Abstract
Background:
The recommended treatment for refractory status epilepticus (RSE) is the use of anesthetic agents, but evidence regarding the agent of choice is lacking. This study was designed to compare target-controlled infusion of propofol versus midazolam for the treatment of RSE regarding seizure control and complications.
Methods:
This prospective, randomized study recruited 23 adult patients with RSE due to any etiology and treated with either propofol or midazolam titrated to clinical seizure cessation and gradual tapering thereafter. The primary outcome measure was seizure control and the secondary outcomes were duration of the Intensive Care Unit stay and duration of mechanical ventilation, occurrence of super RSE (SRSE), and complications.
Results:
We recruited 23 patients (male:female = 18:5) into this study (propofol Group-11; midazolam Group-12). Overall, seizure control was noted in 34.8%, with successful seizure control in 45% of patients in the propofol group and 25% in midazolam group (P = 0.4). Mortality was similar in both the groups (propofol group [8/11; 72.7%] compared to the midazolam group [7/12; 58.3%] [P = 0.667]). The duration of hospital stay was significantly shorter in the propofol group compared to midazolam (P = 0.02). The overall incidence of SRSE was 69.5% in this study. The complication rate was not significantly different between the groups.
Conclusions:
The choice of anesthetic agent does not seem to affect the overall outcome in RSE and SRSE. Target-controlled propofol infusion was found to be equal in its efficacy to midazolam for the treatment of RSE. High mortality might be due to SRSE secondary to the underlying brain pathology.
Keywords: Midazolam, outcome, propofol, refractory status epilepticus, super refractory status, target-controlled infusion
INTRODUCTION
Refractory status epilepticus (RSE) is recognized as one of the most critical neurological emergencies with high mortality and morbidity. RSE develops in approximately 30%–40% of patients with status epilepticus (SE) and the reported mortality ranges around 19%–67% depending on the type of SE.[1] Over the last decade, a major effort has been made in prompt diagnosis and aggressive treatment of SE based on the realization that untreated or undertreated SE can lead to irreversible neuronal damage, independent of metabolic, and systemic consequences.[2]
SE is defined as 5 min or more of (i) continuous clinical and/or electrographic seizure activity or (ii) recurrent seizure activity without recovery (returning to baseline) between seizures.[3] RSE is defined as SE that fails to respond to first-line drugs and any two drugs in the second-line therapy and it is observed in 9%–31% of patients with SE,[4] with mortality ranging from 16% to 39%. The first-line drugs for the treatment of SE are benzodiazepines, specifically lorazepam. The second line includes antiepileptic drugs such as phenytoin, levetiracetam, or valproic acid. A prospective randomized study compared the seizure control and outcome between phenytoin, valproate and levetiracetam and found that all are equally efficacious and no significant difference is noted in the outcome.[5] Super RSE (SRSE) is defined as RSE that continues or recurs 24 h or more after induction of anesthetic coma including those cases where SE recurs on the reduction or withdrawal of anesthetic drugs.[6,7]
RSE is associated with acute, severe, and potentially fatal underlying causes such as encephalitis, massive stroke or rapidly progressive primary brain tumors and is typically accompanied by severe impairment of consciousness.[8] Mortality after RSE is about three times higher than nonrefractory SE During most fatalities, death does not occur during persisting SE; however, after its resolution and is generally attributable to the underlying clinical problem.[9] Despite its clinical and socioeconomic impacts, management of RSE has only been studied in small retrospective reviews and prospective studies without controls.[1] Evidence is lacking regarding which of the two drugs, midazolam or propofol, is better in regarding seizure control and clinical outcome in patients with RSE.[6]
Plasma concentration (Cp) of intravenous (IV) anesthetics are not measured or monitored during routine clinical management of RSE. It is possible that inappropriate pharmacokinetics may be responsible for failure to control seizures in RSE or unacceptable side effects. Target-controlled infusion (TCI), which provides a constant Cp and effect-site concentration of the anesthetic agent might provide insights into the appropriate dose requirement for effective seizure control in RSE.
This prospective, randomized study was carried out with an aim to compare efficacy of TCI of propofol and midazolam in patients with RSE.
METHODS
This prospective, randomized study was conducted after obtaining approval from the Institutional Ethical Committee of a university teaching hospital. Written informed consent was obtained from each of the patient's next of kin. Adult patients (16–60 years) with RSE of any etiology, unresponsive to the first-line IV lorazepam (0.1 mg/kg) and any two of the second-line IV anti-epileptic drugs (phenytoin [15 mg/kg], valproate [20–25 mg/kg], and levetiracetam [30 mg/kg]) were included in the study. Patients with known allergy to the study drug, history of coronary artery disease, documented clinical or two-dimensional-echo cardiac evidence of the left ventricular dysfunction and those in hypotension were excluded from the study. Patients were randomized to one of the two arms (midazolam infusion or target-controlled propofol) based on computer-generated random numbers.
After establishing the diagnosis of RSE, patients underwent a quick, comprehensive examination including the history, physical and systemic examination, and neurological status assessment. The patient's trachea was intubated, standard monitoring (electrocardiography, pulse oximetry and noninvasive blood pressure) was instituted. IV access was secured using a central venous cannulation, and invasive arterial blood pressure monitoring established. IV fluids were administered to the patient based on the hemodynamic parameters. The hemodynamic data were collected 6th hourly and biochemical and hematological investigations along with arterial blood gas analysis were recorded at least once daily and as and when indicated. As a part of the study protocol, second-line antiepileptic drugs were continued during the infusion of propofol or midazolam.
Study drug initiation and escalation protocol
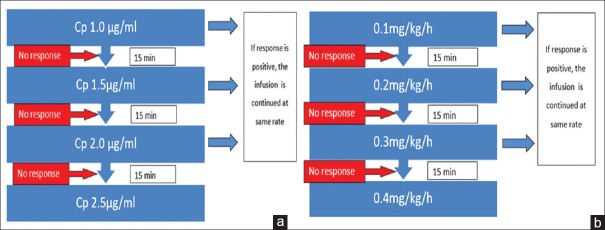
In the propofol group, TCI was started at a Cp of 1.0 μg/ml and escalated based on seizure response [Figure 1a]. If seizures recurred during propofol infusion, 20 mg IV bolus of propofol was administered. If the seizures recurred more than three times in 15 min, or the seizures were not controlled for 15 min, then the dose was escalated by 0.5 μg/ml. The Cp of propofol required for successful seizure control was recorded. If seizures were not controlled even at Cp 2.5 μg/ml, the SE was considered super refractory and study drug treatment failure was recorded. Thereafter, the study drug infusion was terminated and thiopentone administered as a 3 mg/kg bolus followed by a continuous infusion at a rate of 3–5 mg/kg/h.
Figure 1.
Study protocol. (a) Protocol for propofol infusion; (b) protocol for midazolam infusion
In midazolam group, the study drug was administered as a bolus of 0.05 mg/kg followed by an infusion as per the study protocol [Figure 1b]. In case seizures recurring with this dose, 0.05 mg/kg IV bolus was administered. If the seizures recurred >3 times in 15 min, or the seizures were not controlled for 15 min, the dose was escalated to next higher level as shown in Figure 1. If the seizures are not controlled at an infusion dose of 0.4 mg/kg/h, the SE was considered super-refractory, and treatment failure of the study drug was considered. Thereafter, thiopentone infusion was initiated with a bolus of 3 mg/kg followed by a continuous infusion at a rate of 3–5 mg/kg/h.
SE severity score, the need for and the duration of inotropic/vasopressor support and any other complications were noted during therapy.
Study drug tapering protocol
After successful control of seizures for 48 h, the study drugs were tapered as follows: (a) Midazolam was tapered at a rate of 1 mg 12th hourly; (b) Propofol was tapered as Cp decrements by 0.3 μg/ml 12th hourly.
At any stage during tapering, if the seizures recurred, then tapering was halted and the dose increased to the previous level and continued for another 24 h. Tapering was restarted if there was no seizure during this 24 h period. If tapering failed for five times consecutively, the study drug was considered to have failed.
Definitions of outcome
Breakthrough seizures
Recurrence of the seizure despite initial control, resulting in either the escaltion of drug dose or change of the drug itself.
Withdrawal seizures
Recurrence of seizure during or immediately after the tapering or withdrawal of the therapy.
Successful therapy
The SE is completely controlled by the therapy, without breakthrough or withdrawal seizures, or discontinuation due to side-effects, or death during the therapy.
Initial failure
The therapy failed to control SE.[6]
Data collection
Demographic information and data of the seizures and SE among the recruited patients for the study were collected. In the propofol group, Cp, total duration of infusion, number of boluses to control breakthrough seizures and time to seizure control were collected. In midazolam group, the maintenance dose of midazolam, number of midazolam boluses to control breakthrough seizures, total duration of infusion and time to seizure control were collected.
Outcome measures
The primary outcome measure was successful seizure control by the study drug. The secondary outcome measures were as follows: length of the Intensive Care Unit (ICU)/hospital stay, duration of mechanical ventilation; extended Glasgow outcome scale/modified Rankin scale at the time of discharge; and percentage of patients going into super-refractory SE. The following systemic side effects were also recorded-respiratory infection according to CDC guidelines; acute kidney injury according to RIFLE criteria, requirement for inotropic support and the duration of inotropic support, liver dysfunction, and dyselectrolytemia.
Statistical analysis
Quantitative data were analyzed using Mann–Whitney U-test. The difference of qualitative data between the groups was established using Fisher's exact test. Drug stoppage times were analyzed using Kaplan-Meier survival curves and compared between groups using log-rank test. P < 0.05 was considered statistically significant.
RESULTS
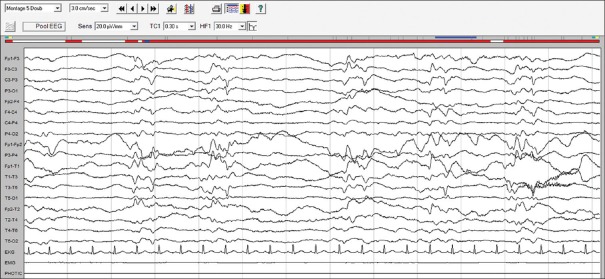
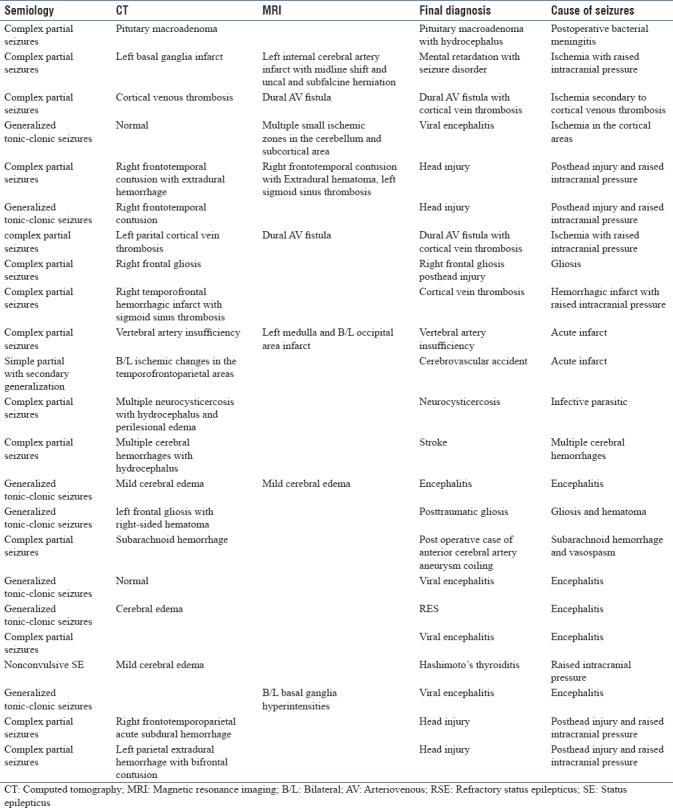
Twenty-four patients were recruited and randomized to two groups, however, one of the patients in the propofol group was lost for follow up; hence, 23 patients were analyzed (propofol n = 11, midazolam n = 12) [Figure 2]. The demographic characteristics were comparable between the two groups [Table 1]. The electroencephalography (EEG) recording (n = 11) showed normal background in 2 patients, slowing of background in 8, and alpha coma in 1 while epileptiform discharges were noted in nine patients, interictal discharges in three, periodic lateralized epileptiform discharges in three [Figure 3] and nonconvulsive SE in three. The clinical and imaging details are presented in Table 2.
Figure 2.
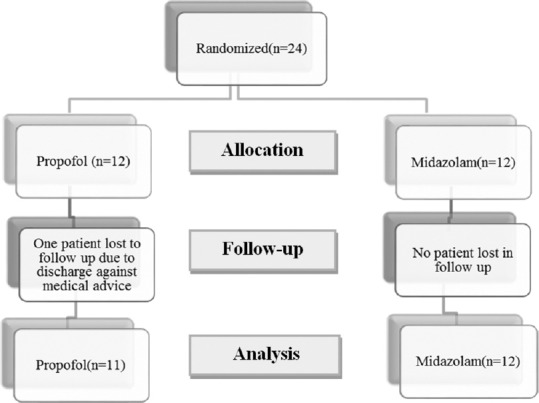
Flow diagram of the study
Table 1.
Demographic and seizure data

Figure 3.
Electroencephalography showing periodic short-interval epileptiform activity from the left hemisphere (temporocentral region) suggestive of periodic lateralized epileptiform discharges
Table 2.
Semiology, imaging, final diagnosis, and the cause of seizure
Doses and serum levels of study drugs
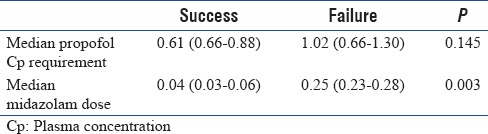
Table 3 shows that there was no difference in the median propofol Cp requirement (0.62 [0.60–0.88] vs. 1.03 [0.67–1.39]) or median midazolam serum levels (0.05 [0.04–0.07] vs. 0.25 [0.24–0.28]) between successfully treated patients and patients in whom study drug has failed.
Table 3.
Median propofol plasma concentration and median midazolam dose between success and failure patients
Seizure outcome
Seizures could be controlled in 34.8% in this cohort with the study anesthetic medications. Successful seizure control was noted in 45% of patients in the propofol group and 25% patients in the midazolam group (P = 0.4). The time taken for seizure control after starting the study anesthetic medications was not significantly different between the two groups. The difference in the duration of infusion before seizure control was not significant (P = 0.083) between the two groups [Table 4].
Table 4.
Secondary outcome parameters between the two groups
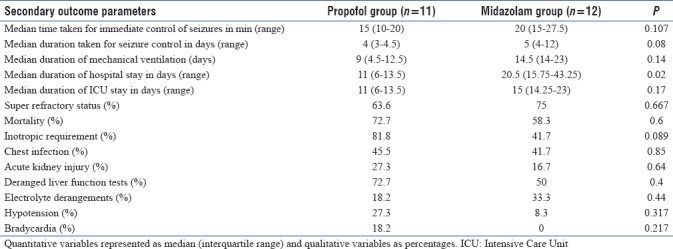
The duration of hospital stay was significantly less in the propofol group compared to the midazolam group (P = 0.02). The duration of mechanical ventilation and the duration of ICU stay was not significantly different between the two groups.
Sixteen out of 23 patients (69.5%) among this cohort developed SRSE, namely uncontrolled SE beyond 24 h in spite of administering the anesthetic medications. Three-fourths of the patients in the midazolam group developed super-refractory SE, whereas 63.6% of the patients in the propofol group developed superrefractory SE (P = 0.667).
Fifteen out of 23 patients (65.2%) succumbed to the illness. The mortality was higher in the propofol group (8/11; 72.7%) compared to the midazolam group (7/12; 58.3%) though the difference was not statistically different (P = 0.6) [Table 4].
Duration for stoppage of medication
Figure 4 shows the Kaplan–Meier curves presenting the median duration for stoppage of the infusion of the drugs. The duration for stopping propofol was longer (median: 120 h; 95% confidence intervals: 98–141 h) compared to midazolam (median: 96 h; 95% confidence intervals: 83–108 h). However, no statistically significant difference exists between the observed values (P = 0.8).
Figure 4.
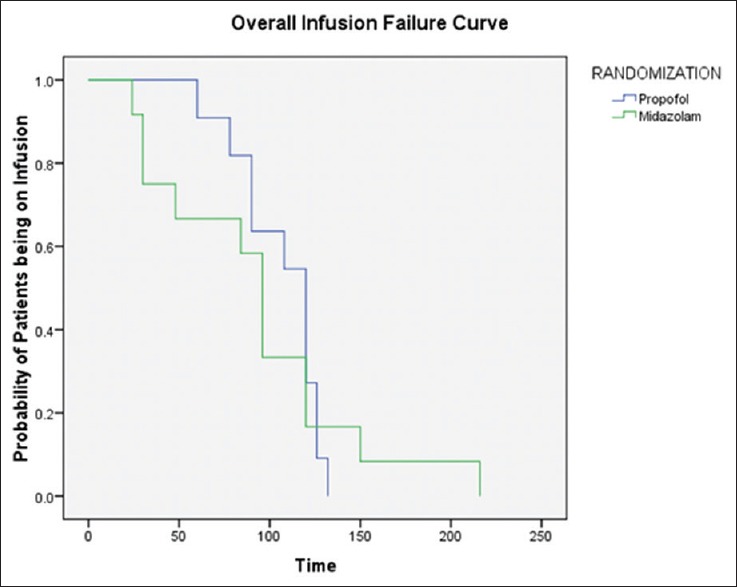
Kaplan–Meier curves for the stoppage of drug infusion
Complications
There was no significant difference between the two groups with regard to the incidence of acute kidney injury (P = 0.64), chest infection (P = 0.85), elevated liver enzymes (P = 0.4), or electrolyte derangements (P = 0.44). Inotropic requirement was higher in the propofol group (81.8%) as compared to the midazolam group (41.7%) with difference trending toward significance (P = 0.089). The incidence of hypotension and bradycardia also did not show any significant difference [Table 4].
DISCUSSION
There are very few prospectively conducted randomized studies in the treatment of patients with the RSE.[10] The present study compared the efficacy of target-controlled propofol and midazolam infusions in the treatment of patients with RSE. This study is unique in that it employed “TCI” using propofol to control the RSE. The Cp recorded using TCI of propofol may be taken as an equivalent of the serum level of propofol as the median performance error with the TCI is <10% in previously published studies.[11,12]
This study did not demonstrate any significant difference between the two study drugs in controlling seizures. Overall, seizure control was achieved in about only one-third of the patients. This could be due to the underlying etiology and the majority developing SRSE seizure control was possible in 45% of patients with propofol, which is comparable with the 50% control reported by Rossetti et al.[10] However, Rossetti et al. titrated the dose of propofol based on the EEG burst suppression where the maximal infusion dose for achieving burst suppression was 5 mg/kg/h. In the present study, we did not use continuous EEG monitoring for seizure control but clinical seizure control was targeted using TCI. In a retrospective study by Prabhakar et al., which compared propofol and barbiturates, seizure control was 42% in the propofol group which is similar to the current study.[13] In another retrospective review comparing propofol and midazolam in RSE, the authors reported a higher success rate of 64% with propofol for clinical seizure control.[14] In a prospective study by Parviainen et al., the authors documented very high effectiveness of propofol in RSE (80%).[15] In our study, the seizure control in midazolam group is 25% whereas in a prospective study, Singhi et al. observed 86% success with midazolam in patients with RSE.[16] However, their study was carried out on pediatric patients with RSE between 2 and 12 years while the cohort in the present study consisted mainly of adult patients in fifth decade. The differences in the outcomes in various studies could be due to heterogeneous etiologies, size of the cohort, age group of the cohort, availability of the EEG monitoring, prospective or retrospective nature of the study, and variation in the comparative study drugs.
Another important observation in this study was that seizure control after starting infusions was 15 min in the propofol group and 20 min in the midazolam group (P = 0.107). A previous study by Stecker et al. on RSE with propofol however, had noted the mean duration between the initiation of propofol and seizure control was 2.6 min.[17] The probable reason for this difference might be the variation in the rate of infusion. In this study, patients were on TCI from the beginning and the loading dose duration was set for 10 min to avoid hypotension. While in their study, protocol dictated IV bolus of 1 mg/kg over 5 min and if seizures are not controlled, another 1 mg/kg was administered over the next 5 min. Anand Kumar et al. reported that in RSE treated with midazolam bolus followed by infusion, the mean duration required for the immediate control of the seizure with midazolam was 1.5 min, which was lesser than in the current study. This is probably due to higher initial bolus dose in their study 0.22 mg/kg (mean value) compared to 0.05 mg/kg in the current study.[18]
The median duration to achieve seizure control in the current study is 4 and 5 days in propofol group and midazolam group, respectively. The difference is not significant (P = 0.083). Rossetti et al.[10] observed 2.5 days in their study. Similar results were noted in a retrospective series where they had observed a seizure control duration of 3 days in propofol-treated patients.[19]
The percentage of patients who developed superrefractory SE among the propofol group was 63.6% where as in midazolam group it was 75%. Due to long duration of convulsion time and underlying etiology of SE, internalization of GABA receptors might have led to the SRSE.[6] Two other retrospective studies,[19,20] on RSE reported that 16.9% and 17%, respectively had SRSE and they concluded that encephalitis was the main determinant for progression of SE to SRSE. The reasons for higher rates of SRSE in the current study might be the longer duration of convulsion time before starting the second-line AEDs, which was 48 h and 24 h in the propofol and midazolam groups, respectively, and the underlying etiology of anoxia and encephalitis in some of them.
The overall mortality in this study was 65.2%. The mortality rate in propofol group was 72.7% and in midazolam group, it was 58.3%; however, the difference was not statistically significant. The high-mortality rate in this study might be because of the higher number of patients with SRSE, which is a known independent predictor of high mortality.[17,18,21] Another important reason could be the long delay in the initiation of treatment which is a predictor of mortality.[22] In a large cohort of 100 patients with fatal SE, prolonged duration of SE, long delay before instituting treatment, poor Glasgow Coma Score at admission to the neurological services, poor drug compliance, and high frequency of neuroinfections were important factors for poor prognosis.[22] Stecker et al. also reported a high mortality of 68%. Their explanation for high mortality was multiorgan failure as indicated by the high Acute Physiology And Chronic Health Evaluation scores.[17] Another retrospective study,[15] comparing propofol and midazolam in RSE patients also found higher mortality in the propofol group (57%) compared to midazolam group (17%). Their patients in the propofol group had acute brain injury and long duration of convulsion before starting the anesthetic drugs.
The percentage of patients who developed chest infection in propofol group was 45.5% compared to 41.7% in the midazolam group (P = 0.85) in the ICU. Interestingly, none with chest infections in the propofol group and 40% in the midazolam group recovered. Acute kidney injury was noted in 27.3% and 16.7% of the patients in propofol and midazolam groups, respectively. The percentage of patients developing liver dysfunction was also similar between the two groups (72.7% vs. 50.0%) but among patients who survived, the liver function recovered completely at discharge. Ionotropic requirement was more frequent in the propofol group (81.8%) compared to midazolam (41.7%) (P = 0.089). Similar results (48%–65%) were seen in the earlier series.[19,23] Other side effects such as arterial hypotension, bradycardia, inotropic requirement, and dyselectrolytemia were comparable in the two arms, thus not supporting assumption that propofol leads to more profound cardiovascular depression compared to the midazolam.[24,25] Stecker et al. did not find any difference in arterial hypotension between patients receiving propofol and barbiturates.[17] Similar results were noted in a retrospective comparison of propofol and midazolam in RSE. The study did not find any difference regarding seizure control, infectious complications, hemodynamic compromise, duration of mechanical ventilation, and mortality.[14]
The length of hospital stay was significantly longer in the midazolam group (20.5 days) compared to propofol group (11 days). The duration of mechanical ventilation and the length of ICU stay was not significantly different between the two groups. Similar results have been reported in a comparative study involving propofol and midazolam.[14] Another prospective randomized study comparing propofol with thiopentone found that the duration of mechanical ventilation was longer in the thiopentone group.[10] Another RSE series found no difference regarding the duration of ICU stay or the incidence of arterial hypotension between patients receiving propofol and those receiving barbiturates.[17] The mean duration of neonatal ICU stay and mechanical ventilation was 17.4 ± 14.5 was 14.4 ± 12.8 days, respectively, in one of the retrospective studies.[23]
The Cp of propofol was comparable in the successful and failed groups. The reason for this may be a low sample size (n = 11). Alternatively, seizure pathogenesis and its amelioration are a dynamic process whose outcome may not be predicted by the dose of anesthetic agent causing initial seizure control. This is reflected by the fact that patients achieving seizure control with a certain Cp of propofol and had recurrence of seizure activity during tapering could not be controlled with the same dose thereafter. Similar result was noted in another prospective study wherein the authors analyzed the pharmacokinetics of propofol between the successful and failed patients.[17] The median dose of midazolam was measured between the successful and failed patients which showed a higher median dose in the failed patients. There are studies which compared high-dose versus low dose midazolam,[26] but none of the studies measured median dose between the successful and failed groups.
We used Kaplan–Meier curves to analyze the median duration of stoppage of infusion. It was 120 h in the propofol group compared to 96 h in the midazolam group (P = 0.8). Kaplan–Meier curves were used because the timing of stoppage of infusion was different among the different patients. The median duration of infusion was shorter in the midazolam group (although not statistically significant), and the cause of infusion termination was either failure to control seizures or refractory hypotension.
This study was intended to gather evidence regarding the efficacy of which anesthetic agent is better for seizure control and had acceptable adverse events. Between the two agents used, seizure control rate was comparable and the complication rates were not different though the length of hospital stay is longer in the midazolam group. There are several limitations of the study, namely small sample size which was mostly due lack of beds in the ICU which has led to early referral of patients to other healthcare facilities, varying referral patterns, the availability of the investigators round the clock and the lack of continuous EEG monitoring which would have given a better idea about the nonconvulsive SE among the patients. From this exploratory randomized study, it might be concluded that the choice of agent does not seem to affect the overall outcome in RSE. Target-controlled propofol infusion was found to be equally efficacious to midazolam for the treatment of RSE. Treatment of RSE, mostly relied on expert opinions and data of low evidence level since several decades.[27] It is necessary to carry out well-designed multicentric studies with a larger number of subjects with consecutive recruitment of patients with both RSE and SRSE comparing various IV anesthetic agents, continuous EEG monitoring and assessment of both short and long-term outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the ICU staff of the NIMHANS hospital for their constant support and cooperation. We also thank the toxicology laboratory staff and the EEG laboratory staff for their support during the study.
REFERENCES
- 1.Kang BS, Jung KH, Shin JW, Moon JS, Byun JI, Lim JA, et al. Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus. J Clin Neurosci. 2015;22:854–8. doi: 10.1016/j.jocn.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Rossetti AO, Logroscino GB. Refractory status epilepticus: Is EEG burst suppressin an appropriate treatment target during drug induced coma. What is the holy grail? Epilepsy Curr. 2006;6:119–20. doi: 10.1111/j.1535-7511.2006.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 4.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF, et al. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–10. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 5.Mundlamuri RC, Sinha S, Subbakrishna DK, Prathyusha PV, Nagappa M, Bindu PS, et al. Management of generalised convulsive status epilepticus (SE): A prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam – Pilot study. Epilepsy Res. 2015;114:52–8. doi: 10.1016/j.eplepsyres.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135:2314–28. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 7.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–18. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: Still more questions than answers. Lancet Neurol. 2011;10:922–30. doi: 10.1016/S1474-4422(11)70187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: A prospective observational study. Epilepsia. 2010;51:251–6. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 10.Rossetti AO, Milligan TA, Vulliémoz S, Michaelides C, Bertschi M, Lee JW, et al. Arandomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14:4–10. doi: 10.1007/s12028-010-9445-z. [DOI] [PubMed] [Google Scholar]
- 11.Cortínez LI, De la Fuente N, Eleveld DJ, Oliveros A, Crovari F, Sepulveda P, et al. Performance of propofol target-controlled infusion models in the obese: Pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2014;119:302–10. doi: 10.1213/ANE.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 12.Lei X, Yan Li Z, Yu Lei G, Jie Z, En You L, Fu Jun Q, et al. Determination of the plasma propofol concentrations in surgical patients and evaluation of the target-controlled infusion system. Pharm Care Res. 2015;15:134–7. [Google Scholar]
- 13.Prabhakar H, Bindra A, Singh GP, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus. Cochrane Database Syst Rev. 2012;8:7–8. doi: 10.1002/14651858.CD009202.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Worrall BB, Bertram EH, Bleck TP. Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia. 2001;42:380–6. doi: 10.1046/j.1528-1157.2001.27500.x. [DOI] [PubMed] [Google Scholar]
- 15.Parviainen I, Uusaro A, Kälviäinen R, Mervaala E, Ruokonen E. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32:1075–9. doi: 10.1007/s00134-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 16.Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17:106–10. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 17.Stecker MM, Kramer TH, Raps EC, O'Meeghan R, Dulaney E, Skaar DJ, et al. Treatment of refractory status epilepticus with propofol: Clinical and pharmacokinetic findings. Epilepsia. 1998;39:18–26. doi: 10.1111/j.1528-1157.1998.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 18.Anand Kumar M, Thomas P, Bleck M. Midaz infusion in refractory status epilepticus. Crit Care Med. 1992;20:483–8. doi: 10.1097/00003246-199204000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti AO, Reichhart MD, Schaller MD, Despland PA, Bogousslavsky J. Propofol treatment of refractory status epilepticus: A study of 31 episodes. Epilepsia. 2004;45:757–63. doi: 10.1111/j.0013-9580.2004.01904.x. [DOI] [PubMed] [Google Scholar]
- 20.Giovannini G, Monti G, Polisi MM, Mirandola L, Marudi A, Pinelli G, et al. Aone-year prospective study of refractory status epilepticus in Modena, Italy. Epilepsy Behav. 2015;49:141–5. doi: 10.1016/j.yebeh.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Jayalakshmi S, Ruikar D, Vooturi S, Alladi S, Sahu S, Kaul S, et al. Determinants and predictors of outcome in super refractory status epilepticus – A developing country perspective. Epilepsy Res. 2014;108:1609–17. doi: 10.1016/j.eplepsyres.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Sinha S, Satishchandra P, Mahadevan A, Bhimani BC, Kovur JM, Shankar SK, et al. Fatal status epilepticus: A clinico-pathological analysis among 100 patients: From a developing country perspective. Epilepsy Res. 2010;91:193–204. doi: 10.1016/j.eplepsyres.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Parviainen I, Uusaro A, Kälviäinen R, Kaukanen E, Mervaala E, Ruokonen E, et al. High-dose thiopental in the treatment of refractory status epilepticus in Intensive Care Unit. Neurology. 2002;59:1249–51. doi: 10.1212/01.wnl.0000032253.88378.d7. [DOI] [PubMed] [Google Scholar]
- 24.Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: An 11-year clinical experience. Crit Care Med. 2009;37:3024–30. doi: 10.1097/CCM.0b013e3181b08ac7. [DOI] [PubMed] [Google Scholar]
- 25.Sinha S, Prashantha DK, Thennarasu K, Umamaheshwara Rao GS, Satishchandra P. Refractory status epilepticus: A developing country perspective. J Neurol Sci. 2010;290:60–5. doi: 10.1016/j.jns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez A, Lantigua H, Lesch C, Shao B, Foreman B, Schmidt JM, et al. High-dose midazolam infusion for refractory status epilepticus. Neurology. 2014;82:359–65. doi: 10.1212/WNL.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]