Abstract
Cassia occidentalis is an annual tropical shrub causing toxicity in cattle. However, human case reports of its poisoning are scarce. We, here, report three young children, residents of Western Uttar Pradesh in North India, who presented with lethargy, jaundice, and altered sensorium after consumption of Cassia seeds. The toxidrome was defined as hepatomyoencephalopathy. The children were resuscitated, managed for acute liver failure, and subsequently discharged without sequel. Although few studies have previously documented this association, this is the first such case series documenting a direct causal relationship of Cassia to hepatomyoencephalopathy syndrome. Public and clinician awareness regarding this syndrome mimicking viral encephalitis has the potential to prevent further outbreaks.
Keywords: Accidental poisoning, acute hepatomyoencephalopathy syndrome, Cassia occidentalis, phytotoxin, plant poisoning
INTRODUCTION
Plant poisoning accounts for 1.7% of all cases of poisoning reported at the National poison information Center from India; commonly implicated plants include Datura, yellow oleander, and cannabis.[1] Cassia occidentalis/coffee senna (family: Fabaceaesae) is a ubiquitous tropical shrub, abundant in Asia, Africa, Australia, and United States and used in Indian Traditional Medicine.[2] Literature is replete with reports of poisoning with Cassia spp. in cattle and animals, but data on human poisoning are sparse. Few published human case reports are mainly epidemiologic observations.[3,4,5,6] We share our experience with three cases of C. occidentalis poisoning in young children at a tertiary-level Pediatric Intensive Care Unit.
CASE REPORTS
Case 1
A 4.5-year-old male child, resident of Western Uttar Pradesh, was brought with a history of consumption of plant seeds called locally as “Kasoundi” in September. The consumption happened 2 days ago and was followed by lethargy, reduced oral acceptance, and altered sensorium with irrelevant talking and self-mutilating behavior. There was no history of fever, cough, vomiting, convulsions, rash, bleeding, animal/snake bite, or head injury. There was a history of similar ingestion of seeds by younger sibling who expired few hours after ingestion. This child was initially treated at district hospital for 1 day and subsequently referred to our center. An uprooted plant was also brought by caregivers as sample for identification.
On initial examination, he was afebrile with stable vitals, altered sensorium (glasgow coma scale, 13/15 - E3V4M6), bilateral mid-dilated sluggishly reactive pupils with normal fundus examination, no meningeal signs, normal tone, slightly reduced power (4/5 Medical Research Council scale), brisk deep tendon reflexes, and bilateral extensor plantar response. The rest of general and systemic examination was within normal limits.
Blood investigations revealed mild anemia, leukopenia, thrombocytopenia with normal kidney function test, serum electrolytes, and blood sugar levels [Table 1]. Liver function tests (LFTs) were deranged with high serum bilirubin (total 1.8 mg/dl and direct-1.1 mg/dl), marked transaminitis (serum glutamic oxaloacetic transaminase, 2282 IU/L; serum glutamate pyruvate transaminase, 1800 IU/L; and alkaline phosphatase 225 IU/L) and deranged coagulation profile (international normalized ratio, 2.83 and prothrombin time, 31.5 s). Serum creatinine phosphokinase and lactate dehydrogenase levels were elevated. The arterial blood gas analysis including serum lactate and urine examination was normal. Investigations for viral serology (Japanese encephalitis, herpes encephalitis, and HIV) and malarial antigen were negative.
Table 1.
Clinical and biochemical profile of cases with Cassia seeds poisoning
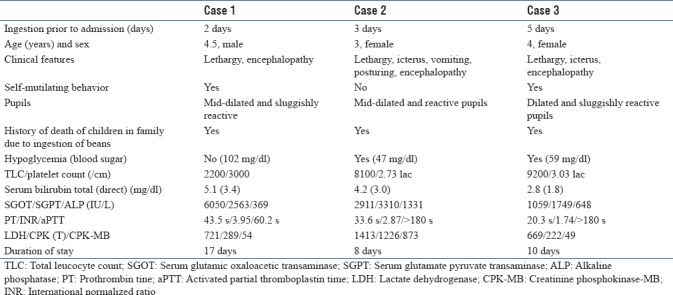
On the basis of clinical and biochemical abnormalities, the toxidrome was defined as hepatomyoencephalopathy syndrome. The alleged plant was identified as C. occidentalis. General resuscitative measures for airway, breathing, and circulation were immediately instituted and gastric lavage was done with charcoal. Standard management for liver failure and hepatic encephalopathy was instituted with dextrose infusion, high bowel wash, lactulose, Vitamin K, platelet transfusion, and antibiotics. On day 3, LFTs were worsened [Table 1] with concomitant worsening of hepatic encephalopathy to Stage 3. Supportive measures and intensive care management of acute hepatic failure were continued. The child started showing improvement in encephalopathy and LFTs on the 5th day of admission. Sensorium and hematologic abnormalities normalized and oral feeds were started by day 7. LFTs normalized on the 15th day and the child was discharged on day 17 with no sequel. The family was counseled before discharge to prevent the recurrences of poisoning.
Cases 2 and 3
Following the first case, we received two more children in next 1 month from the same geographical area with a history of consumption of seeds from “Kasoundi” plant. Both the children had jaundice, hepatic failure, and encephalopathy with a history of sibling death at home in both cases due to beans consumption. Blood investigations revealed deranged LFTs and markers of muscle involvement, with normal hematologic parameters [Table 1]. The children were immediately resuscitated, managed for acute hepatic failure, and discharged without complications. The demographic, clinical, and biochemical profile of all three cases is summarized in Table 1.
DISCUSSION
This series highlights the potential for life-threatening intoxication with seeds of Cassia spp. in children. Most reported cases of human poisoning coincide with fruiting season which is between September and November.[6] Cases were initially misdiagnosed as encephalitis, Reye's syndrome, and mystery disease till Vashishtha et al. showed an epidemiological relationship between consumption of seeds and illness in children.[4]
All cases had a history of sibling death at home. Children consumed seeds while playing and those with large intake probably succumbed at home. There is a dose–response relationship between intake and symptoms; consumption of 6–7 pods (250–300 seeds) may cause severe symptoms and consumption of seeds equal to cupped hand of a child may be fatal.[6]
Cassia poisoning affects multiple organ systems, but three predominantly affected systems are liver, muscle, and brain and hence the toxidrome of hepatomyoencephalopathy. In an animal experimental model, Panigrahi et al. established the toxicity of Cassia seeds in rats, demonstrating these three as the target organs.[7] Pathological changes in liver include diffuse hydropic change and ballooning in hepatocytes and focal or perivenular necrosis in acinar zone 2/3. Muscle changes include small, focal areas of sarcoplasmic degeneration, hyalinization, lysis, with mild-to-moderate proliferation of sarcolemmal nuclei. Brain tissues show mild spongiosis with focal gliosis.[3] In a recent study, chemo toxic profiling of Cassia seeds has implicated five anthraquinone aglycones (physcion, emodin, rhein, aloe-emodin, and chrysophanol) as the toxic moieties responsible for symptomatology in Cassia poisoning.[8]
The acute symptoms of this toxidrome include fever, vomiting preceding unconsciousness, lethargy and agitation, blood pressure fluctuations, teeth grinding/self-mutilation, dilated or poorly reacting pupils, seizures, and respiratory abnormality.[3] Children with low dose prolonged exposure suffer from lethargy, weakness, recumbency, learning disability, and reduced memory. Children with these milder symptoms may not seek medical attention and hence the actual prevalence of poisoning may be much higher than that reported.[7] The plant contains multiple toxins and no specific antidote is available; however, most affected children can be salvaged by aggressive supportive management for acute liver failure as highlighted in this series. Aggressive public awareness campaigns can reduce the burden of poisoning as shown by a successful multimodal campaign resulting in disappearance of disease from Saharanpur district, Uttar Pradesh.[9]
CONCLUSION
Our case series demonstrates clinical and biochemical spectrum of Cassia seeds poisoning and highlights the importance of keeping Cassia poisoning as a differential for acute liver failure and encephalopathy in children even when a definitive history of consumption is unavailable. More awareness is needed not only in community regarding deleterious effect of plant but also at referral centers for rapid diagnosis and appropriate management of this potentially treatable disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Srivastava A, Peshin SS, Kaleekal T, Gupta SK. An epidemiological study of poisoning cases reported to the National Poisons Information Centre, All India Institute of Medical Sciences, New Delhi. Hum Exp Toxicol. 2005;24:279–85. doi: 10.1191/0960327105ht527oa. [DOI] [PubMed] [Google Scholar]
- 2.Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S, et al. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia. 2010;81:223–30. doi: 10.1016/j.fitote.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Vashishtha VM, Nayak NC, John TJ, Kumar A. Recurrent annual outbreaks of a hepato-myo-encephalopathy syndrome in children in Western Uttar Pradesh, India. Indian J Med Res. 2007;125:523–33. [PubMed] [Google Scholar]
- 4.Vashishtha VM, Kumar A, John TJ, Nayak NC. Cassia occidentalis poisoning causes fatal coma in children in Western Uttar Pradesh. Indian Pediatr. 2007;44:522–5. [PubMed] [Google Scholar]
- 5.Kumar A. Cassia poisoning behind mysterious disease in children in Uttarakhand. Indian Pediatr. 2008;45:423. [PubMed] [Google Scholar]
- 6.Vashishtha VM, John TJ, Kumar A. Clinical & pathological features of acute toxicity due to Cassia occidentalis in vertebrates. Indian J Med Res. 2009;130:23–30. [PubMed] [Google Scholar]
- 7.Panigrahi G, Tiwari S, Ansari KM, Chaturvedi RK, Khanna VK, Chaudhari BP, et al. Association between children death and consumption of Cassia occidentalis seeds: Clinical and experimental investigations. Food Chem Toxicol. 2014;67:236–48. doi: 10.1016/j.fct.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Panigrahi GK, Ch R, Mudiam MK, Vashishtha VM, Raisuddin S, Das M, et al. Activity-guided chemo toxic profiling of Cassia occidentalis (CO) seeds: Detection of toxic compounds in body fluids of CO-exposed patients and experimental rats. Chem Res Toxicol. 2015;28:1120–32. doi: 10.1021/acs.chemrestox.5b00056. [DOI] [PubMed] [Google Scholar]
- 9.Panwar RS. Disappearance of a deadly disease acute hepatomyoencephalopathy syndrome from Saharanpur. Indian J Med Res. 2012;135:131–2. doi: 10.4103/0971-5916.93436. [DOI] [PMC free article] [PubMed] [Google Scholar]


