Abstract
Context:
Both intubation and extubation are associated with pressor response.
Aims:
We aimed to evaluate if ropivacaine 0.25% nebulization would prevent hemodynamic and cough responses to intubation and extubation and compared it with lignocaine 2% and saline group.
Settings and Design:
This was a randomized double-blind clinical trial.
Materials and Methods:
A total of 75 patients classified as the American Society of Anaesthesiologists physical status Classes I and II belonging to 18–60 years were included in the randomized double-blind trial and divided into three groups; Group 1: received 5 ml of normal saline, Group 2: received 5 ml of 0.25% ropivacaine, Group 3: received 5 ml of 2% lignocaine through nebulization before the induction. Patients were then administered general anesthesia. Mean arterial pressure (MAP), heart rate (HR), and saturation were recorded at baseline (T1), at intubation (T2), upon anesthetic withdrawal (T3), upon eye opening (T4), upon extubation (T5), and 2 min after extubation (T6). Cough response was recorded at emergence and extubation.
Statistical Analysis Used:
Repeated measures analysis of variance were used to compare hemodynamic variables and Chi-square test to compare the grades of cough between the two groups.
Results:
The drug ropivacaine was found to be effective in reducing the hemodynamic responses to both intubation and extubation when compared to saline (P < 0.05). At extubation, though the mean values of HR and MAP were lower in ropivacaine compared to lignocaine group, the difference did not achieve statistical significance (P = 0.103 and 0.153 respectively). Only 40% of patients who received ropivacaine had cough at extubation (P < 0.001).
Conclusion:
Ropivacaine when used through nebulization preinduction effectively reduced both intubation and extubation responses when compared to saline. However, there was no significant difference between the ropivacaine and lignocaine on extubation response.
Keywords: Extubation, intubation, lignocaine, nebulisation, pressor response, ropivacaine
INTRODUCTION
Both intubation and extubation can increase the concentration of catecholamines in the blood resulting in hemodynamic changes and complications such as myocardial infarction, arrhythmias and cerebrovascular hemorrhage.[1,2] During intubation, agents such as propofol and opioids given at the time of induction can effectively inhibit airway stimulation by endotracheal tube. During extubation, withdrawal of these anesthetics and emergence stimulate sympathetic nervous system, causing cough and hemodynamic responses.[3,4] Various drugs such as α2 agonists, short-acting opioids, local anesthetics, and beta blockers are used to attenuate the response to extubation.[3] Among the local anesthetics administered topically, lignocaine has widely been used both at intubation and extubation.[5,6] However, because of its limited duration of action, the effect of lignocaine given preinduction may not last up to extubation and hence may warrant repeat dose of local anesthetic at the time of extubation. Hence, we aimed at using ropivacaine through nebulization, a longer acting local anesthetic, which has been reported to significantly attenuate histamine-induced bronchospasm given topically.[7] We aimed to study and to compare the hemodynamic responses to intubation and extubation in topical ropivacaine 0.25% group, lignocaine 2% group and control group. We hypothesized that ropivacaine when administered as aerosol preoperatively would maintain topical anesthesia of the airway till the time of emergence and thus reduce the hemodynamic responses and cough occurring at extubation.
MATERIALS AND METHODS
The study is a randomized double-blind controlled trial conducted on 75 adult patients aged 18–60 years undergoing elective surgery under general anesthesia with endotracheal intubation in Pondicherry Institute of Medical Sciences. The study has been registered with Clinical Trial Registry of India (CTRI registration number: CTRI/2017/10/010254). Patients with history of risk factors for perioperative aspiration of gastric contents, history of chronic obstructive pulmonary disease, asthma, history of recent respiratory infections, anticipated difficult intubation and duration of surgery more than 3 h were excluded from the study. Institutional Ethical Committee clearance and informed written consent were obtained from all patients. Patients were premedicated both night before and morning of surgery with tablet ranitidine, tablet diazepam, and tablet perinorm. After transferring to the operating room, the American Society of Anesthesiologists standard monitors were attached and blood pressure mean arterial pressure (MAP), heart rate (HR), electrocardiography, and saturation of blood oxygen (SPO2) were continuously monitored. All patients received 5 ml/kg of Ringer lactate solution over a 10 min period before induction of anesthesia and thereby maintenance infusion continued. Based on the predicted duration of surgery, patients were divided into two strata. Patients in each stratum (i.e. <1.5 h and ≥1.5 h) were then randomized using block randomization into three study groups using computer-generated randomization chart [Figure 1]. Patients assigned to groups were recorded and sealed within sequentially numbered envelopes. The primary anesthesiologist blinded to the assignments administered nebulization to patients in all groups. The second blinded anesthesiologist recorded the hemodynamic changes. The study drug was prepared by a third anesthesiologist, the one who had originally opened the envelope, as a uniform 5 ml solution. Thus, participants as well as anesthesiologists preparing the drug, administering the drug, and recording outcomes were blinded to the study.
Figure 1.
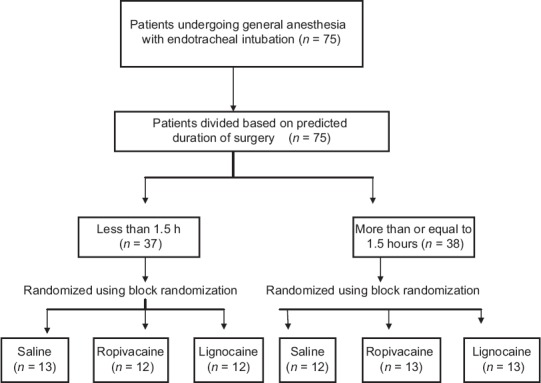
Consolidated standards for reporting of trials flow diagram showing enrollment and method of allocation. n: Number of patients
Group 1: Received 5 ml of plain normal saline
Group 2: Received 5 ml of 0.25% ropivacaine nebulization
Group 3: Received 5 ml of 2% lignocaine via nebulization.
Drug was administered as aerosol through nebulization. Nebulization was done with a nebulizer by an insufflations gas (100% oxygen at a flow rate of 10 L/min from a wall mount oxygen port) through a 200 cm tubing connected from the oxygen port to the face mask attached with a nebulizer. Patients were propped up and advised to take normal tidal volume breaths. Nebulization was continued until the complete solution in the nebulizer got aerosolized (10–12 min).
Ten min after completion of nebulization, patients were preoxygenated with 100% oxygen for 5 min and then induced with injection midazolam 0.02 mg/kg, fentanyl 2 μg/kg, thiopentone 5 mg/kg, and vecuronium 0.1 mg/kg. After 3 min of bag and mask ventilation, patients were intubated with the appropriate size endotracheal tube. Anesthesia was maintained with O2:N2O mixture in the ratio of 1:2 and isoflurane. Tidal volume was controlled at 7 mg/kg, the respiratory rate was set at 12/min, and the inspiration/expiration ratio was set at 1:2. Supplementary analgesia was provided by injection morphine in a dose of 0.1 mg/kg. Neuromuscular blockade was maintained with maintenance doses of vecuronium. At the end of the case, oropharynx suctioned, inhalational agent was stopped, and patient-administered 100% oxygen. After return of spontaneous respiration, neuromuscular blockade was reversed with 0.05 mg/kg of neostigmine and 0.01 mg/kg of injection glycopyrrolate. Trachea was extubated when patient demonstrated the ability to follow verbal commands or showed purposeful movements in addition to resumption of regular spontaneous respiration. The anesthetist blinded to the study recorded the following parameters:
-
MAP, HR, and SPO2 at;
- Baseline (T1)
- Upon tracheal intubation (T2)
- Upon anesthetic withdrawal (T3)
- Upon eye-opening on verbal commands (T4)
- Upon tracheal extubation (T5)
- Two min after extubation (T6).
-
Severity of cough[8]
- Mild - single cough
- Moderate - More than one episode of unsustained cough (<5 s)
- Severe- sustained ≥5 s bout of coughing.
-
Time to extubation:
- Time from anesthetic withdrawal to the removal of endotracheal tube.
Any rise in HR >120/min or a MAP >120 mmHg during extubation was treated with appropriate dose of beta blocker (injection esmolol 0.25–0.5 mg/kg) in discretion with the consultant anesthesiologist.
Statistical analysis
The results on continuous measurements were presented as mean ± standard deviation and results on categorical data were presented as number (percentages). Statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 20 (IBM Corp, Armonk, NY). 2011 was used for analysis. Repeated measures analysis of variance was used to find the significance of hemodynamic parameters among the three groups at various time points followed by Post hoc Tukey test to find pairwise significance. The number of patients who experienced cough in each group was reported as percentages and compared using Chi-square test. All statistical analysis was carried out at 5% level of significance.
Taking difference in the MAP between the ropivacaine and control group to be the effect size from a previous similar study,[8] a power analysis indicated that a minimum of 18 patients in each group would be needed to reach 80% power with an alpha error of 0.05 to reject null hypothesis. A total of 25 patients were included in each group to allow for withdrawal/dropout due to various reasons.
RESULTS
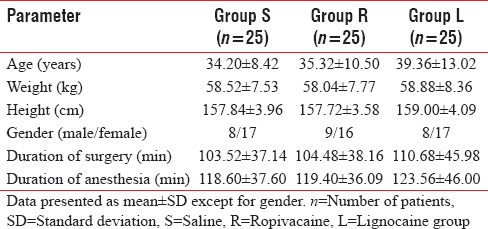
A total of 75 patients were included in the study. There were no significant differences between the groups with respect to demographic data (age, height, weight, and gender), duration of anesthesia, or duration of surgery [P > 0.05 each, Table 1].
Table 1.
Demographic data of the three study groups
The hemodynamic parameters (HR, MAP, and SPO2) were compared between the three groups at various time points. There was an overall statistically significant difference among the three groups regarding HR and MAP at intubation (T2), emergence (T4), and at extubation (T5) (P < 0.05) [Tables 2 and 3].
Table 2.
Comparison of heart rate between the study groups at various time points
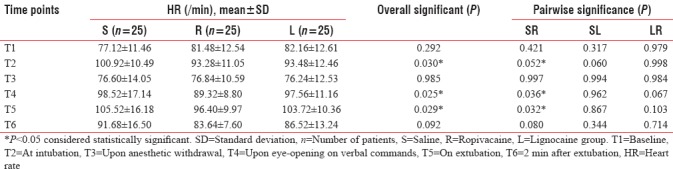
Table 3.
Comparison of mean arterial pressure between the study groups at various time points
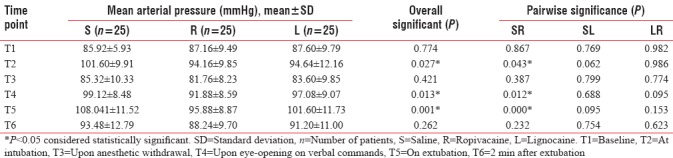
The drug ropivacaine was found to significantly reduce both HR and MAP at intubation and extubation when compared to normal saline (P = 0.052, 0.043, respectively, at intubation and P = 0.032, <0.001 respectively, at extubation).
On comparing the two local anesthetics, there was no difference between them at intubation. However, at extubation, though the mean values of HR and MAP were lower in ropivacaine compared to lignocaine group (96.40 ± 9.97 vs. 103.72 ± 10.36 and 95.88 ± 8.87 vs. 101.60 ± 11.73, respectively), the difference did not achieve statistical significance (P = 0.103 and 0.153, respectively) [Tables 2 and 3].
Only 40% of patients who received ropivacaine had cough (mild to moderate) at extubation as compared to 92% in saline and 88% in lignocaine (P < 0.001) [Figure 2].
Figure 2.
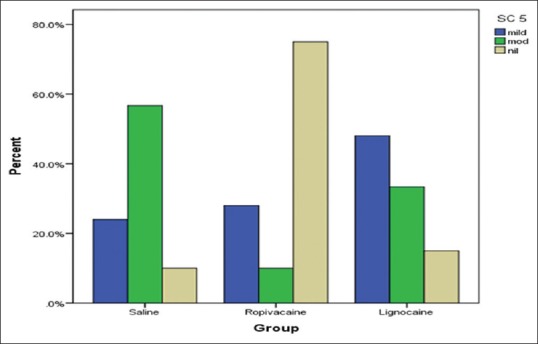
Bar chart showing distribution of patients with cough on extubation among the three groups. 40% of patients in ropivacaine group had mild-to-moderate cough (P < 0.001) SC: Severity of a cough at extubation (T5)
There was no difference in mean extubation time between the three groups.
Only one patient in saline group received one dose of 20 mg injection esmolol to control his HR at extubation. None of the other patients received any dose of antihypertensive at any time point intraoperatively.
DISCUSSION
Insertion of laryngoscope and endotracheal tube during intubation and withdrawal of anesthetics during emergence and extubation is often associated with an increase in sympathetic nervous system discharge leading to increase in HR and MAP.[9] Although tolerated by many patients, in some it causes dangerous complication such as myocardial infarction, arrhythmias, and cerebrovascular hemorrhage. Various techniques and drugs such as α2 agonists, short-acting opioids, local anesthetics and beta-blockers are used to attenuate the airway and circulatory reflexes to extubation.[10,11,12,13] However, administration of these drugs before extubation can delay postoperative awakening. A large number of research has been done using local anesthetic as the choice of drug for this purpose. Although there are innumerable studies demonstrating lignocaine to be effective in reducing these pressor responses,[14,15,16,17,18] literature review did not reveal many studies that had used ropivacaine topically for the same purpose.[8,19]
In a study done by Jain and Khan,[20] a bolus dose of 1.5 mg/kg of 2% lignocaine followed by an infusion administered intravenously was found to be effective in reducing intubation response (P < 0.05) in 60 patients undergoing elective laparoscopic cholecystectomy. In addition, lignocaine administered topically for airway anesthesia in the form of endotracheal instillation, intracuff lignocaine, and lignocaine sprays have been found to reduce pressor response associated with intubation.[4,21,22,23] In our study, though MAP and HR values during intubation were lower in lignocaine group than in the saline group, the difference achieved only a partial statistical significance (P = 0.062 and P = 0.060, respectively). On the other hand, ropivacaine reduced intubation response effectively compared to saline (P < 0.05). There was no difference between the two local anesthetics, lignocaine, and ropivacaine with regard to intubation responses (P = 0.998 and P = 0.986 for HR and MAP, respectively). The results were similar to a study done by Gao et al.,[8] who showed no significant difference between dicaine and ropivacaine at intubation. Notably, both lignocaine and ropivacaine were effective in stopping neural conduction and inhibiting the airway reflexes caused by contact with an endotracheal tube. Since the difference lies only in the duration of topical anesthesia caused by each (lignocaine 45–60 min, ropivacaine over 2 h), at intubation (which occurred almost within 20 min of administration of nebulization), there was no difference between the two local anesthetic groups.
The effect of ropivacaine, a newer amide and a longer acting local anesthetic, is due in large part to its blockade of sympathetic nerves distributed in the airway.[8,24,25] When extubation response was observed in our study, we found lignocaine to be no different from saline, i e., it did not reduce extubation response. Considering the fact that duration of lignocaine is short (45–60 min), it given preinduction may not last up to the time of extubation. On the other hand, ropivacaine, being longer acting effectively, reduced MAP and HR at extubation compared to saline (P < 0.05 each). Gao et al. demonstrated a similar decrease in HR and MAP at extubation in the group which received ropivacaine through trans cricothyroid injection before induction. Meng et al.[19] conducted a randomized study to evaluate the effect of topical ropivacaine anesthesia on hemodynamic responses to extubation and found HR and MAP were significantly lower in the ropivacaine than in the lignocaine and saline group (P < 0.05 each). In contrast to this, in our study, at extubation, though MAP and HR values were lower in the ropivacaine than in the lignocaine group, the difference could not achieve statistical significance (96.40 ± 9.97 vs. 103.72 ± 10.36 and 95.88 ± 8.87 vs. 101.60 ± 11.73 respectively. P = 0.103 and 0.153, respectively). Perhaps, it is because we used a lower concentration of ropivacaine (0.25%) through nebulization as against Meng, who used a much higher concentration (0.75%) of ropivacaine.[19]
As patients emerge from general anesthesia, the stimulating effect of positive pressure ventilation and endotracheal tube on mechanosensitive receptors on the trachea and larger bronchi may provoke coughing.[26,27,28]
Only 40% of patients who received ropivacaine had cough (mild to moderate) at extubation as compared to 92% in saline and 88% in lignocaine (P < 0.001). Ropivacaine effectively suppressed cough at extubation compared to both lignocaine and saline (P < 0.001 each). Gao et al. also demonstrated a similar decrease in incidence of coughing compared to dicaine at extubation. Compared with the effects of lignocaine, those of ropivacaine last longer. Considering the duration of anesthetizing effect of lignocaine to be limited to 45–60 min, lignocaine was not effective in suppressing cough at extubation.
Watkins et al.[29] found lignocaine given before the induction through the laryngotracheal kit prolonged the mean extubation times by nearly 2 min. They stated the reason to be inability of the patients to perceive the stimulating effect of tracheal tube because of topical anesthesia induced by higher concentration of lignocaine (4%) used in their study. In our study, we found no difference in mean extubation times between the three groups. None of the local anesthetics were found to have prolonged the extubation time. Perphaps, it is because of the low concentrations of the local anesthetics which we had used in our study.
In conclusion, ropivacaine was found effective in reducing both intubation and extubation response in patients undergoing general anesthesia with endotracheal intubation. Although we hypothesized ropivacaine to be better than lignocaine in reducing extubation response in view of its longer duration of action, we did not achieve a significant difference between the two local anesthetics at extubation. Probably, it was because of the lowest concentration of ropivacaine which we had used in our study. Further future studies may be needed with higher concentration of topical ropivacaine to achieve a significant reduction in extubation response when compared to other local anesthetics.
Limitations
In our study, the concentration of ropivacaine used was 0.25%. The results which obtained cannot be extrapolated to other higher available concentration of ropivacaine. We did not measure the plasma concentration levels of local anesthetic assuming the levels would be very minimal since the drug was administered topically.
CONCLUSION
Ropivacaine when used topically for airway anesthesia through nebulization pre induction effectively reduced both intubation and extubation responses when compared to saline. However, there was no significant difference between the ropivacaine and lignocaine on extubation response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh S, Smith JE. Cardiovascular changes after the three stages of nasotracheal intubation. Br J Anaesth. 2003;91:667–71. doi: 10.1093/bja/aeg240. [DOI] [PubMed] [Google Scholar]
- 2.Asai T, Koga K, Vaughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80:767–75. doi: 10.1093/bja/80.6.767. [DOI] [PubMed] [Google Scholar]
- 3.Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM, et al. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108:1157–60. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 4.Estebe JP, Delahaye S, Le Corre P, Dollo G, Le Naoures A, Chevanne F, et al. Alkalinization of intra-cuff lidocaine and use of gel lubrication protect against tracheal tube-induced emergence phenomena. Br J Anaesth. 2004;92:361–6. doi: 10.1093/bja/aeh078. [DOI] [PubMed] [Google Scholar]
- 5.Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004;99:1253–7. doi: 10.1213/01.ANE.0000132779.27085.52. [DOI] [PubMed] [Google Scholar]
- 6.Baraka A. Intravenous lidocaine controls extubation laryngospasm in children. Anesth Analg. 1978;57:506–7. doi: 10.1213/00000539-197807000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Groeben H, Grosswendt T, Silvanus MT, Pavlakovic G, Peters J. Airway anesthesia alone does not explain attenuation of histamine-induced bronchospasm by local anesthetics: A comparison of lidocaine, ropivacaine, and dyclonine. Anesthesiology. 2001;94:423–8. doi: 10.1097/00000542-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Gao W, Xi JH, Ju NY, Cui GX. Ropivacaine via trans-cricothyroid membrane injection inhibits the extubation response in patients undergoing surgery for maxillary and mandibular fractures. Genet Mol Res. 2014;13:1635–42. doi: 10.4238/2014.March.12.16. [DOI] [PubMed] [Google Scholar]
- 9.Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–9. doi: 10.1093/bja/59.3.295. [DOI] [PubMed] [Google Scholar]
- 10.Adcock JJ, Douglas GJ, Garabette M, Gascoigne M, Beatch G, Walker M, et al. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138:407–16. doi: 10.1038/sj.bjp.0705056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandazi AK, Louizos AA, Davilis DJ, Stivaktakis JM, Georgiou LG. Inhalational anesthetic technique in microlaryngeal surgery: A comparison between sevoflurane-remifentanil and sevoflurane-alfentanil anesthesia. Ann Otol Rhinol Laryngol. 2003;112:373–8. doi: 10.1177/000348940311200414. [DOI] [PubMed] [Google Scholar]
- 12.Ayuso A, Luis M, Sala X, Sánchez J, Traserra J. Effects of anesthetic technique on the hemodynamic response to microlaryngeal surgery. Ann Otol Rhinol Laryngol. 1997;106:863–8. doi: 10.1177/000348949710601010. [DOI] [PubMed] [Google Scholar]
- 13.Matot I, Sichel JY, Yofe V, Gozal Y. The effect of clonidine premedication on hemodynamic responses to microlaryngoscopy and rigid bronchoscopy. Anesth Analg. 2000;91:828–33. doi: 10.1097/00000539-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Wieczorek PM, Schricker T, Vinet B, Backman SB. Airway topicalisation in morbidly obese patients using atomised lidocaine: 2% compared with 4% Anaesthesia. 2007;62:984–8. doi: 10.1111/j.1365-2044.2007.05179.x. [DOI] [PubMed] [Google Scholar]
- 15.Xue FS, Liu HP, He N, Xu YC, Yang QY, Liao X, et al. Spray-as-you-go airway topical anesthesia in patients with a difficult airway: A randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg. 2009;108:536–43. doi: 10.1213/ane.0b013e31818f1665. [DOI] [PubMed] [Google Scholar]
- 16.Simmons ST, Schleich AR. Airway regional anesthesia for awake fiberoptic intubation. Reg Anesth Pain Med. 2002;27:180–92. doi: 10.1053/rapm.2002.30659. [DOI] [PubMed] [Google Scholar]
- 17.Gefke K, Andersen LW, Friesel E. Lidocaine given intravenously as a suppressant of cough and laryngospasm in connection with extubation after tonsillectomy. Acta Anaesthesiol Scand. 1983;27:111–2. doi: 10.1111/j.1399-6576.1983.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinhaus JE, Gaskin L. A study of intravenous lidocaine as a suppressant of cough reflex. Anesthesiology. 1963;24:285–90. doi: 10.1097/00000542-196305000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Meng YF, Cui GX, Gao W, Li ZW. Local airway anesthesia attenuates hemodynamic responses to intubation and extubation in hypertensive surgical patients. Med Sci Monit. 2014;20:1518–24. doi: 10.12659/MSM.890703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain S, Khan RM. Effect of peri-operative intravenous infusion of lignocaine on haemodynamic responses to intubation, extubation and post-operative analgesia. Indian J Anaesth. 2015;59:342–7. doi: 10.4103/0019-5049.158733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee D, Park SY. Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Anesth Analg. 2003;96:293–7. doi: 10.1097/00000539-200301000-00058. [DOI] [PubMed] [Google Scholar]
- 22.Fagan C, Frizelle HP, Laffey J, Hannon V, Carey M. The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth Analg. 2000;91:201–5. doi: 10.1097/00000539-200007000-00038. [DOI] [PubMed] [Google Scholar]
- 23.Bousselmi R, Lebbi MA, Bargaoui A, Ben Romdhane M, Messaoudi A, Ben Gabsia A, et al. Lidocaine reduces endotracheal tube associated side effects when instilled over the glottis but not when used to inflate the cuff: A double blind, placebo-controlled, randomized trial. Tunis Med. 2014;92:29–33. [PubMed] [Google Scholar]
- 24.Groban L, Deal DD, Vernon JC, James RL, Butterworth J. Does local anesthetic stereoselectivity or structure predict myocardial depression in anesthetized canines? Reg Anesth Pain Med. 2002;27:460–8. doi: 10.1053/rapm.2002.35166. [DOI] [PubMed] [Google Scholar]
- 25.Stewart J, Kellett N, Castro D. The central nervous system and cardiovascular effects of levobupivacaine and ropivacaine in healthy volunteers. Anesth Analg. 2003;97:412–6. doi: 10.1213/01.ANE.0000069506.68137.F2. [DOI] [PubMed] [Google Scholar]
- 26.Sant'Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- 27.Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998;87:1170–4. doi: 10.1097/00000539-199811000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Diachun CA, Tunink BP, Brock-Utne JG. Suppression of cough during emergence from general anesthesia: Laryngotracheal lidocaine through a modified endotracheal tube. J Clin Anesth. 2001;13:447–51. doi: 10.1016/s0952-8180(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 29.Watkins J, Lee D, White WA, Jr, Mundy S. Effects of topical lidocaine on successful extubation time among patients undergoing elective carotid endarterectomies. AANA J. 2012;80:99–104. [PubMed] [Google Scholar]


