Abstract
Objectives:
To compare the effects of ketamine-fentanyl (KF) and ketamine-midazolam (KM) combinations on hemodynamic parameters, recovery properties, pain, and side effects in pediatric patients undergoing extracorporeal shock wave lithotripsy (ESWL) procedure.
Methodology:
In this double-blinded, randomized trial, 60 pediatric patients aged between 1 and 13 years with American Society of Anesthesiologists physical status Classes I and II, who scheduled for ESWL procedure, were included in the study. Patients were randomly divided into two groups: Group KM received 0.1 mg/kg of midazolam +1–1.5 mg/kg of ketamine and Group KF received 1 μg/kg of fentanyl +1–1.5 mg/kg of ketamine intravenously.
Results:
There were similar demographic properties, recovery, and discharge times between groups. No statistically significant difference was found in peripheral oxygen saturation, mean and diastolic blood pressure, Ramsey sedation scores, modified Aldrete recovery scores, side effects, and recovery times (Group KM, 16.067 ± 1.2 min; Group KF, 19.46 ± 0.86 min) between groups (P > 0.05).
Conclusion:
KF combination offers better hemodynamic properties, less side effects with lower visual analog scores, and face, legs, activity, cry, and consolability scores than KM in the pediatric ESWL procedure.
Keywords: Child, fentanyl, ketamine, lithotripsy, midazolam, pain
INTRODUCTION
The prevalence of pediatric renal stones is increasing.[1,2] The reason of this increase is unclear in pediatric population. Before the entry of noninvasive technique like extracorporeal shock wave lithotripsy (ESWL) that has been introduced for treatment of pediatric renal and ureteral calculi since 1986 into practice, majority of the upper urinary tract stones have been treated with open surgery.[3] Although ESWL is a noninvasive technique, the shock wave causes a temporary, discomforting, deep, and visceral sharp pain.[4] During ESWL procedure, pain can occur by two mechanisms: external pain resulting from direct contact of shock waves on the pain receptors in the cutaneous and subcutaneous tissue and visceral pain caused by the stretching of the renal capsule around the affected area, fragmented stones, and the internal pressure increasing exponentially in the kidney.[5] Hence, during painful ESWL procedure, analgesia and sedation are needed for pain management, cardiovascular stability, comfort, and immobility for optimize shock wave delivery to better target renal stones.
The ideal sedation regime during ESWL procedure should be safe and effective with rapid onset, earlier recovery, and minimal drug side effects. There are many sedation and analgesic drug options applied with single or combinations regimes such as midazolam, opioids, ketamine, barbiturate, acetaminophen, dexmedetomidine, and propofol.[4,6,7] Ketamine, which is a NMDA antagonist, provides dissociative anesthesia with an analgesic effect. Ketamine has minimal effects on cardiovascular and respiratory system. Combination of ketamine with midazolam (KM), a short-acting benzodiazepine, providing muscle relaxation, anxiolysis, anterograde, and retrograde amnesia but lacking analgesic effect, results in better sedation properties.[8] Fentanyl, a lipid-soluble synthetic opioid analgesic, has rapid onset, high potency, and short duration of action. Respiratory depression, apnea, nausea, and vomiting may occur with fentanyl.
In our study, we aimed to investigate the effects of ketamine/fentanyl (KF) and KM combinations on hemodynamics, sedative, analgesic, respiratory function, and pain scores in pediatric patients who underwent ESWL procedures.
METHODOLOGY
This randomized, prospective, double-blind controlled study was approved by Dicle University Medical Faculty Institutional Review Board and Ethics committee (Registry Url: 2012/42); the written informed consent was obtained from the parents. The patients aged between 1 and 13 years, American Society of Anesthesiologists (ASA) Physical Status I–II, 60 patients who underwent elective ESWL were recruited in the study. Exclusion criteria were refusal of parents, ASA physical status III and IV patients, patients aged over than 14 years, respiratory tract infections, allergy to drugs (midazolam, ketamine, and fentanyl), obesity (body mass index >30), history of sleep apnea, airway and respiratory failure, hemodynamic instability, hemostatic, cardiovascular, pulmonary (asthma), renal and neurological disorders.
Patients were randomly divided into two groups by computer system support as Group KM received 0.1 mg/kg of midazolam (Dormicum, 1 mg/ml, 5 ml; Deva Holding, Istanbul, Turkey) +1–1.5 mg/kg of ketamine (Ketalar, 50 mg/ml, 10 ml; Pfizer, Sandwich, UK) and Group KF 1 μg·kg − 1 of fentanyl (Mylan, USA) +1–1.5 mg/kg of ketamine intravenously. The midazolam was given slowly over 1 min; after 2 min, ketamine was given slowly over 1 min in KM group. The same intravenous (IV) application was used for the drugs in KF group. If additional doses were needed, 0.75 mg/kg (maximum mg/kg) of ketamine were added to initial doses intravenously due to pain, restlessness, patient movement, or excessive crying. If this supplement is not enough, propofol (1 mg/kg) was added. The study drugs were drew into syringes and labeled as “study drug” by an investigator who did not participate in this study. Then, the drugs were handed to anesthesiologists. All the investigators and observers were blinded to groups.
The patients fasted for at least 4 h before the surgery. The parents were allowed to be with them, and they were explained about procedure applied to the children. Patients were taken to the recovery room 30 min before the procedure. They were monitored with 3-lead surface electrocardiogram (DII), peripheral oxygen saturation (SpO2), and noninvasive arterial blood pressure (PETAS KMA® S/5 Anesthesia Monitor, Turkey). Sixteen-gauge IV cannula was inserted on antecubital veins on dorsum of the hand. 5 ml/kg of isotonic saline solution was infused. After replacing the nonrebreather oxygen masks to patients (2 L/min), 0.01 mg/kg atropine was administered as pharmacological medication before procedure. In all patients, systolic blood pressure (SBP), diastolic blood pressure, mean blood pressure, heart rate (HR), SpO2 (peripheral oxygen saturation), respiratory rate, duration of the procedure, and recovery period were recorded at the beginning, after the drug administration, and for every 5 min. The sedation levels of the patients were evaluated with Ramsey sedation scores (RSS) throughout the procedure.[9] The procedure was started after an RSS of 3–4. The degree of pain of the patients was evaluated with Face, Legs, Activity, Cry, and Consolability (FLACC) 10 and visual analog scale (VAS). At the end of the procedure, the time that modified Aldrete score reached[9,10] was measured as the discharge criteria from recovery area by an observer blinded to anesthetic medications and possible complications (nausea, vomiting, and respiratory depression) were recorded.[11] Emergence reactions (agitation, nightmares, hallucinations, and delirium) were recorded. Hypotension was defined as a 30% or more decrease in SBP from baseline. Hypotension was treated first with IV volume expansion (saline infusion at 1–2 ml/kg/h). Bradycardia was defined as a HR <60 bpm and was treated with 0.01 mg/kg of IV atropine. Respiratory depression (<10 breaths/min) and desaturation (oxygen saturation <90%) were treated with verbal stimuli, jaw extension, and supplemental oxygen (2–3 L/min).
Metoclopramide (Metpamid, 10 mg/2 ml, 2 ml; Sifar, Istanbul, Turkey) (0.15–0.25 mg/kg) was administered to patients with nausea and vomiting intravenously as antiemetic agent, and it was recorded.
The patients' analgesic requirement and analgesic consumption were recorded again during recovery. Metamizole sodium (Novalgin, 1 g/2 ml, 2 ml; Sanofi, Istanbul, Turkey) (15 mg/kg) were administered intravenously to patients with ≥5 VAS and/or ≥5 FLACC score as pain management. Following analgesic infusion patients having VAS and FLACC scores less than 5 were transferred to their clinics. All personnel included throughout the study and data analysis periods were unaware of each patient group assignment.
Statistical analysis
The power analysis was calculated by PASS software (Power Analysis of Sample Size, http://www.ncss.com/software/pass/). The sample size of 29 patients per group was analyzed with the alpha error level set at 0.05 and 80% power to detect significant difference in the time of recovery between groups. We planned 30 patients for each group. SPSS 15 (Statistical Package for the Social Sciences, Chicago, Illinois, USA) was performed for data analysis. Continuous variables were expressed as mean and (±) standard deviation, whereas categorical variables as frequencies and percentages and one-way ANOVA test were used for baseline characteristics. Student's t-test was used for parametric data, and Mann–Witney U-test was used for nonparametric data. P < 0.05 was taken as statistically significant.
RESULTS
Sixty patients were recruited in the study (30 patients in each group). All of the patients completed the study uneventfully. The demographic data are shown in Table 1. The mean age were 7.17 ± 3.8 years in Group KM and 6.77 ± 3.9 years in Group F (P = 0.69). The gender of patients was comparable among groups. There were no differences between the two groups with regard to age, weight, ASA physical status, and the duration of procedure (P > 0.05). The duration of the procedure in minutes (17 ± 2.3 for Group KM and 17 ± 3.2 for Group KF) and recovery time in minutes (16.067 ± 1.2 for Group KM and 19.46 ± 0.86 for Group KF) were found to be comparable among groups [Table 1].
Table 1.
Demographic characteristics of patients

Comparing the average HR between groups, statistically significant decrease was observed in 5th, 10th, and 15th min at procedure time and 5th, 10th, 15th, and 20th min at recovery time in Group KF than Group KM, P = 0.003, P = 0.0001, P = 0.0001, P = 0.0001, P = 0.001, P = 0.006, and P = 0.01, respectively. There was no statistically significant differences for HR between groups at the beginning of the procedure (baseline) (P > 0.05) [Table 2].
Table 2.
Hemodynamic and respiratory parameters

The desaturation and hypoventilation in the operation did not occur in both groups. The SBP was significantly higher in Group KM at 15th during procedure and 10th and 20th min during recovery period (P < 0.05) [Table 2].
The FLACC scores were statistically lower in Group F at the beginning, 5th, 10th, and 15th min during procedure; P = 0.004, P = 0.007, P = 0.013, and P = 0019, respectively.
No significant difference was found in VAS at the beginning and 5th min between groups (P = 0.002). VAS was significantly lower in Group F [Figure 1].
Figure 1.
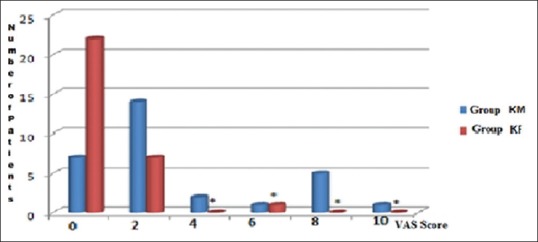
The distribution of visual analog scale assessment: mean values of the groups according to the number of patients
No statistically difference was found in terms of mean RSS values between groups (P > 0.05) [Figure 2]. There was not statistically difference between groups in modified Aldrete recovery scores (P > 0.05) [Figure 3].
Figure 2.

The distribution of Ramsay sedation score assessment: mean values of the groups according to the number of patients
Figure 3.

The distribution of modified Aldrete scale assessment: mean values of the groups according to the number of patients
Comparing side effects between groups, no statistically difference was found (P = 0.1). The nausea and vomiting were noted in 3 patients, agitation in 2 patients, and both nausea and vomiting and agitation were recorded in 1 patient in Group KM. In Group KF, agitation was seen in only 1 patient. The respiratory depression was not observed in both groups [Table 3].
Table 3.
Side effects in group ketamine/midazolam and ketamine/fentanyl

The two groups were examined in terms of the additional dose of analgesic according to pain, restlessness, FLACC scores ≥5, and/or VAS score ≥4. Seven patients were administered 15 mg/kg dose of metamizole sodium in Group KM and 1 patient in Group KF. There was statistically significant differences between groups in supplemental analgesic needed (P = 0.03). There was one patient with inraoperatif movement and restlessness in Group KF. Three patients were administered metoclopramide, and one patient was administered metoclopramide + metamizole sodium after the procedure in Group KM. We did not observe any side effects of the SOS medicines applied.
DISCUSSION
The aim of this study was to compare the effects of KF and KM combinations on hemodynamic parameters and recovery and to see the sedative effectiveness with side effects in pediatric ESWL procedures. Both sedation regimens provided effective sedation during ESWL procedure. The main findings of this study are that fentanyl-ketamine combination in the pediatric population for ESWL procedure can be a safe alternative to KM combination with lower VAS scores and FLACC scores with better hemodynamic properties between groups.
ESWL is a safe and effective choice for the treatment of urinary stones in children. The success rates of ESWL was reported between 68% and 97.6%.[6,12] The demand for anesthetic modalities is increasing for pediatric ESWL procedures.[13] Muslumanoglu et al. reported that 35% of lithotripsy including children older than 3 years were anesthetized. Sedoanalgesia technique is common in children undergoing the lithotripsy process through ESWL technique. It provides cost-effectiveness and safe and satisfactory operating with less side effects.[14] Moreover, early recovery and rapid home discharge are other important benefits of sedoanalgesia for ambulatory practice.
We found that fentanyl (1–1.5 μg/kg) and midazolam (0.1 mg/kg) coadministered with ketamine provided similar hemodynamic stability for ESWL procedure. In terms of SBP values, a significant decrease was observed in 15th at the procedure and 10th and 20th min at recovery in Group KF. We think that this is related to deep sedoanalgesia effects of fentanyl along with ketamine and/or the reduction related with fentanyl-induced vascular resistance. The anesthetic induction with fentanyl provides minimal changes in all hemodynamic parameters (HR, blood pressure, cardiac output, systemic and pulmonary vascular resistance, and pulmonary wedge pressure) even at high doses in children with low cardiac reserve. Compared with baseline HR values in both groups, there was significantly decrease in Group KF. No HR decrease was observed at the beginning of anesthetic induction The reason may be due to sympathomimetic effect of ketamine and/or sympathetic effect due to anxiety at the beginning of procedure. Pellier et al. and Gottschling et al. did not find any changes of cardiovascular parameters with KM combination[15,16] whereas Marx et al. reported tachycardia in 66.7% of patients and hypertension in 38.9% of patients in study comparing KM combination with meperidine-midazolam combination as sedation regimens for painful procedures in pediatric oncology patients.[8] In our study, we observed that there was hemodynamic stability with KM combination despite increase in HR values.
We used Ramsay sedation scale to follow sedation level, VAS, and FLACC score for pain evaluation and modified Aldrete score for recovery in our study. We found no difference with respect to RSS statistically between the groups (P > 0.05). We accepted an RSS of 4 for ESWL procedure. We found similar procedure time; sedation levels with the study of Koruk et al. compared KM versus dexmedetomidine/midazolam in children with ESWL procedure.[4] Gottschling et al. and Kennedy et al. reported adequate sensation levels with KM combinations in emergent orthopedic procedures.[16,17] Tosun et al. did not find any difference between RSS of propofol/ketamine and propofol/fentanyl during wound dressing changes in pediatric burn patients but with higher RSS scores according to our study.[18] The recovery times were shorter in Group KM (16.067 ± 1.2 min vs. 19.46 ± 0.86 min; P > 0.005), though not statistically significant. This result may be due to shorter half-life of midazolam than fentanyl. The recovery time in our KM group was shorter than that reported by Godambe et al. and Gottschling et al.[16,19]
In our study, FLACC scores at 0th, 5th, 10th, and 15th min were lower in Group KF (P = 0.004, P = 0.007, P = 0.013, and P = 0.019). Moreover, VAS scores were significantly lower in Group KF (Graphic 1). Fentanyl is a potent opioid providing analgesia for intense pain of short duration and with ketamine combination getting synergistic effects. Elshammaa et al. compared the effects of 1 μg/kg of fentanyl (F1), 2 μg/kg of fentanyl (F2), 0.5 mg/kg of ketamine (K1) and fentanyl 1 μg/kg plus ketamine 0.5 mg/kg (KF) on postoperative analgesia for children undergoing tonsillectomy and found lower FLACC scores with Fentanyl –ketamine (KF) group. They concluded that coadministration of KF improves postoperative pain control.[20] In our study, we used less supplemental analgesic for postoperative pain in Group KF (P = 0.005, 1 patient in Group KF vs. 7 patients in Group KM). The analgesic effect of fentanyl showed this difference because midazolam produces no analgesia.
Minor side effects were seen lower in KF group, though not statistically significant, such as agitation (2 patients in KM group versus agitation in 1 patient in KF group), nausea and vomiting (3 patients in KM group), and both nausea and vomiting and agitation in 1 patient Group KM. Ketamine is a safe and effective sedative agent which has short duration of action, rapid onset, and protecting laryngeal reflexes.[21] Despite these benefits, emergence phenomena (hallucinations, delirium, agitation, and unpleasant dreams) is a problem when ketamine administered alone, given in large doses or rapidly injection.[21] Reports from some studies suggest that concurrent administration of a benzodiazepine with ketamine decreases emergency reactions,[17,22] while Sherwin reported that midazolam did not decrease recovery agitation and concluded that concurrent administration is unnecessary.[23] There was not any respiratory problems such as desaturation, respiratory depression, laryngospasm, or apnea in our study. Other studies reported 6%–7.3% oxygen desaturations with KM sedation regimens in orthopedic emergencies.[17,24] This difference can be due to slow IV administration of ketamine which can cause central apnea with rapid bolus,[22] administration of atropine to prevent hypersalivation, shorter sedation time, and excluding patients with active infections before study. Restlessness during procedure was seen just in 1 patient in Group KF. In our study, nausea/vomiting was observed in 10% patients (3 patients) in Group KM during recovery period. The rate of nausea/vomiting in our study is comparable with the study of Wathen et al. (9.6%). Midazolam has been shown to decrease nausea and vomiting in patients after tonsillectomy.[24,25] Despite it is known that opioids can increase postoperative nausea and vomiting, we did not observe this side effect in our study.
A limitation of this study is that we did not use end tidal carbon dioxide monitoring during sedation for ESWL, though we performed strict importance to screening for potential adverse events such as airway problems. Another limitation is the definition of the emergency phenomena like nightmares or hallucinations in children, and long-term follow-up may be needed for recurrent hallucinations and adverse effects due to ketamine. Further limitation of our study was the relatively small number of patients and heterogeneous age groups due to the rarity of the ESWL in our hospital. In addition, patients younger than 1 year of age and ASA III–IV patients were excluded from study, so possible effects and complications in this age group and this ASA status could not be considered. Another limitation is that anesthetic depth could be evaluated with BIS. We used RSS to evaluate anesthetic depth.
CONCLUSION
Consequently, in pediatric patients undergoing ESWL procedure, both KM and KF sedation regimens can be used efficiently with adequate safety precautions. These both regimens provided effective analgesia and sedation during procedure while optimizing the conditions for performing ESWL procedure. We found that fentanyl/ketamine combination in the pediatric population was superior to KM for sedoanalgesia with less side effects (nausea/vomiting), lower VAS scores, FLACC scores, and better hemodynamic parameters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS, Lieske JC, Rule AD, et al. Temporal trends in incidence of kidney stones among children: A 25-year population based study. J Urol. 2012;188:247–52. doi: 10.1016/j.juro.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandervoort K, Wiesen J, Frank R, Vento S, Crosby V, Chandra M, et al. Urolithiasis in pediatric patients: A single center study of incidence, clinical presentation and outcome. J Urol. 2007;177:2300–5. doi: 10.1016/j.juro.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Onal B, Citgez S, Tansu N, Emin G, Demirkesen O, Talat Z, et al. What changed in the management of pediatric stones after the introduction of minimally invasive procedures? A single-center experience over 24 years. J Pediatr Urol. 2013;9:910–4. doi: 10.1016/j.jpurol.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Koruk S, Mizrak A, Gul R, Kilic E, Yendi F, Oner U, et al. Dexmedetomidine-ketamine and midazolam-ketamine combinations for sedation in pediatric patients undergoing extracorporeal shock wave lithotripsy: A randomized prospective study. J Anesth. 2010;24:858–63. doi: 10.1007/s00540-010-1023-1. [DOI] [PubMed] [Google Scholar]
- 5.Torrecilla Ortiz C, Rodríguez Blanco LL, Díaz Vicente F, González Satué C, Marco Pérez LM, Trilla Herrera E, et al. Extracorporeal shock-wave lithotripsy: Anxiety and pain perception. Actas Urol Esp. 2000;24:163–8. doi: 10.1016/s0210-4806(00)72423-5. [DOI] [PubMed] [Google Scholar]
- 6.Aksoy Y, Ozbey I, Atmaca AF, Polat O. Extracorporeal shock wave lithotripsy in children: Experience using a mpl-9000 lithotriptor. World J Urol. 2004;22:115–9. doi: 10.1007/s00345-003-0385-5. [DOI] [PubMed] [Google Scholar]
- 7.Eker HE, Cok OY, Ergenoğlu P, Ariboğan A, Arslan G. IV paracetamol effect on propofol-ketamine consumption in paediatric patients undergoing ESWL. J Anesth. 2012;26:351–6. doi: 10.1007/s00540-012-1335-4. [DOI] [PubMed] [Google Scholar]
- 8.Marx CM, Stein J, Tyler MK, Nieder ML, Shurin SB, Blumer JL, et al. Ketamine-midazolam versus meperidine-midazolam for painful procedures in pediatric oncology patients. J Clin Oncol. 1997;15:94–102. doi: 10.1200/JCO.1997.15.1.94. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 2006;16:258–65. doi: 10.1111/j.1460-9592.2005.01773.x. [DOI] [PubMed] [Google Scholar]
- 11.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs. 1998;13:148–55. doi: 10.1016/s1089-9472(98)80044-0. [DOI] [PubMed] [Google Scholar]
- 12.Demirkesen O, Onal B, Tansu N, Altintaş R, Yalçin V, Oner A, et al. Efficacy of extracorporeal shock wave lithotripsy for isolated lower caliceal stones in children compared with stones in other renal locations. Urology. 2006;67:170–4. doi: 10.1016/j.urology.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 13.Muslumanoglu AY, Tefekli AH, Altunrende F, Karadag MA, Baykal M, Akcay M, et al. Efficacy of extracorporeal shock wave lithotripsy for ureteric stones in children. Int Urol Nephrol. 2006;38:225–9. doi: 10.1007/s11255-005-4792-y. [DOI] [PubMed] [Google Scholar]
- 14.Birch BR, Anson KM, Miller RA. Sedoanalgesia in urology: A safe, cost-effective alternative to general anaesthesia. A review of 1020 cases. Br J Urol. 1990;66:342–50. doi: 10.1111/j.1464-410x.1990.tb14952.x. [DOI] [PubMed] [Google Scholar]
- 15.Pellier I, Monrigal JP, Le Moine P, Rod B, Rialland X, Granry JC, et al. Use of intravenous ketamine-midazolam association for pain procedures in children with cancer. A prospective study. Paediatr Anaesth. 1999;9:61–8. [PubMed] [Google Scholar]
- 16.Gottschling S, Meyer S, Krenn T, Reinhard H, Lothschuetz D, Nunold H, et al. Propofol versus midazolam/ketamine for procedural sedation in pediatric oncology. J Pediatr Hematol Oncol. 2005;27:471–6. doi: 10.1097/01.mph.0000179238.37647.91. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy RM, Porter FL, Miller JP, Jaffe DM. Comparison of fentanyl/midazolam with ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics. 1998;102:956–63. doi: 10.1542/peds.102.4.956. [DOI] [PubMed] [Google Scholar]
- 18.Tosun Z, Esmaoglu A, Coruh A. Propofol-ketamine vs propofol-fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changes. Paediatr Anaesth. 2008;18:43–7. doi: 10.1111/j.1460-9592.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 19.Godambe SA, Elliot V, Matheny D, Pershad J. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a pediatric emergency department. Pediatrics. 2003;112:116–23. doi: 10.1542/peds.112.1.116. [DOI] [PubMed] [Google Scholar]
- 20.Elshammaa N, Chidambaran V, Housny W, Thomas J, Zhang X, Michael R, et al. Ketamine as an adjunct to fentanyl improves postoperative analgesia and hastens discharge in children following tonsillectomy – A prospective, double-blinded, randomized study. Paediatr Anaesth. 2011;21:1009–14. doi: 10.1111/j.1460-9592.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 21.Green SM, Rothrock SG, Lynch EL, Ho M, Harris T, Hestdalen R, et al. Intramuscular ketamine for pediatric sedation in the emergency department: Safety profile in 1022 cases. Ann Emerg Med. 1998;31:688–97. doi: 10.1016/s0196-0644(98)70226-4. [DOI] [PubMed] [Google Scholar]
- 22.Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, review and implications. Ann Emerg Med. 1990;19:1033–46. doi: 10.1016/s0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin TS, Green SM, Khan A, Chapman DS, Dannenberg B. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:229–38. doi: 10.1016/s0196-0644(00)70073-4. [DOI] [PubMed] [Google Scholar]
- 24.Wathen JE, Roback MG, Mackenzie T, Bothner JP. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled, emergency department trial. Ann Emerg Med. 2000;36:579–88. doi: 10.1067/mem.2000.111131. [DOI] [PubMed] [Google Scholar]
- 25.Splinter WM, MacNeill HB, Menard EA, Rhine EJ, Roberts DJ, Gould MH, et al. Midazolam reduces vomiting after tonsillectomy in children. Can J Anaesth. 1995;42:201–3. doi: 10.1007/BF03010676. [DOI] [PubMed] [Google Scholar]


