Abstract
Introduction:
Lack of restorative sleep and altered sleep-wake cycle is a frequent problem among patients admitted to the Intensive Care Unit (ICU). This study was conducted to estimate the prevalence of poor sleep and patient's perspective of factors governing poor sleep in the ICU.
Materials and Methods:
A cross-sectional study was performed in medical ICU of a tertiary care hospital. A total of 32 patients admitted to the ICU for at least 24 h were recruited. A 72-h actigraphy was done followed by a subjective assessment of sleep quality by the Richards-Campbell Sleep Questionnaire (RCSQ). Patient's perspective of sleep quality and quantity and possible risk factors for poor sleep were recorded.
Results:
Poor sleep (defined as RCSQ <50, sensitivity 88% and specificity 87%) was found in 15 out of the 32 patients (47%). The prevalence of poor sleep was higher among patients on mechanical ventilation (n = 15) (66.7% vs. 33.3%, P < 0.05). Patients with poor sleep had higher age (median age [in years] 42.8 vs. 31.4, P = 0.008), acute physiology, and chronic health evaluation II score (mean 14 ± 5.15 vs. 9.3 ± 5.64, P = 0.02), SAPS 3 score (62.7 ± 8.9 vs. 45.6 ± 10.5, P ≤ 0.0001), and worse actigraphy parameters. Only 55.63% of total sleep time was in the night (2200–0600). All patients had discomfort from indwelling catheters and suctioning of endotracheal tubes. All patients suggested that there be a minimum interruption in the sleep for interventions or medications.
Conclusion:
There is a high prevalence of poor sleep among patients admitted to the ICU. There is a dire need to minimize untimely interventions and design nonpharmacological techniques to allow patients to sleep comfortably.
Keywords: Actigraphy, Intensive Care Unit, Richards-Campbell Sleep Questionnaire, sleep quality
INTRODUCTION
Sleep is a naturally occurring periodic, reversible state of reduced consciousness, and response to external stimuli. Normal human sleep consists four to six 90–100 min blocks, during which nonrapid eye movement and rapid eye movement (REM) sleep alternate in a cyclical fashion accounting for a total sleep duration of 7–8 h/night.[1]
Sleep is an indispensable physiological need often underestimated and disregarded especially in critically ill patients.[2,3,4] Sleep in them is highly fragmented; therefore, they lack deep restorative REM sleep. Around 38.5% of the patients who survived critical illness and were on mechanical ventilation (MV) for at least 48 h reported not being able to sleep well, 40% of the study group remembered frequent awakenings in the night, and 35% recalled having had difficulty falling asleep during their Intensive Care Unit (ICU) admission.[5] Sleep deprivation has been associated with the release of inflammatory cytokines, worse cardiovascular outcomes, poorer immunological response, etc.[6] Sleep disruption induces a catabolic state, impairs cellular and humoral immune response,[7] and causes respiratory dysfunction due to muscle fatigue and central respiratory.[8] Sleep disturbances are known to impair consolidation of memory and cognitive function.[9,10,11,12,13]
Various factors implicated to cause sleep deprivation in ICU setting, such as delirium because of organic causes, underlying disease state, noise, and change in environment, MV and sedatives, unavailability of familial faces, etc.[3,10,14,15]
Polysomnography (PSG) is the gold standard for measuring sleep; but in the ICU setting, it is cumbersome and impractical.[13,16] Actigraphy, which is a validated substitute for PSG,[17] has been widely used in the ICU due to its low cost and minimally invasive nature.[18,19,20]
The primary objective of our study was to assess the quantity and quality of sleep in patients admitted to the ICU using actigraphy and Richards-Campbell Sleep Questionnaire (RCSQ) respectively. Our secondary objective was to know patient's perspective of factors related to poor sleep.
MATERIALS AND METHODS
A cross-sectional study was conducted in an eight-bed adult medical ICU of a tertiary care hospital in North India. The ICU caters to patients with sepsis, poisoning, multisystem dysfunction, and acute respiratory distress syndrome; there is a separate ICU for cardiology, gastroenterology, neurology, postoperative, and trauma patients which were not included as part of this study. There are eight nursing officers on duty at a time, with the patient: nurse ratio varying from 2:1 to 3:1 depending on the workload. There are two house physicians on duty at a time, with rounds being conducted once daily by the consultant physician, usually between 0930 and 1130. There is no barrier to compartmentalize each patient bed. There were only two beds by the window and no nonpharmacological aid-like earplugs and soothing mask available. Entry of one family relative was allowed every day between 1700 and 1800. Patients in the age group of 18–60 years who were able to give informed written consent and admitted for at least 24 h in ICU before actigraphy were included in the study. If intubated at the time of admission, s/he was recruited 24 h after extubation. Patients were screened by confusion assessment method-ICU questionnaire [21] for delirium, Modified Berlin Questionnaire [22] for premorbid sleep illness(s) and Epworth Sleepiness Score [23] for obstructive sleep apnea; those tested positive or found to be at an elevated risk were excluded. In addition, those with kidney disease and past or current history of the cerebrovascular accident were excluded as they are some of the known risk factors that alter sleep-wake cycle.[24,25] Ethical clearance was obtained from the Institutional ethics committee. Data were collected from January 2014 to June 2014.
Evaluation of the patients at the baseline
After admission to the ICU, data were collected regarding clinical and laboratory parameters at the time of admission; acute physiology and chronic health evaluation II (APACHE II) score,[26] and simplified acute physiology score 3 (SAPS 3)[27] scores were calculated (higher score associated with higher chances of mortality). After 24 h of ICU admission, patients were re-assessed on a routine basis and if inclusion criteria were met, 72-h actigraphy was done. After actigraphy study, assessment of sleep quality was done using RCSQ.[28] The researcher was not aware of the actigraphy results of the patient. Information regarding factors causing discomfort during ICU stay was collected with a preformed questionnaire. Individual suggestions to improve sleep quality in the ICU were also taken from the participants.
Actigraphy
The actigraph SOMNOwatch™ was worn on the nondominant arm by the participant. The activity was measured in epochs of 15 s and plotted as an actigraphy profile. The watch has seven channels including six internal channels (body position, three activity sensors, ambient light and patient marker, and one external signal input (AUX). It has a 12 analog to digital converter, with an adjustable sampling rate of 256/s to 1/s and adjustable storage rates of 1/120s to 256/s. It has internal data storage of 8 MB, has a size of 45 mm × 16 mm and weighs 30 g (including battery). DOMINOlight 1.3.0 version was used for analysis.
Scoring and sleep determination
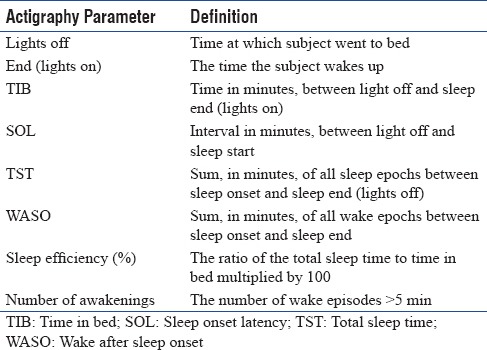
Automatic scoring using the event marker points (lights off and lights on) was checked by the same scorer to determine the participants' time in bed (TIB). Usually, through the integrated light sensor, the relevant measurement period (TIB) is determined. However, this method cannot be applied in ICU because the intensity of light is different from time to time and does not correlate with the sleeping periods of the patient. Therefore, patients were asked to press the marker button just before going to sleep and immediately after waking up from sleep. This was marked as lights off and lights on, respectively. The time between lights off and lights on was taken as total TIB. Based on this, actigraph gives us other sleep parameters [Listed in Table 1]. Usually, the circadian rhythm will be automatically calculated by actigraph by the light input sensor. But since, this was irrelevant in the ICU, we calculated circadian rhythm by dividing 24 h into three 8 h periods, (2200–0600, 0600–1400, and 1400–2200). The total sleep time during each phase was calculated and thus the distribution of sleep epochs was determined.
Table 1.
Definition of various actigraphy parameters
The overall perception of the sleep was collected by asking the question “How was your sleep during the ICU stay?” with a dichotomous response collected, i.e. either good or poor. Since judgment by the patient is the best way to assess sleep, we considered the overall self-perception of sleep as the standard. Richards Campbell sleep questionnaire (RCSQ) was then applied to objectively evaluate sleep quality. It consists of a 5-item questionnaire evaluating sleep depth, latency, number of awakenings, efficiency, and quality (with a sixth parameter, noise added in modified RCSQ). The technique is the same as described by Kamdar et al.[29]
Based on these qualitative and quantitative answers, we generated a cut in value for RCSQ score. Based on this new standard of RCSQ score, we defined the poor sleep as RCSQ score below the derived cut off value.
Sleep disturbance definition
A total RCSQ score of ≥50 was used to define good sleep (sensitivity 88.24%, specificity 86.67%) (receiver operating characteristic [ROC] area- 0.92, 95% confidence interval [CI]). Patients with total RCSQ score <50 were considered to have poor sleep.
Statistical analysis
Statistical analysis was performed using STATA version 12.0 (STATA Corporation, College Station Road, Houston, Texas, USA). The data analyzer was aware of the patient group. A sample size of convenience was taken. Two-tailed P < 0.05 was considered statistically significant. The statistical tests used are the same as listed by the author(s) previously.[30]
RESULTS
A total of seventy patients were screened, out of whom forty fulfilled the criteria of the study. Of the patients who fulfilled the inclusion criteria, 35 gave informed consent. Of the 35 patients, three could not complete the study, because of early transfer out from ICU to the ward [Figure 1]. The characteristics of the enrolled patients are given in Table 2. The mean age of the study population was 36.8 ± 12.7 years. There were 18 males and 14 females in the study population. The mean APACHE II score of the study group was 11.5 ± 5.8.
Figure 1.
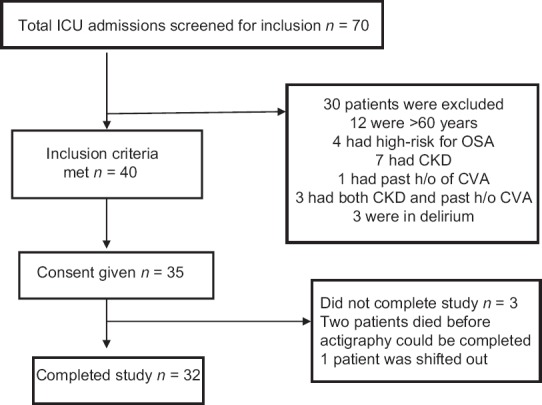
Study flow diagram
Table 2.
Baseline characteristics of the population
Actigraphy
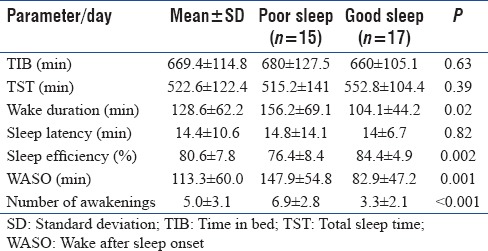
The actigraphy parameters among those with good sleep (RCSQ score ≥50 mm) and with poor sleep (RCSQ score <50 mm) are shown in Table 3. Although TIB and TST did not differ in the two groups, there was a significant difference in sleep efficiency, WASO, and a total number of awakenings.
Table 3.
Actigraphy parameters
Richards-Campbell Sleep Questionnaire
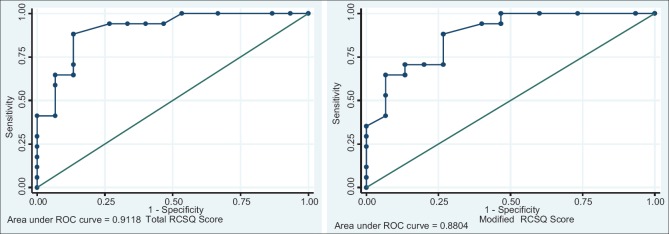
The mean RCSQ score of our study population was 51.6 ± 13.5 mm. An RCSQ score of ≥50 mm had a sensitivity of 88.2% and specificity of 86.7% in determining patients with good sleep as assessed by patient perception (ROC area 0.91, CI 95%) [Figure 2]. Patients with RCSQ score ≤50 mm were considered to have poor sleep. According to this definition, the prevalence of poor sleep among studied patients in medical ICU (MICU) 46.8% (15/32) [Table 4].
Figure 2.
Receiver operating characteristic curve of total Richards-Campbell Sleep Questionnaire score and modified Richards-Campbell Sleep Questionnaire score
Table 4.
Richards-Campbell Sleep Questionnaire parameters
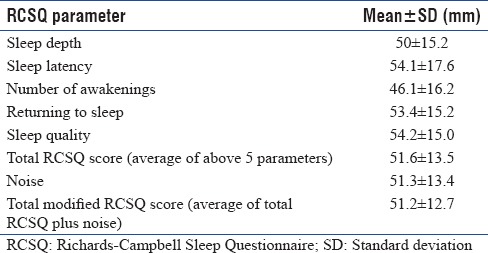
A sixth parameter used in previous studies along with the five parameters of RCSQ was noise. The mean modified RCSQ score was 51.2 ± 12.7 mm. A cutoff score of ≥48 mm of modified RCSQ had a sensitivity 88.2% and specificity of 73.3% (ROC area 0.88, CI: 95%) in defining patients with good sleep. The prevalence of poor sleep by modified RCSQ score was 40.6% (13/32).
The patients with good sleep were younger than those with poor sleep (mean age 31.4 ± 12.3 vs. 42.8 ± 10.6 years, P = 0.008). There was no significant difference in perception of poor sleep with regards to gender distribution (47.1% vs. 40.0%, P = 0.69). A total of 15 patients (46.9%) were mechanically ventilated during the ICU stay and had received sedative medication. The percentage of poor sleep was higher in patients who were mechanically ventilated, in comparison to those who were not (66.7 % vs. 33.3%, P = 0.035). Patients with poor sleep had a significantly higher APACHE II score when compared to patients with good sleep (14 ± 5.2 vs. 9.4 ± 5.6, P = 0.02). Similarly, patients with poor sleep had significantly higher SAPS 3 score when compared to patients with good sleep (62.7 ± 8.9 vs. 45.6 ± 10.5, P < 0.0001). Body Mass Index (BMI, in kg/m2) in patients with good sleep was higher than patients with poor sleep; however, it was statistically insignificant (23.62 ± 3.5 vs. 22.9 ± 3.8, P > 0.05). Except for total bilirubin and pH, the hematological and biochemical parameters were comparable at the time of admission in patients with poor sleep and good sleep.
There was no significant difference in the median duration of hospital stay before actigraphy between those with poor and good sleep (8.4 days [interquartile range (IQR): 2.5–9.2] vs. 11 days [IQR: 5.8–19.8], P > 0.05).
Circadian rhythm and sleep disturbances
Mean TST over three days was 11.4 ± 4.1 h; divided into the three periods of 2200–0600, 0600–1400 and 1400–2200 as 6.3 ± 1.7 h, 2.7 ± 1.1 h and 2.4 ± 1.3 h respectively; showing that only 55.63% of TST was in the night (2200-0600). This pattern of altered sleep-wake cycle was found in both mechanically ventilated and other patients.
Patient-perceived risk factors for poor sleep and suggestions
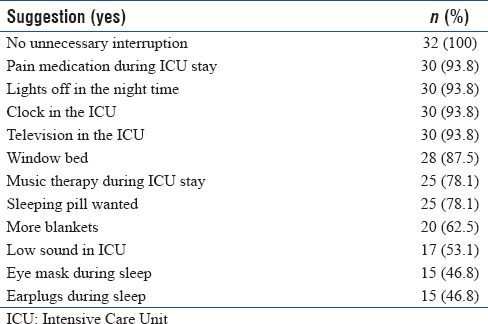
Factors causing discomfort during the ICU stay were assessed and have been listed in Table 5. Indwelling catheters (central line, Foleys catheter, and nasogastric tube) and endotracheal tube suctioning were the most common factors causing maximum discomfort. Rounds conducted by the medical team were the least discomforting factor. The suggestions given by the study population to improve the ICU care are listed in Table 6.
Table 5.
Factors associated with sleep disturbance
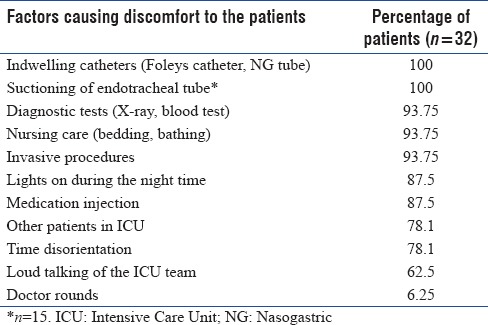
Table 6.
Suggestions from participants to improve sleep (n=32)
DISCUSSION
This study characterizes sleep quality and quantity using both qualitative and quantitative techniques in ICU patients. There was a high prevalence of poor sleep which was fragmented with altered sleep-wake cycle; more so among patients who were on mechanical ventilation. Despite recent advances in critical care medicine and a lot of research showing adverse events related to sleep deprivation, this area has been tough to tackle with minimal improvement over the years.
While there is a high level of agreement between actigraphy and PSG in healthy participants,[17] a study done by Beecroft et al.[31] in 14 MV patients showed poor agreement between the two because of the high level of inactivity in patients who are critically ill. The accuracy using automatic threshold in the same study was only 51%.[31] Total sleep time correlation between actigraphy and PSG varies from 0.72 to 0.98.[32] Mean TIB and TST were 11 ± 2 and 8.7 ± 2, respectively, reflecting that the patients were asleep one-third of the time. Although the duration of sleep was within normal range, it was fragment with frequent awakenings and alteration in circadian rhythm. Only 55.6% of TST was in the night (2200–0600). This altered sleep-wake cycle has been well-documented previously in ICU patients.[4,3,33] This is reflective of the poor RCSQ score which is a subjective way of sleep assessment.
The mean score (51.6 ± 13.5 mm) obtained was lower than the mean score obtained (60 ± 27 mm) in Richard's original validation study in 70 male ICU patients.[28] This is probably due to less sick patients included by Richard in the study group (none of the patients were on MV), who, therefore, required fewer procedures, indwelling catheters, and medication that are the major risk factors affecting sleep quality. In a study done in Sweden among 31 surgical patients admitted in the emergency ICU, mean RCSQ and modified RCSQ were 45.5 mm and 66.5 mm, respectively.[34] This study had a large number of patients >75 years of age (8/31), and it is a well-known fact that perception of sleep quality decreased with advancing age.[35] In another study among 104 postsurgical patients admitted in the ICU, mean RCSQ on the first postoperative night was 51.42 mm.[36] Our study, was, however, conducted in a medical ICU, and therefore, the risk factors and the patient profile was different; the results, therefore, cannot be compared. Our results are similar to that reported by Kamdar et al.,[29] where mean RCSQ among 33 patients (92 paired responses over 137 patient-days) admitted in medicine ICU was 57 (±30) mm. while RCSQ is a standardized and easy way to subjectively assess sleep quality, application, and interpretation of RCSQ should be taken with a pinch of salt. In an interventional study done by Bourne et al.,[37] 20% patients were not able to complete RCSQ, primarily because of delirium. In another study [36] among ICU patients, the failure rate of around 50% was observed.
Our results of worse sleep quality in higher APACHE II score was similar to the study done by Gabor et al.-[38] a shortened sleep time, reduced SWS and increased awakening index was seen in patients with higher APACHE II score compared to healthy participants. Fanfulla et al.[39] found high SAPS score and alkalosis to be important causes of sleep disturbance in critically ill patients. Patients with higher APACHE II score at admission require more medications, catheters, and interventions which are important factors related to comfortable sleep.
Robust data collection, making cutoff for RCSQ and gathering information on patient's perception of sleep and related factors are the merits of our study. There are, however, important limitations to our study. A sample size of convenience was taken. The patients were not followed up by repeat actigraphy in ward or discharge, and therefore, the contribution of ICU admission to the sleep disturbance cannot be judged independently. We did not measure noise and light levels, dose, and duration of sedatives used, number of catheters and procedures carried out per patient per day; which would have helped us objectively assess risk factors and compare with other studies in the field. We assessed sleep quality as a dichotomous variable, although it occurs in continuum. Sleep staging was not possible through actigraphy, and therefore, it is unknown what the sleep distribution was. Other limitations for the use of actigraphy in ICU population include lack of validated criteria to define poor sleep. We need further studies with a larger sample size to validate actigraphy in ICU population and to determine the cutoff values of various actigraphy parameters.
CONCLUSION
There is a high prevalence of sleep disturbance among patients admitted to the ICU, especially those on MV. Knowledge about the factors related and designing methods to minimize these factors can help in improving quality of medical care and stay in ICU. Further studies are required to see whether poor sleep is associated with increased mortality and can effectively sleep avert such adverse outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Collop NA, Salas RE, Delayo M, Gamaldo C. Normal sleep and circadian processes. Crit Care Clin. 2008;24:449–60. v. doi: 10.1016/j.ccc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: Findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29:310–6. doi: 10.1016/j.iccn.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Parthasarathy S, Tobin MJ. Sleep in the Intensive Care Unit. Intensive Care Med. 2004;30:197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- 4.Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical Intensive Care Unit: Are our patients sleeping? J Trauma. 2007;63:1210–4. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- 5.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the Intensive Care Unit syndrome. Heart Lung. 1980;9:464–8. [PubMed] [Google Scholar]
- 6.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmblad J, Cantell K, Strander H, Fröberg J, Karlsson CG, Levi L, et al. Stressor exposure and immunological response in man: Interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20:193–9. doi: 10.1016/0022-3999(76)90020-9. [DOI] [PubMed] [Google Scholar]
- 8.White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–6. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 9.Salas RE, Gamaldo CE. Adverse effects of sleep deprivation in the ICU. Crit Care Clin. 2008;24:461. doi: 10.1016/j.ccc.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the Intensive Care Unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 11.Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: Its role in physical and psychological recovery. J Intensive Care Med. 2012;27:97–111. doi: 10.1177/0885066610394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BaHammam A. Sleep in acute care units. Sleep Breath. 2006;10:6–15. doi: 10.1007/s11325-005-0044-8. [DOI] [PubMed] [Google Scholar]
- 13.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: An observational study. Crit Care. 2013;17:R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krachman SL, D'Alonzo GE, Criner GJ. Sleep in the Intensive Care Unit. Chest. 1995;107:1713–20. doi: 10.1378/chest.107.6.1713. [DOI] [PubMed] [Google Scholar]
- 15.Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF, et al. Sleep in the Intensive Care Unit. Am J Respir Crit Care Med. 2015;191:731–8. doi: 10.1164/rccm.201411-2099CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA, et al. Feasibility study of unattended polysomnography in medical Intensive Care Unit patients. Heart Lung. 2014;43:445–52. doi: 10.1016/j.hrtlng.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 18.Raj R, Ussavarungsi K, Nugent K. Accelerometer-based devices can be used to monitor sedation/agitation in the Intensive Care Unit. J Crit Care. 2014;29:748–52. doi: 10.1016/j.jcrc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Mistraletti G, Taverna M, Sabbatini G, Carloni E, Bolgiaghi L, Pirrone M, et al. Actigraphic monitoring in critically ill patients: Preliminary results toward an “observation-guided sedation”. J Crit Care. 2009;24:563–7. doi: 10.1016/j.jcrc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Grap MJ, Borchers CT, Munro CL, Elswick RK, Jr, Sessler CN. Actigraphy in the critically ill: Correlation with activity, agitation, and sedation. Am J Crit Care. 2005;14:52–60. [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–80. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 25.Schuiling WJ, Rinkel GJ, Walchenbach R, de Weerd AW. Disorders of sleep and wake in patients after subarachnoid hemorrhage. Stroke. 2005;36:578–82. doi: 10.1161/01.STR.0000154862.33213.73. [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 27.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3 – From evaluation of the patient to evaluation of the Intensive Care Unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–55. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131–44. [PubMed] [Google Scholar]
- 29.Kamdar BB, Shah PA, King LM, Kho ME, Zhou X, Colantuoni E, et al. Patient-nurse interrater reliability and agreement of the Richards-Campbell sleep questionnaire. Am J Crit Care. 2012;21:261–9. doi: 10.4037/ajcc2012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Gupta K, Tripathy S, Dhooria S, Ranjan S, Pandey RM, et al. Nevirapine- versus efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and tuberculosis infections in India: A multi-centre study. BMC Infect Dis. 2017;17:761. doi: 10.1186/s12879-017-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ, et al. Sleep monitoring in the Intensive Care Unit: Comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34:2076–83. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 32.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 34.Nicolás A, Aizpitarte E, Iruarrizaga A, Vázquez M, Margall A, Asiain C, et al. Perception of night-time sleep by surgical patients in an Intensive Care Unit. Nurs Crit Care. 2008;13:25–33. doi: 10.1111/j.1478-5153.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 35.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neurosci Biobehav Rev. 1995;19:553–71. doi: 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 36.Frisk U, Nordström G. Patients' sleep in an Intensive Care Unit – Patients' and nurses' perception. Intensive Crit Care Nurs. 2003;19:342–9. doi: 10.1016/s0964-3397(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 37.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: Research and clinical implications. Crit Care. 2007;11:226. doi: 10.1186/cc5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the Intensive Care Unit. Curr Opin Crit Care. 2001;7:21–7. doi: 10.1097/00075198-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Fanfulla F, Ceriana P, D'Artavilla Lupo N, Trentin R, Frigerio F, Nava S, et al. Sleep disturbances in patients admitted to a step-down unit after ICU discharge: The role of mechanical ventilation. Sleep. 2011;34:355–62. doi: 10.1093/sleep/34.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]