Abstract
Background and Aims:
The diaphragm is considered the main respiratory muscle and difficulty in weaning can occur because of impaired diaphragmatic function. Hence, monitoring diaphragmatic function is important. The aim of this study is to assess the ability of various lung ultrasound (US) indices and the rapid shallow breathing index (RSBI) to predict the outcome of the weaning process and compare them with RSBI.
Materials and Methods:
This was a prospective study conducted on patients admitted to critical care unit at a tertiary care hospital in north India from February 2017 to June 2017. Patients were put to spontaneous breathing trial (SBT) when they met the weaning criteria. Initial US was done immediately after putting the patient on SBT to check anatomy of the diaphragm and rule out patients according to exclusion criteria. This was followed by complete lung US (LUS) after 20 min of SBT.
Results:
The RSBI performed better than all other parameters, with an area under the curve (AUC) of 0.996. The sensitivity and specificity is 100%. Only comparable to RSBI is the speed of diaphragmic contraction (DC) which has AUC of 0.93. All other parameters had an AUC <0.8. Moreover, the DC and LUS score are strongly positively correlated with RSBI, whereas diaphragmic excursion and diaphragmic thickness fraction (DTF %) are weakly correlated.
Conclusion:
In Intensive Care Unit, RSBI is the best clinical tool for weaning, and DC is found to be the best parameter for weaning among the US-based weaning parameters. It can even be a substitute for RSBI, in today's world of real-time monitoring methods.
Keywords: Extubation predictor, lung ultrasound, rapid shallow breathing index, weaning process
INTRODUCTION
Weaning has always been one of the most difficult tasks for intensivists. Since ages many papers have been published regarding weaning trials. Still it remains an entity of dilemma for clinical practitioners.[1] It has been seen that even after applying all weaning criteria, nearly 20% of patients have difficulty in weaning and extubation.[2]
Multiple indices have been devised to assess the patient's ability to regain spontaneous breathing during weaning such as:[3]
Minute ventilation; Maximum inspiratory pressure; Breathing frequency. (rate); Rapid shallow breathing index (RSBI) = respiratory frequency/tidal volume; Tracheal airway occlusion pressure; Compliance, rate, oxygen pressure index; Esophageal and gastric pressure monitoring.
Ely et al. in their article about weaning trial said that subjective decisions about weaning are often wrong.[4] Stroetz and Hubmayr found that clinical prediction of extubation success or failure with the decision to extubate are often incorrect. Thus, regular assessments of breathing frequency, minute ventilation, and negative inspiratory force have little impact in improving the timing of successful extubation.[5] A more recent parameter, RSBI provides a successful guide for timing of extubation with spontaneous breathing trials (SBTs).[6]
The diaphragm is considered the main respiratory muscle and difficulty in weaning can occur due to impaired diaphragmatic function. Hence, monitoring diaphragmatic function is very important during weaning.[7] All the usual methods for diaphragmatic assessment such as fluoroscopy, phrenic nerve conduction, and transdiaphragmatic pressure measurements, have limitations and disadvantages, especially inside the Intensive Care Unit (ICU) due to ionizing radiation exposure, not widely available methods in practice and the need for patient transportation to the respective unit is also a big setback.[8]
Ultrasound (US) is a well-established bedside radiological tool. Different parameters have been described for the diaphragmatic US, namely the diaphragmatic excursion (DE) and diaphragmatic muscle thickening fraction (DTF).[9] Furthermore, lung US (LUS) can be used in the assessment of lung aeration which can be useful and helpful during the weaning process as it reflects the aeration loss and subsequently predicts the post-extubation distress.[10]
The aim of this study is to assess the ability of various diaphragmatic indices and LUS as new additive parameters to predict the outcome of the weaning process and compare them with RSBI.
MATERIALS AND METHODS
This was a prospective study conducted on patients admitted in critical care unit at a tertiary care hospital in north India from February 2017 to June 2017,
-
Inclusion criteria
- Age between 18 and 50 years
-
Exclusion criteria
- Patients <18 years old
- Any patient in whom the primary US revealed unilateral/bilateral absent diaphragmatic mobility, or known neuromuscular disorder.
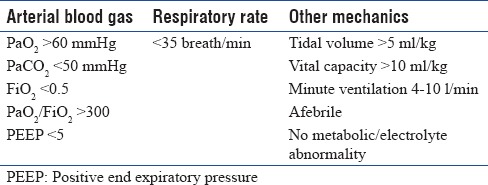
All patients were mechanically ventilated through the endotracheal tube. Consent was obtained according to the rules of the ethical committee. Patients were put to SBT when they met the following criteria [Table 1].
Table 1.
Criteria for weaning
Initial US was done immediately after putting the patient on SBT to check anatomy of the diaphragm and rule outpatients according to exclusion criteria. This was followed by complete diaphragmatic and LUS after 20 min of SBT.
Diaphragmatic ultrasound
US machines: Siemens Acuson X300.
Diaphragmatic thickness fraction assessment
The linear US probe was placed intercostally perpendicular to the chest wall in the 8th or 9th intercostal space between the anterior and midaxillary line. The thickness was measured during the end inspiration and the end expiration.
Diaphragmatic thickness fraction calculation = (thickness at the end inspiration– thickness at the end expiration)/thickness at the end expiration
Subsequently, we found out the DTF % by multiplying it with 100.
Diaphragmatic excursion and speed of diaphragmatic contraction
The convex probe is placed subcostally parallel to the intercostal space to measure the range of the diaphragmatic movement. In the M mode, the diaphragmatic excursion (displacement, cm), the speed of diaphragmatic contraction (slope, mm/s) is measured.
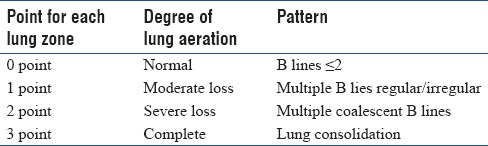
Lung ultrasound score
Each lung was divided into three zones underwent examination anteriorly and posteriorly using
B-mode to assess the degree of lung aeration with total 12 zones to be examined.
Total score from 0 to 36 (adding all points in 12 lung zones) [Table 2].
Table 2.
Ultrasound Score and Lung Zones
Analysis of data
The patients were divided into two groups successful group and failed group (FG) (re-intubation within 48 h after extubation) accordingly to their response to weaning trials. The DE, DTF %, diaphragmic contraction (DC), and LUS score measurements were collected for each group and then were correlated with RSBI.
All data were analyzed with statistical tools “MedCalc.” Data were presented as mean with standard deviation or proportions as appropriate. Further, the following statistical significance tests were applied.
Student's paired t-test was used as the statistical tool to test for significance of observed mean differences
Statistical analysis was performed using “Chi-square test”
Unpaired t-test was employed to compare for different means
Karl Pearson's correlation coefficient was used for finding correlation between two continuous data
A “P value” is considered to be nonsignificant if >0.05 and significant if <0.05.
RESULTS
We studied a total of 53 patients from March to June 2017. Of the 53 patients, 30 were male and 23 were female. There was statistically no significant difference among the patients for their age, with P = 0.7803 (P > 0.05) [Table 3]. The number of patients in the successful weaning group was 40 and FG was 13 (P = 0.0029, P < 0.05). The result was statistically significant [Table 4]. The different weaning parameters studied along with their mean and standard deviations for successful and failed weaning are shown in Table 5. All the parameters have a significant difference between the two groups with P value < 0.001. A receiver operating characteristic curve was also constructed for the RSBI, DE, DTF %, DC, and LUS [Figure 2]. The areas under the curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value and proposed cutoffs are in Table 6 and Figure 1. The RSBI performed better than all other parameters, with an AUC of 0.996. The sensitivity and specificity is 100%. Only comparable to RSBI is DC which has AUC of 0.93. All other parameters had an AUC <0.8. Subsequently, we found the correlation between different US-based weaning parameters with RSBI and found DC and LUS are strongly positively correlated, whereas DE and DTF % are weakly correlated. Finally, we found the cutoff values (scores for successful weaning/failed weaning) for all US-based parameters [Table 7].
Table 3.
Age distribution among the patients (central tendency)

Table 4.
Final outcome of patients (successful or failed group)

Table 5.
Illustration of results of different weaning parameters in between two groups
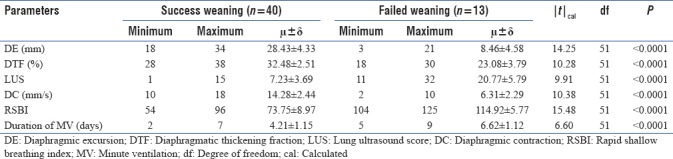
Figure 2.
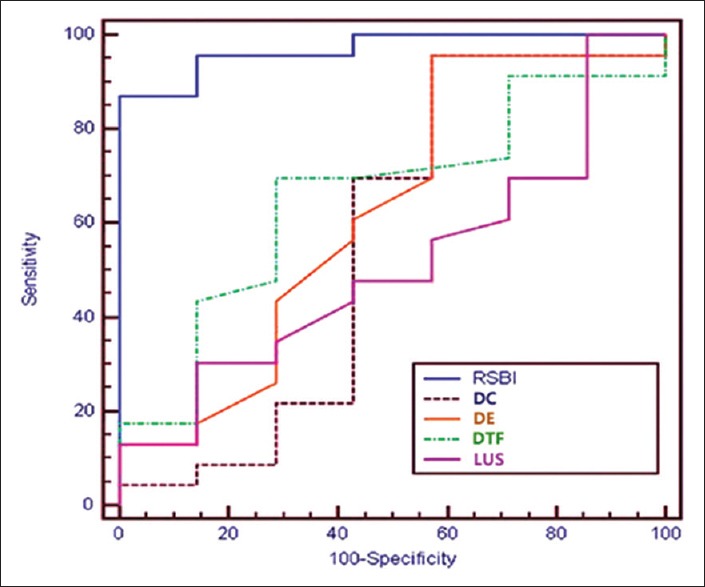
Receiver operating characteristics
Table 6.
Agreement for different parameters to predict outcome of success
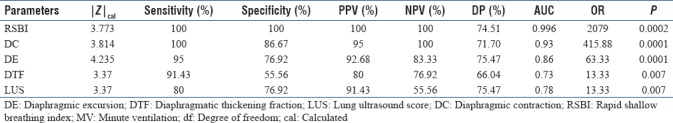
Figure 1.
Agreement for different parameters to predict outcome of success
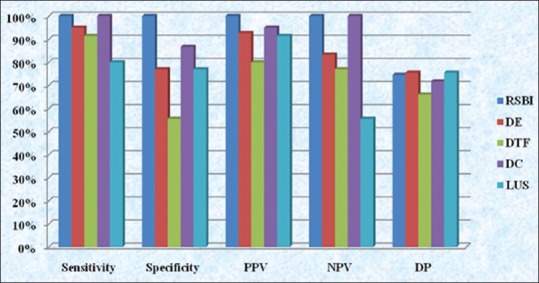
Table 7.
Suggested score system of diaphragmic excursion, diaphragmatic thickening fraction, diaphragmic contraction and lung ultrasound score when used as weaning parameters
DISCUSSION
Weaning is one of the most important concerns in ICU. Thus, it is important to extubate at the right time, to prevent failed weaning, increase the length of hospital stay, and mortality.[1] Patients who have a history of difficult weaning and very long intubation period have to increase readmission rates in ICU also.[1]
Since the paper published by Yang and Tabun, RSBI has become one of the most important predictors for weaning indices.[3] Other indices such as compliance rate oxygenation and pressure index, simplified weaning indices have also been proposed but did not get much importance because of low acceptance rate.
The RSBI is an integrative function of respiratory load and inspiratory muscle capacity. It reflects the function of all inspiratory muscles including the diaphragm and the nondiaphragmic muscles. If the diaphragm is failing, the nondiaphragm inspiratory muscles will compensate to preserve the tidal volume, and the presence of diaphragm weakness may be masked. However, the nondiaphragmatic muscles are more prone to fatigue and weaker than the diaphragm, and will not be able to sustain adequate ventilation for a long time. Hence, it is proposed that RSBI can give false positive result as extubation criteria and extubation failure may occur despite an initially adequate tidal volume and good clinical extubatable condition.[11,12]
In this study, we found RSBI as a very good marker of extubation criteria having a very high sensitivity (100%) and specificity (100%) with cutoff <104 breath/TV and AUC (0.996). This is contradictory with many old studies as explained above. Our result can be explained by the fact that we have taken all the measurements after 20 min of giving SBT. RSBI expresses the end product of the balance between strength and load of all muscles, and by 20 min, when all accessory muscles also fail to contribute the required tidal volume, RSBI correctly determines which patient can be extubated and which cannot.
In a large study conducted on 210 healthy individuals, Boussuges et al. determined normal values for DE during quiet and deep breathing as 1 and 4.7 cm, respectively.[13] Lerolle et al. in their study evaluated diaphragmatic dysfunction in intubated postcardiac surgery patients and found that DE of <2.5 cm was a predictor of prolonged intubation.[14]
The present study did not find DE as a very useful parameter in predicting weaning outcome (AUC: 0.86) when compared with the RSBI (AUC 0.99) [Table 6]. The success rate of weaning with cutoff value >11 mm [Table 7] has a sensitivity of 95%, but the specificity of 76.92% [Table 6]. Saeed et al. reported 86.4% sensitivity and 87.5% specificity, whereas Baess et al. found 69.5% sensitivity and 71.4% specificity.[15,16]
Similarly for DTF % also our study did not find it to be as good as RSBI. The success rate of weaning with cutoff value >28% [Table 7] has a sensitivity of 91.43%, but the specificity of 51.56% for positive weaning [Table 6]. Baess et al. and Di Nino et al. who reported 30% DTF cutoff with sensitivity about 69.57%, 88% and specificity about 71.43%, 71%, respectively.[16,17]
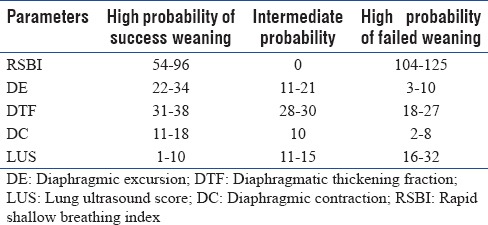
Although the AUC of both DTF % and DE are >0.7, [Table 7] we can still assume they can predict extubation with fair accuracy, because of such differences in all values as cited above, we cannot consider DE and DTF % as good predictive markers for extubation. When compared with RSBI they are not strongly correlated [Table 8].
Table 8.
Correlation of rapid shallow breathing index with other parameter
The intra-analyzer and interanalyzer reproducibility differences for the measurements as depicted by Vivier et al. can be one of the most important reasons for the above. Coefficients of repeatability ranged around 7%–8% for intra-or interanalyzer repeatability and around 15%–18% for intra- or inter-observer repeatability.[18] Hence, this variability can account for inaccurate interpretation. One more reason given by Ayman I, Baess et al. is probably because of use of much lower resolution of low-frequency probes that make determining the exact boundaries of the muscle sheet challenging.[19]
The speed of diaphragmatic contraction (slope, mm/s) was also measured [Figure 3], during the assessment of the diaphragmic excursion (DE). It relates with the short, sharp inspiratory effort and it is thought to reproduce the quantitative assessment of diaphragmatic strength although its role in the respiratory assessment of ICU patients is still not extensively studied.[20] The slope (speed) of diaphragmatic contraction, during quiet breathing, has been measured at 1.3 ± 0.4 cm/s in 40 healthy individuals without any significant differences between males and females.[21] The study showed the speed of DC with a cutoff value speed of >8 mm/sec has a very good sensitivity (100%); specificity (86.67%) with an AUC of 0.93 and when compared with RSBI it is strongly positively correlated. Hence, we can conclude the speed of DC as one of the best monitors to determine the patient who can be extubated. It can determine patients who can have problems with weaning failure because of pathologies such as sepsis, ventilator-induced diaphragmatic dysfunction, and ICU neuromyopathy. Although the speed of DC has not been previously studied to a great extent, we after our study can emphasize on the fact that it is a better indicator of acquired diaphragmic palsy in ICU.
Figure 3.
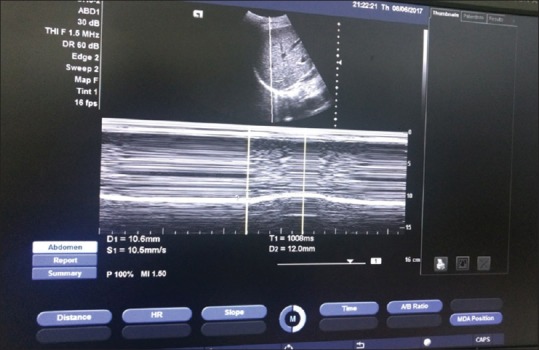
The method of finding the speed of diaphragmic contraction is shown here. The speed of diaphragmic contraction (slope) is 10.5 mm/sec (S1)
One more parameter we tried to correlate is the degree of lung aeration. The amount of lung aeration loss can be quantified via LUS during different clinical conditions including the weaning process. Lung aeration loss can be estimated using by a validated score called the LUS score.[10]
Soummer et al. showed that a LUS <13 at the end of a SBT is predictive of extubation success. On the other hand, a LUS >17 is highly predictive of postextubation distress and extubation failure.[10]
In our study, the LUS also depicted good sensitivity (80%) and specificity (76.92%) [Table 6] for cutoff value <16 LUS [Table 7] but is lower than RSBI. Although it is positively correlated with RSBI because of its very low-negative predictive value (55.56%) [Table 6], it should not be used as a weaning index.
CONCLUSION
In ICU, RSBI is the best clinical tool for weaning of patients if done after 20 min of SBT, for assured extubation and prevention of reintubation. When other US-based weaning parameters are compared with each other speed of DC was found to be the best parameter for weaning. Speed of DC can be more reliably used as an additional indices of weaning parameter, and can even be a substitute of RSBI, in today's world of real-time monitoring methods. We have taken only a small sample of 53 patients. More studies are required to proof the facts as concluded in our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Epstein SK. Extubation. Respir Care. 2002;47:483–92. [PubMed] [Google Scholar]
- 2.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA. 2002;287:345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 3.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–50. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Baker AM, Evans GW, Haponik EF. The prognostic significance of passing a daily screen of weaning parameters. Intensive Care Med. 1999;25:581–7. doi: 10.1007/s001340050906. [DOI] [PubMed] [Google Scholar]
- 5.Stroetz RW, Hubmayr RD. Tidal volume maintenance during weaning with pressure support. Am J Respir Crit Care Med. 1995;152:1034–40. doi: 10.1164/ajrccm.152.3.7663780. [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, Hui KP, Chan TB, Tan WC, Lim TK. Rapid shallow breathing (frequency-tidal volume ratio) did not predict extubation outcome. Chest. 1994;105:540–3. doi: 10.1378/chest.105.2.540. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F, et al. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J. 2014;6:8. doi: 10.1186/2036-7902-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39:801–10. doi: 10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- 9.Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A, et al. Ultrasonography evaluation during the weaning process: The heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42:1107–17. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
- 10.Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–72. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 11.Hershenson MB, Kikuchi Y, Tzelepis GE, McCool FD. Preferential fatigue of the rib cage muscles during inspiratory resistive loaded ventilation. J Appl Physiol (1985) 1989;66:750–4. doi: 10.1152/jappl.1989.66.2.750. [DOI] [PubMed] [Google Scholar]
- 12.Hershenson MB, Kikuchi Y, Loring SH. Relative strengths of the chest wall muscles. J Appl Physiol (1985) 1988;65:852–62. doi: 10.1152/jappl.1988.65.2.852. [DOI] [PubMed] [Google Scholar]
- 13.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 14.Lerolle N, Guérot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest. 2009;135:401–7. doi: 10.1378/chest.08-1531. [DOI] [PubMed] [Google Scholar]
- 15.Saeed A, El Assal G, Ali TM. Role of ultrasound in assessment of diaphragmatic function in chronic obstructive pulmonary disease patients during weaning from mechanical ventilation. Egypt J Bronchol. 2016;10:167–72. [Google Scholar]
- 16.Baess A, Abdallah TH, Emara DM. Diaphragmatic ultrasound as a predictor of successful extubation from mechanical ventilation: Thickness, displacement, or both? Egypt J Bronchol. 2016;10:162–6. [Google Scholar]
- 17.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69:423–7. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 18.Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38:796–803. doi: 10.1007/s00134-012-2547-7. [DOI] [PubMed] [Google Scholar]
- 19.Baess AI, Abdallah TH, Emara DM, Hassan M. Diaphragmatic ultrasound as a predictor of successful extubation from mechanical ventilation: Thickness, displacement, or both? Egypt J Bronchol. 2016;10:162–6. [Google Scholar]
- 20.Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–80. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soilemezi E, Tsagourias M, Talias MA, Soteriades ES, Makrakis V, Zakynthinos E, et al. Sonographic assessment of changes in diaphragmatic kinetics induced by inspiratory resistive loading. Respirology. 2013;18:468–73. doi: 10.1111/resp.12011. [DOI] [PubMed] [Google Scholar]


