Abstract
Context:
Ventilator associated pneumonia is one the most common nosocomial infection encountered in the ICU patients. Despite of the implementation of the VAP prevention bundle, the incidence remains high. This can be attributed to the peritubal leak and the aspiration of the oropharyngeal secretions. The secretions further forms a nidus for the growth of organisms in the lower respiratory tract. In this study, a specialised tube, named ‘suction above cuff endotracheal tube’ is used, which has an additional suction port opening above the cuff. This is to facilitate timely aspiration of the secretion which pent-up above the cuff and gradually trickles down the trachea resulting in pneumonia.
Aim:
to compare the incidence of VAP with standard endotracheal tube (SETT) and suction above cuff endotracheal tube (SACETT) in neurological post-operative patients and its impact on clinical outcome.
Settings and Design:
60 patients of post-operative neurological cases aged ≥ 18 years and requiring intubation and/or ventilation and anticipated to remain on ETT for ≥48 h were randomized to receive either SETT or SACETT.
Results:
In this study involving neurological population, there was no significant difference in incidence of clinical and microbiological VAP between SETT and SACETT group, when other strategies for VAP prevention were similar. Other outcomes were similar with use of either tube for intubation.
Keywords: Neurological, nosocomial, subglottic secretions, subglottic secretions, suction above cuff endotracheal tube, ventilator-associated pneumonia
INTRODUCTION
Providing ventilatory support to Intensive Care Unit (ICU) patients is often associated with both ventilator- and patient-related complications. Ventilator-associated pneumonia (VAP) is one such complication, accounting for about 56% of mortality and morbidity in such patients, apart from their primary illness.[1,2,3] Several measures as suggested by various studies such as oral intubation, use of nasogastric tubes, early enteral nutrition, stress ulcer prophylaxis, maintenance of cuff pressure between 20 and 30 cm H2O, and semi-recumbent patient position (commonly termed as VAP bundle) were used for decreasing the incidence of VAP. Despite implementation of VAP bundle, prevention of VAP still remains nightmare situation for intensivists. It has been considered in many similar studies that the peritubal leak and prolonged intubation with bad maintenance of oral hygiene are responsible for VAP to occur.[4,5] Prolonged intubation leads to penting up of secretions above the cuff which forms a nidus for bacterial colonization that gradually trickles down the lower respiratory tract resulting in pneumonia. Therefore, a specially designed “suction above cuff” endotracheal tube (SACETT), which helps in intermittent suctioning of the secretions above cuff, was used in this study [Figure 1]. Here, VAP is assessed both clinically and microbiologically. The study group consists of postoperative neurological patients expected to require prolonged mechanical ventilation. This study group is chosen so as to eliminate other causes of raised leukocytic counts other than VAP. The primary aim of the study is the compare the incidence of VAP between the two groups, i.e., one group with conventional ETT and the other with SACETT. Other parameters such as duration of intubation, mechanical ventilation, and ICU stay are also assessed.
Figure 1.
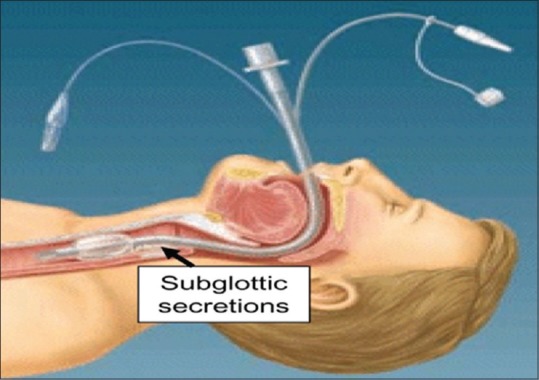
Suction above cuff endotracheal tube
MATERIAL AND METHODS
Study design
After institutional ethical committee approval and written informed consent from patient's guardian, this study was undertaken at a tertiary referral center during a period of 12 months. Sixty patients were randomly allocated using computer-generated randomization into any of two groups (30 each) after fulfillment of the inclusion criteria.
Group A: Patients receiving standard ETT (SETT)
Group B: Patients receiving SACETT.
Patients who underwent surgery for neurological illness, >18 years of age, without any significant comorbidity, requiring endotracheal intubation and mechanical ventilation, and anticipated to remain intubated for >48 h were included in the study, whereas those patients who arrived in ICU already intubated and reintubated during the study period and patients with tracheostomy and on prior ventilation were excluded from the study. The design of the study is such that blinding for physicians and nurses was not possible. However, the microbiologist who analyzed the tracheal suction samples was blinded.
Intensive Care Unit care
On admission to ICU following the surgery, all the baseline investigations including the chest X-ray were obtained within 24 h of intubation. The patients received similar modes of ventilation to avoid any disparity. The uses of neuromuscular blockade to facilitate ventilation were avoided. The components of VAP bundle were similarly adhered to in both the groups. All the recruited patients were screened for the occurrence of clinical VAP daily. Other components which would influence the incidence of VAP were also recorded such as concomitant illness, preemptive antibiotic therapy, and ventilatory modes.
Assessment of ventilator-associated pneumonia
Clinical diagnosis of VAP was based on the presence of a new or progressive infiltrate in chest X-ray, at least 48 h after intubation. Along with this, at least one of the following criteria should be present: temperature >38°C or <36°C, total leukocyte count >11,000 or <4000/cumm, change in character or volume of endotracheal secretions, or worsening of oxygenation (decreased PaO2/FiO2).
The diagnosis of microbiological VAP was done by analyzing nonbronchoscopically performed mini-bronchoalveolar lavage (mini-BAL).[6] For this procedure, a suction catheter of 16 Fr, cut to the size of ETT, was introduced inside ETT aseptically. After this, a second suction catheter of 8 Fr was introduced inside the first one, extending 10–15 cm beyond its distal tip and aspiration was done. These mini-BAL samples were collected after 24 h and thereafter every 48 h and sent for microbiological examination. In the second Group B (SACETT), apart from the routine endotracheal suctioning, the subglottic secretions were aspirated from the special port every 2 h. The subglottic secretions were also sent for microbiological analysis at the same time and reports of both the culture obtained. The study protocol was maintained for at least 7 days of mechanical ventilation or tracheal extubation or tracheostomy or whichever occurred earlier. Therefore, this study analyzes the data and reports pertaining to the occurrence of only “early” VAP.
End points of the study
The primary end point of the study was the incidence of early-onset clinical and microbiological VAP in the SACETT and SETT groups. Secondary outcome variables were durations of intubation, mechanical ventilation, and ICU stay.
Statistical analysis
To express categorical variables, number and percentage were used, whereas for quantitative variables, mean ± standard deviation was used. Both the groups were compared by analysis of variance test for continuous variables and Chi-square test for categorical ones. The statistical analysis was done using SPSS version 16 (Statistical Package for the Social Sciences version 16 (SPSS Inc., Chicago, IL, USA)). P < 0.05 was considered significant.
RESULTS
Ten patients were excluded from the study, out of which four did not comply with the study protocol, three expired during the study, and three left against medical advice. Thereby, the results of the analysis were performed on fifty patients. Demographically, both the groups had comparable parameters, as shown in Table 1.
Table 1.
Demographic parameters and potential risk factors for ventilator-associated pneumonia
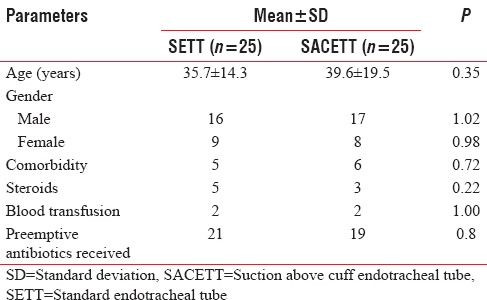
The neurological diagnoses of the study population predominately consisted of surgically managed neurological patients and the distribution is shown in the pie chart, Figure 2.
Figure 2.
Distribution of neurological cases
The incidence of clinical VAP in this study was 20% (5/25) in SETT group and 12% (3/25) in SACETT group (P = 0.70) [Figure 3].
Figure 3.
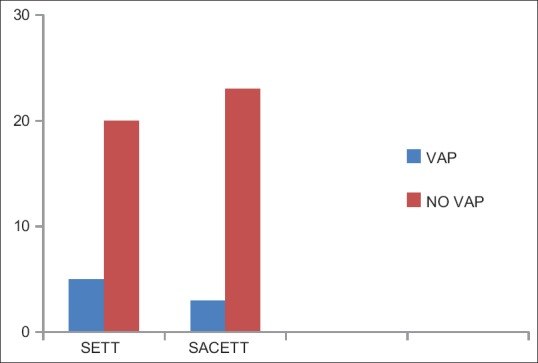
Incidence of clinical ventilator-associated pneumonia in both the groups
The incidence of microbiological VAP was higher in the SETT group (13/25; 52%) as compared to the SACETT group (11/25; 44%) but not statistically significant (P = 0.78) [Figure 4].
Figure 4.
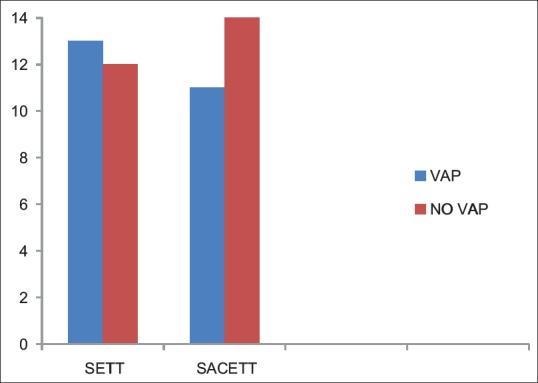
Incidence of microbiological ventilator-associated pneumonia in both the groups
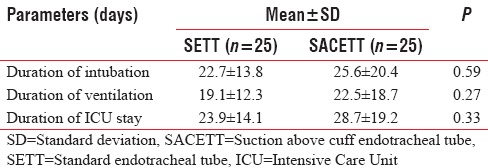
The absolute reduction in clinical and microbiological VAP was 8% each with the use of SACETT. The relative risk reduction for clinical VAP was 40%, and for microbiological VAP, it was 15% with the use of SACETT. There was no difference in the potential risk factors studied (diagnosis, concomitant illness, preemptive antibiotic therapy, steroids, and blood transfusion) between the two groups. The ventilatory mode and type of humidification were similar in all the patients during the duration of the study. There was no difference between the two groups for measured outcomes such as duration of intubation, mechanical ventilation, and ICU stay [Table 2].
Table 2.
Outcome parameters in neurological patients
DISCUSSION
Nosocomial infection accounts for a large section of deaths caused in ICU.[7] Out of these, the major contributor to the list is VAP in patients who are intubated and consequently put on ventilator. Several precautionary measures have been advised to reduce the incidence of VAP, the “VAP prevention bundle” being the most common. It includes oral intubation, use of nasogastric tubes, early enteral nutrition, stress ulcer prophylaxis, maintenance of cuff pressure between 20 and 30 cm H2O, and semi-recumbent patient position. However, despite implementation of VAP prevention bundle, the incidence remains high.
Therefore, this study advocates the use of SACETT which helped in frequent aspiration of the oropharyngeal secretions. These secretions result in peritubal leak forming a nidus for the organisms to grow in the lower respiratory tract. Subglottic secretion drainage using a specially designed ETT (SACETT) with a separate lumen above the cuff has shown to reduce the occurrence of VAP in some studies.[8,9] However, Kollef et al. conducted a study in cardiac surgical patients examining that the effect of continuous drainage of subglottic secretions has not shown difference in VAP rate.[10] One reason for differences in incidence of VAP could be heterogeneous study populations involving medical and surgical patients of different pathologies (sepsis, trauma, neurological, cardiac, pulmonary, etc.) in these studies. The current study evaluated the incidence of VAP in homogeneous population, contrary to other studies. The advantage of this homogeneity was to rule out other causes of sepsis and raised leukocytic counts, other than pneumonia. The incidence of clinical VAP in our population was lower, but the incidence of microbiological VAP was similar to previous studies. However, both were insignificant. Earlier studies involving neurological patients have documented an incidence of clinical VAP in 28% of patients with stroke.[1] Other studies, such as those conducted by Prendergast et al.[11] and Zygun et al.,[12] involving patients of mixed neurological diagnoses (trauma, tumor, and traumatic brain injury) have shown an incidence of 24% and 45%, respectively. In our study, the incidence of clinical VAP was lower than that reported in these previous studies. The lower VAP rate in our study could be attributed to the strict adherence to all the components of VAP prevention bundle with the exception of study intervention (subglottic secretion drainage). The incidence of VAP also varies depending on how the sample is collected. Protected bronchial sampling and BAL are bronchoscopic-guided techniques of obtaining samples and are considered to be more specific for VAP. The technique used in this study is user-friendly and yet provides a representative sample of bronchoalveolar secretions for the diagnosis of VAP. In this study, although incidence of VAP was less in SACETT group as compared to ETT group, the values were statistically insignificant. The reason for this lack of difference in VAP between the two groups could be the following; first, the volume of secretions aspirated from the subglottic region using the special tube was negligible. This may be due to thick secretions, small channel of the tube, and occlusion of the aspiration port by the mucous membrane of the trachea during negative aspiration. Second, the volume of oropharyngeal secretions in both groups was not clinically different. Yet, another point to be considered is that out of the 11 patients who acquired microbiological VAP, merely two of them had the microbiological growth similar as that obtained from the subglottic secretions. This suggests that the subglottic organisms may not have contributed to VAP in majority of the cases. The incidence of VAP also varies depending on how the sample is collected. The limitations of this study were that the effect of late-onset VAP could not be assessed, and second, the cost and difficulty involved in predicting which patient is likely to develop VAP to recommend the selective use of this special tube; the overall benefit remains questionable.
CONCLUSION
From the above study conducted in homogeneous neurological patients, it was observed that a trend exists in both the clinical and microbiological VAP with the use of SACETT and SETT for intubation, adhering to the VAP prevention bundle similarly in both the groups. However, this was statistically insignificant. Other secondary parameters were also comparable between the two groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kasuya Y, Hargett JL, Lenhardt R, Heine MF, Doufas AG, Remmel KS, et al. Ventilator-associated pneumonia in critically ill stroke patients: Frequency, risk factors, and outcomes. J Crit Care. 2011;26:273–9. doi: 10.1016/j.jcrc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–93. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 5.Oikkonen M, Aromaa U. Leakage of fluid around low-pressure tracheal tube cuffs. Anaesthesia. 1997;52:567–9. doi: 10.1111/j.1365-2044.1997.149-az0153.x. [DOI] [PubMed] [Google Scholar]
- 6.Rouby JJ, Rossignon MD, Nicolas MH, Martin de Lassale E, Cristin S, Grosset J, et al. A prospective study of protected bronchoalveolar lavage in the diagnosis of nosocomial pneumonia. Anesthesiology. 1989;71:679–85. doi: 10.1097/00000542-198911000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Li HY, Li SJ, Yang N, Hu WL. Evaluation of nosocomial infection risk using APACHE II scores in the neurological Intensive Care Unit. J Clin Neurosci. 2014;21:1409–12. doi: 10.1016/j.jocn.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 8.Mahul P, Auboyer C, Jospe R, Ros A, Guerin C, el Khouri Z, et al. Prevention of nosocomial pneumonia in intubated patients: Respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992;18:20–5. doi: 10.1007/BF01706421. [DOI] [PubMed] [Google Scholar]
- 9.Smulders K, van der Hoeven H, Weers-Pothoff I, Vandenbroucke-Grauls C. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–62. doi: 10.1378/chest.121.3.858. [DOI] [PubMed] [Google Scholar]
- 10.Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest. 1999;116:1339–46. doi: 10.1378/chest.116.5.1339. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast V, Hallberg IR, Jahnke H, Kleiman C, Hagell P. Oral health, ventilator-associated pneumonia, and intracranial pressure in intubated patients in a neuroscience Intensive Care Unit. Am J Crit Care. 2009;18:368–76. doi: 10.4037/ajcc2009621. [DOI] [PubMed] [Google Scholar]
- 12.Zygun DA, Zuege DJ, Boiteau PJ, Laupland KB, Henderson EA, Kortbeek JB, et al. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocrit Care. 2006;5:108–14. doi: 10.1385/ncc:5:2:108. [DOI] [PubMed] [Google Scholar]


