Miller's Anesthesia. 8th ed. Vol. 2. Philadelphia: Churchill Livingstone, Elsevier Saunders; 2015. p. 2984. [Google Scholar]
- 3.Arlock P. Actions of three local anaesthetics: Lidocaine, bupivacaine and ropivacaine on guinea pig papillary muscle sodium channels (Vmax) Pharmacol Toxicol. 1988;63:96–104. doi: 10.1111/j.1600-0773.1988.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 4.McCrae AF, Jozwiak H, McClure JH. Comparison of ropivacaine and bupivacaine in extradural analgesia for the relief of pain in labour. Br J Anaesth. 1995;74:261–5. doi: 10.1093/bja/74.3.261. [DOI] [PubMed] [Google Scholar]
- 5.Brockway MS, Bannister J, McClure JH, McKeown D, Wildsmith JA. Comparison of extradural ropivacaine and bupivacaine. Br J Anaesth. 1991;66:31–7. doi: 10.1093/bja/66.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Korula S, George GM, Ipe S, Abraham SP. Epidural anesthesia and post-operative analgesia for bilateral inguinal mesh hernioplasty: Comparison of equipotent doses of ropivacaine and bupivacaine. Saudi J Anaesth. 2011;5:277–81. doi: 10.4103/1658-354X.84101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister GC, D'Angelo R, Owen M, Nelson KE, Gaver R. A comparison of epidural analgesia with 0125% ropivacaine with fentanyl versus 0125% bupivacaine with fentanyl during labor. Anesth Analg. 2000;90:632–7. doi: 10.1097/00000539-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Wildsmith JA, Brown DT, Paul D, Johnson S. Structure-activity relationships in differential nerve block at high and low frequency stimulation. Br J Anaesth. 1989;63:444–52. doi: 10.1093/bja/63.4.444. [DOI] [PubMed] [Google Scholar]
- 9.Muldoon T, Milligan K, Quinn P, Connolly DC, Nilsson K. Comparison between extradural infusion of ropivacaine or bupivacaine for the prevention of postoperative pain after total knee arthroplasty. Br J Anaesth. 1998;80:680–1. doi: 10.1093/bja/80.5.680. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen H, Fomsgaard JS, Dirks J, Wetterslev J, Dahl JB. Effect of continuous epidural 02% ropivacaine vs. 0.2% bupivacaine on postoperative pain, motor block and gastrointestinal function after abdominal hysterectomy. Br J Anaesth. 2000;84:144–50. doi: 10.1093/oxfordjournals.bja.a013394. [DOI] [PubMed] [Google Scholar]
- 11.Owen MD, D’Angelo R, Gerancher JC, Thompson JM, Foss ML, Babb JD, et al. 0.125% ropivacaine is similar to 0.125% bupivacaine for labor analgesia using patient-controlled epidural infusion. Anesth Analg. 1998;86:527–31. doi: 10.1097/00000539-199803000-00015. [DOI] [PubMed] [Google Scholar]


