Abstract
During early literacy skills development, rhyming is an important indicator of the phonological precursors required for reading. To determine if neural signatures of rhyming are apparent in early childhood, we recorded event-related potentials (ERPs) from 3- to 5-year-old, preliterate children (N = 62) in an auditory prime-target nonword rhyming paradigm (e.g., bly-gry, blane-vox). Overall, nonrhyming targets elicited a larger negativity (N450) than rhyming targets over posterior regions. In contrast, rhyming targets elicited a larger negativity than nonrhyming targets over fronto-lateral sites. The amplitude of the two rhyming effects was correlated, such that a larger posterior effect occurred with a smaller anterior effect. To determine whether these neural signatures of rhyming related to phonological awareness, we divided the children into two groups based on phonological awareness scores while controlling for age and socioeconomic status. The posterior rhyming effect was stronger and more widely distributed in the group with better phonological awareness, whereas differences between groups for the anterior effect were small and not significant. This pattern of results suggests that the rhyme processes indexed by the anterior effect are developmental precursors to those indexed by the posterior effect. Overall, these findings demonstrate early establishment of distributed neurocognitive networks for rhyme processing.
Keywords: Rhyming effect, Event-related potentials, Phonological awareness, Preschoolers, Nonword processing
1. Introduction
The term ‘phonological awareness’ encompasses skills involved in recognizing and manipulating the sounds of language, from basic rhyme recognition to complex phoneme deletion (e.g., Adams, 1990). Behavioral studies have shown that early phonological awareness skills like rhyming ability develop during the pre-school years (e.g., Wood and Terrell, 1998). However, few studies have explored pre-school development of rhyming abilities from a neurocognitive perspective. Event-related potentials (ERPs) provide an on-line index of real-time neural processing, and allow for measurement of rhyme processing without the potential confounds of a behavioral response. To our knowledge, the present study is the first ERP investigation of rhyming in preschoolers.
1.1. Rhyming and reading: behavioral measures
The development of phonological awareness is typically divided into three stages: syllable awareness, onset-rime awareness, and phonemic awareness (Cisero and Royer, 1995). The awareness of rhyme, as tested by asking children to produce or judge rhyming syllables, typically first appears around 3 or 4 years of age (Hayes et al., 2000; Hayes et al., 2009; Wood and Terrell, 1998). Recent studies have reported a relationship between speech decoding skills and rhyming skill in preliterate 4-year olds (Janssen et al., 2016; van Goch et al., 2014), and rhyming ability predicts later word understanding and word production (Tsao et al., 2004). Remarkably, rhyming ability is also directly predictive of reading ability (e.g., Bradley and Bryant, 1983). This causal link may be because rhyming words often share spelling patterns (e.g., beak and peak) that children who are able to rhyme can take advantage of (Goswami, 1988, Goswami, 1994; Wood and Farrington-Flint, 2001). There is also an indirect link in that rhyming ability is strongly related to the development of phoneme-level awareness, which in turn is critical for decoding grapheme-phoneme associations (Bryant et al., 1990). Overall, through these links, behavioral studies have indicated that the ability of preliterate children to detect rhyme is one of the best predictors of initial reading development (Ellis and Large, 1987; Gathercole et al., 1991; Maclean et al., 1987; Wood and Terrell, 1998).
1.2. Rhyming: electrophysiological measures
ERPs index neural processing as it unfolds millisecond by millisecond, and can therefore index rhyme processing not captured by behavioral tasks − tasks that young children might be unwilling or unable to perform. Yet few studies have capitalized on the sensitivity of ERPs to investigate the neural basis of early rhyming abilities, particularly within the preschool age range when rhyming skills are beginning to develop.
In one of the first developmental ERP studies of rhyming, Coch et al. compared auditory rhyme processing in adults, adolescents, and children as young as age 7 (Coch et al., 2002). Both reaction times (RTs) for rhyme judgments (via button press) and ERPs were recorded to word pairs presented as primes and nonrhyming or rhyming targets. RTs were significantly longer in children than adults. In contrast, there were no differences in a posterior ERP rhyming effect between groups: in each age group, the negativity peaking around 450 ms was larger (more negative) for nonrhyming compared to rhyming targets, particularly over occipitoparietal regions. Given the similarities in the electrophysiological index of rhyme processing across groups, the longer RTs observed in children may have been due to immature motor skills or slower overt judgments, rather than slower processing of phonological information. Regardless, this study did not address the development of neural rhyme processing before first grade.
This classic posterior ERP rhyming effect – a larger N450 for nonrhyming than rhyming targets – has been replicated across a number of studies with auditory and visual stimuli, in adults (e.g., Coch et al., 2008a; Coch et al., 2008b; Davids et al., 2011) and children as young as age 6 (Ackerman et al., 1994; Coch et al., 2002; Coch et al., 2005; Coch et al., 2011; Grossi et al., 2001; Lovrich et al., 1996, Lovrich et al., 2003; Perre et al., 2009; Wagensveld et al., 2012a; Weber-Fox et al., 2003; Weber-Fox et al., 2008). Moreover, some studies with children, using both auditory nonword (Coch et al., 2005) and printed letter (Coch et al., 2008a) stimuli, have reported relationships between the posterior rhyming effect and behavioral measures of phonological awareness.
In addition, a subset of these studies has reported a polarity reversal of the posterior effect within the same time window over frontal regions (e.g., Coch et al., 2002; Coch et al., 2005; Grossi et al., 2001). The amplitude of this anterior effect (rhyming targets elicit a larger negativity than nonrhyming targets) was not correlated with the amplitude of the posterior rhyming effect (e.g., Coch et al., 2002), suggesting that the two ERP effects index unrelated aspects of rhyme processing (see Khateb et al., 2007; Mohan and Weber, 2015, for a similar account).
Studies with children as young as age 6 have identified ERP rhyming effects using real word (e.g., Coch et al., 2002), nonword (e.g., Coch et al., 2005), and single letter (e.g., Coch et al., 2008a) stimuli. Importantly, the use of nonwords as stimuli avoids confounding phonological awareness with vocabulary size, since nonwords are not lexical items and thus are equally unfamiliar to all participants (Wagensveld et al., 2012b). Using nonword stimuli, in comparison to words, results in longer RTs in rhyme judgment tasks and smaller, later ERP rhyming effects, presumably because nonwords are more difficult to process (Dumay et al., 2001; Praamstra and Stegeman, 1993; Rugg, 1984; Wagensveld et al., 2012c).
Whereas these studies have investigated phonological processing in young children and adults in terms of rhyming (at the onset-rime level), other developmental ERP studies have explored phonological processing at the phonemic level in terms of the phonological mismatch negativity (MMN) (e.g., Lovio et al., 2009; Pihko et al., 2008). As with the ERP rhyming effect, the MMN effect is reduced for pseudowords, as compared to words (e.g., Pulvermüller et al., 2001; Pulvermüller et al., 2004). However, the MMN is greatest under conditions of rare deviance within a stream of common standards, and most rhyming studies have been designed with equal frequency of a rhyming or nonrhyming stimulus pair; therefore, it is unlikely that MMN effects intermingle with ERP rhyming effects in standardly designed rhyming studies.
1.3. The present study
The reliable ERP rhyming effects observed in primary-school children raise the possibility that electrophysiological measures might provide an index of rhyme processing in even younger children. Here, we modified the nonword rhyming paradigm employed by Coch et al. (2005) for use with preschoolers: children watched an animated movie rather than a crosshair and an explicit judgment was requested on only 18% of the trials. This resulted in a short and interesting paradigm that maintained the engagement of 3- to 5-year-olds. We predicted both posterior and anterior ERP rhyming effects in preschoolers who could demonstrate the ability to rhyme behaviorally. Further, we expected significant correlations between the size of the posterior ERP rhyming effect and scores on standardized measures of phonological awareness (cf. Coch et al., 2005; Coch et al., 2008a).
2. Materials and methods
2.1. Participants
Children in the current study were part of a larger study involving 117 3- to 5-year-olds recruited from Head Start schools (early childhood education centers for low income children). The study was conducted in accordance with the Declaration of Helsinki. Parents gave informed consent (approved by the Institutional Review Board of the University of Oregon) prior to the child’s participation. The overall study included multiple ERP paradigms composing a recording session lasting about one hour. From this larger group, children were excluded according to our screening criteria: insufficient ERP data in the rhyming paradigm (fewer than 10 trials/condition, n = 15), language impairment (i.e., lower than 17th percentile on the receptive language test while still within one standard deviation of the mean on the nonverbal IQ measure, n = 3), low language proficiency (i.e., lower than 25th percentile on the receptive language test, n = 15), handedness (left-handed, n = 1), missing behavioral data (n = 5), and not possible to match into the two phonological awareness groups (n = 16). Thus, the final sample of participants was composed of 62 3- to 5-year-old children (see Table 1). All participants who contributed data to analyses were native English speakers, were right-handed according to parental report on a questionnaire (Oldfield, 1971), had normal or corrected-to-normal vision and normal hearing, were screened for childhood behavioral and neurological problems, and were paid for their participation. Maternal education was collected as a measure of socioeconomic status (SES; refer to Table 1).
Table 1.
Group demographics.
| 3- to 5-year-olds | LPA | HPA | |
|---|---|---|---|
| N (Females) | 62 (38) | 31 (21) | 31 (17) |
| Age (SD) | 4;8 (0;6) | 4;8 (0;6) | 4;8 (0;5) |
| Range | 3;7−5;5 | 3;8−5;4 | 3;7−5;5 |
| SESa (SD) | 4.5 (0.9) | 4.5 (0.8) | 4.5 (1.0) |
| Range | 2–7 | 2–5 | 2–7 |
Note. Age shown in years; months. LPA = Lower Phonological Awareness; HPA = Higher Phonological Awareness.
The seven-point socioeconomic scale (SES) taken from (Hollingshead, 1975) included (1) less than 7 years of education, (2) between 7 and 9 years of education, (3) 10–11 years of education (part of high school), (4) high school graduate, (5) 1–3 years at college (also business school), (6) four-year college graduate (BA, BS, BM), and (7) a professional degree (e.g., MA, MS, ME, MD, PhD).
2.2. Behavioral testing
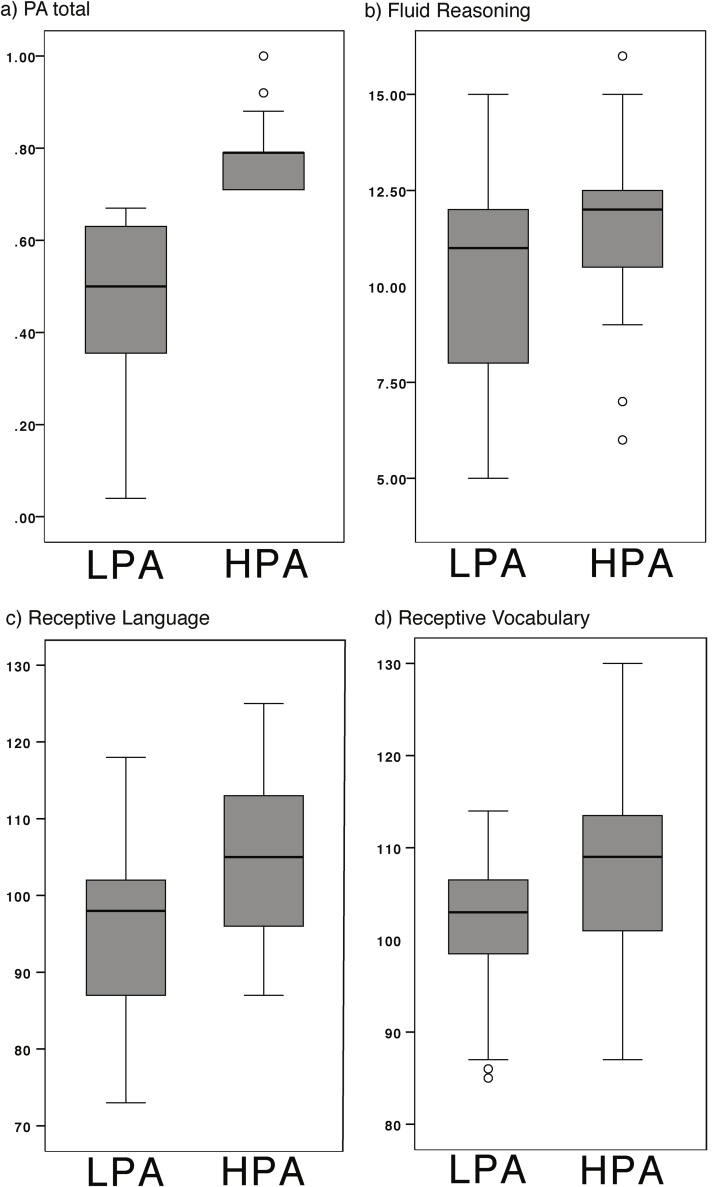
The receptive language subtest of the preschool version of the Clinical Evaluation of Language Fundamentals test (CELF-P; Wiig et al., 2004); the Peabody Picture Vocabulary Test, a measure of receptive vocabulary (PPVT-III; Dunn and Dunn, 1997); and the nonverbal fluid reasoning subtest of the Stanford Binet Intelligence Scales (SB-5; Roid, 2003) were administered to participants. In addition, the phonological awareness subtests of the Comprehensive Test of Phonological Processing (CTOPP; Wagner et al., 1999) were administered. On the basis of the CTOPP Phonological Awareness composite score, children were divided by median split (cut-off score 0.70) into lower (LPA, range 0.04–.67) and higher (HPA, range 0.71–1.00) phonological awareness groups (see Table 2 and Fig. 1). Groups were matched on age and SES (see Table 1).
Table 2.
Language proficiency, nonverbal processing, and phonological awareness by group.
| Test | 3- to 5-year-olds | LPA | HPA | t(60) |
|---|---|---|---|---|
| Receptive Language | 100.5 (11.7) | 96.3 (11.3) | 104.6 (10.7) | 2.94** |
| Receptive Vocabulary | 104.6 (9.6) | 101.9 (7.4) | 107.3 (10.8) | 2.30* |
| Fluid Reasoning | 10.9 (2.4) | 10.2 (2.5) | 11.6 (2.2) | 2.33* |
| CTOPP subtests: | ||||
| Blending Compounds | 0.76 (0.34) | 0.61 (0.41) | 0.90 (0.17) | 3.63** |
| Blending Syllables | 0.77 (0.33) | 0.61 (0.40) | 0.94 (0.13) | 4.30*** |
| Segmenting Sentences | 0.64 (0.26) | 0.51 (0.27) | 0.77 (0.16) | 4.70*** |
| Segmenting Syllables | 0.55 (0.35) | 0.40 (0.29) | 0.70 (0.34) | 3.81*** |
| Detect Rhyme | 0.67 (0.26) | 0.54 (0.27) | 0.80 (0.16) | 4.59*** |
| Produce Rhyme | 0.38 (0.36) | 0.17 (0.28) | 0.59 (0.32) | 5.52*** |
| PA composite score | 0.63 (0.20) | 0.47 (0.17) | 0.78 (0.07) | 9.41*** |
Note. Receptive Language and Receptive Vocabulary: Standardized means reported such that scores between 85 and 115 are within normal range. Scores between 9 and 13 are within normal range on the test of Fluid Reasoning. CTOPP: Means of percentile ranks. For all tests, standard deviation given in parentheses. LPA = Lower Phonological Awareness, HPA = Higher Phonological Awareness. Independent-samples t-tests were used to compare LPA and HPA group means. When variance scores violated equality of means as measured by Levene’s test for equality of variance, corrected p-values and uncorrected degrees of freedom are reported.
p < .05.
p < .01.
p < .001.
Fig. 1.
Box plots depicting standardized test scores by group (see Table 2). Plots show that a) all individuals in the higher phonological awareness (HPA) group had higher composite PA scores on the CTOPP than individuals in the lower phonological awareness (LPA) group. The two groups also differed in performance on the b) fluid reasoning, c) receptive language, and d) receptive vocabulary measures. However, individuals in the HPA and LPA groups had overlapping scores on these measures.
2.3. Auditory stimuli
The auditory stimuli included 88 pairs of rhyming nonwords that followed the phonological rules of English (from Coch et al., 2005; Appendix A). Nonrhyming pairs were created by associating the prime from one rhyming pair with the target of another rhyming pair such that each nonword was used as both a rhyming and a nonrhyming token. Each nonword was stored in a separate file with 10 ms of silence before sound onset. Sounds varied in length from 361 ms (gee) to 906 ms (stide) with an average length of 516 ms (SD = 93.5). Mean fundamental frequency was 186 Hz (SD = 25.3). By virtue of rhyming, rhyming pairs shared more phonemes and were acoustically slightly more similar to each other than nonrhyming pairs. Specifically, on average rhyming pairs differed in length by 91 ms (SD = 70.3) whereas nonrhyming pairs differed in length by 107 ms (SD = 89.8). For rhyming pairs, mean intensity differed by 1.3% of maximum (SD = 1.1) whereas for nonrhyming pairs, mean intensity differed by 1.9% of maximum (SD = 1.4). Finally, differences in both average pitch for rhyming pairs (13 Hz, SD = 20.1) and pitch change within nonwords for rhyming pairs (132 Hz, SD = 155.7) were smaller than those for nonrhyming pairs (difference in average pitch = 17 Hz, SD = 16.7; difference in pitch change = 167 Hz, SD = 179.1). The small differences in acoustic similarity of rhyming and nonrhyming pairs were far less salient than the rhyming relationships themselves. During ERP recording, the nonwords were presented from a speaker located 57 inches directly in front of the participant. Stimulus onset asynchrony between primes and targets was 1167 ms. Stimuli were presented at a comfortable listening level of 65 dB SPL (A-weighted).
2.4. Visual presentation
To increase attentiveness, we designed pairs of novel creatures that seemed to speak either the prime or the target in simple movies. The movies appeared on a monitor 57 inches in front of the participant. The creatures entered the screen across a 900 ms span and then remained in position for 2000 ms; they exited the screen across a 1000 ms span, beginning 1500 ms after the onset of a target. The specific creature and presentation side for prime and target were counterbalanced for rhyming and nonrhyming pairs, ensuring that any differences in ERP rhyming effects were not due to visuospatial attention or otherwise related to the animation. Sixteen additional probe movies in which a creature asked “did they sound alike?” or “what did they say?” were created in order to maintain attention and assess overt judgments.
2.5. Procedure
After procedures were explained to participants and accompanying parents or guardians, the electrode cap was prepared and children were seated in a comfortable chair in a sound-attenuating and electrically shielded booth. An experimenter was seated in the booth with the child to provide instructions, monitor eye movements, and control the pace of trial presentation.
Children were first asked if they knew what rhyming was and were then given examples of nonwords that did and did not rhyme. Subsequently, they were instructed to listen carefully to what the creatures said. The 44 rhyming and 44 nonrhyming pairs were then presented in random order. Simultaneously, the videos were presented in an independently randomized order, with probe question movies presented after about every fifth pair of nonwords. The rhyming portion of the ERP recording session lasted about 20 min.
2.6. ERP recording
EEG was recorded continuously during auditory nonword pair presentation from 29 tin electrodes mounted in an elastic cap (Electro-Cap International, Eaton, Ohio). These included three midline sites (Fz, Cz, and Pz) and 13 pairs of lateral sites (FP1/2, F7/8, FT7/8, F3/4, FC5/6, C3/4, C5/6, T3/4, CP5/6, P3/4, T5/6, TO1/2, and O1/2). Data from the midline and frontopolar sites (FP1/2) were not included in analyses (but were used in the creation of the topographical figures). Electrodes were also placed beneath the lower right eye to detect blinks and at the outer canthi of the left and right eyes to monitor eye movements. Each scalp electrode was referenced to the right mastoid during recording; data were re-referenced to the averaged mastoids during offline processing. Eye electrode impedances were maintained below 10 kΩ and mastoid and scalp electrode impedances below 5 kΩ.
The EEG was amplified with Grass 7P511 amplifiers (band-pass 0.01–100 Hz) and digitized at a sampling rate of 250 Hz. Off-line, ERPs time-locked to presentation of targets were segmented out of the continuous EEG separately for each participant at each electrode site over 1100 ms epochs, using a 100 ms pre-stimulus baseline. ERP processing was conducted using EEGLAB (Delorme and Makeig, 2004).
2.7. Artifact rejection
Trials containing large or paroxysmal artifacts, movement artifacts, or amplifier saturation were identified by visual inspection and excluded from further analysis. Subsequently, a digital low-pass 40 Hz filter was applied to reduce high-frequency noise and a digital high-pass filter of 0.1 Hz was applied to reduce drift. Data were then submitted to the extended ‘runica’ routine of EEGLAB software. Ocular artifacts were identified from scalp topographies and the component time series and were removed. Independent component analysis (ICA)-processed data were then subjected to a final manual artifact rejection step to detect any residual or atypical ocular artifacts not completely removed with ICA. Overall, after artifact rejection, the average number of rhyming trials (29.16, SD = 6.36) did not differ from the average number of nonrhyming trials (29.61, SD = 6.92) included in individual averages (paired samples t(61) = 1.369, p = 0.176). Number of trials averaged also did not vary with PA group (ps > .362).
2.8. Statistical analyses
Since young participants were expected to show more trial-to-trial variability in the timing of the ERP effects, perhaps resulting in temporal smear (Luck, 2014), mean amplitude was measured in three 200-ms time windows (100–300, 300–500, and 500–700 ms) as well as a longer, later epoch (700–1000 ms). The three early time windows overlap with those used in previous studies (e.g., Coch et al., 2002; Coch et al., 2011; Grossi et al., 2001).
Based on both a priori hypotheses consistent with previous studies (e.g., Coch et al., 2005) and visual inspection, the anterior rhyming effect was measured across frontal, fronto-temporal, and temporal sites and the posterior rhyming effect was measured across central, parietal, and occipital sites. All mean amplitude measurements were subjected to repeated measures ANOVAs with four within-subjects factors: rhyme condition (rhyme/nonrhyme), hemisphere (right/left), lateral/medial position, and anterior/posterior position (anterior effects: F/FT/T; posterior effects: C/P/O). Phonological awareness group (LPA/HPA) was the between-subjects factor in these analyses; planned within-subjects ANOVAs were also conducted on data from each group separately. Interactions between rhyme condition and electrode position factors in omnibus ANOVAs motivated additional analyses on data from subsets of electrodes. Those step-down analyses used Bonferroni-corrected p-values based on the number of subsets of electrodes that were considered separately. The Greenhouse-Geisser correction was applied to all statistics with more than two levels of a factor. Corrected p-values and uncorrected degrees of freedom are reported. Effect sizes for the ERP rhyming effects are reported as partial eta squared (ηp2) values.
Pearson’s correlations were calculated to examine hypothesized associations between ERP mean amplitudes and behavioral measures. For these correlation analyses, ERP rhyming effects were calculated as the difference in amplitude (nonrhyme − rhyme) for each participant at the sites and in the time windows where there was a main effect of rhyme condition in the omnibus ANOVAs. This approach replicates the analyses used in a previous study (Coch et al., 2008a).
3. Results
3.1. Behavioral tests and measures
3.1.1. 3- to 5-year-old children as a group
Generally (see Table 2), children scored within or above normal range on the proficiency measures, including receptive language (CELF-P), receptive vocabulary (PPVT-III), and fluid reasoning (SB-5). Scores on the following tests were significantly correlated: receptive language and vocabulary (r = 0.51, p < .001), and receptive language and fluid reasoning (r = 0.50, p < .001). Normed scores on the proficiency measures did not correlate with age (all ps > .055).
Scores were also within or above normal range on the phonological awareness measure (CTOPP; see Table 2). The composite score of phonological awareness was positively correlated with receptive language (r = 0.27, p < .05), receptive vocabulary (r = 0.32, p < .05), and fluid reasoning (r = 0.26, p < .05) scores. Positive correlations with age were driven by older children’s better performance on blending syllables, one of the CTOPP subtests (r = 0.27, p < .05), and having higher phonological awareness overall (composite scores, r = 0.28, p < .05). SES did not correlate with any behavioral measure (all ps > .218).
Participants were asked eight times, randomly, during the ERP task whether a just-presented target and prime nonword pair sounded alike. This proved to be a difficult task: The children as a group were not significantly better than chance, t(60) = 0.43, p = .402 (with one child from the HPA group missing data). Children were also asked eight times, randomly, throughout the ERP task to recall what the creatures had just said in the previous trial. This was also difficult, with children as a group able to recall only about 4 items (M = 4.15, SD = 1.52) of the possible 16 (8 primes, 8 targets).
3.1.2. Phonological awareness groups
Children with higher phonological awareness (HPA) scored better than children with lower phonological awareness (LPA) on every administered subtest of the CTOPP (see Table 2 and Fig. 1), confirming separable groups based on phonological awareness skill. As suggested by the correlations reported above, the HPA group also outperformed the LPA group on fluid reasoning measures and the two language proficiency tests, receptive language and receptive vocabulary (see Table 2).
Performance did not differ between proficiency groups for either task during the ERP session: reporting whether the previous pair sounded alike, t(59) = 1.199, p = .235 (HPA: M = 4.40, SD = 1.61; LPA, M = 3.94, SD = 1.41), or recalling what the previous pair had been, t(59) = 0.535, p = .594 (HPA: M = 4.43, SD = 3.99; LPA, M = 3.87, SD = 4.21). Overall, the ERP tasks were too difficult for our young participants and performance did not differ between groups.
3.2. ERP rhyming effects
3.2.1. Posterior rhyming effect
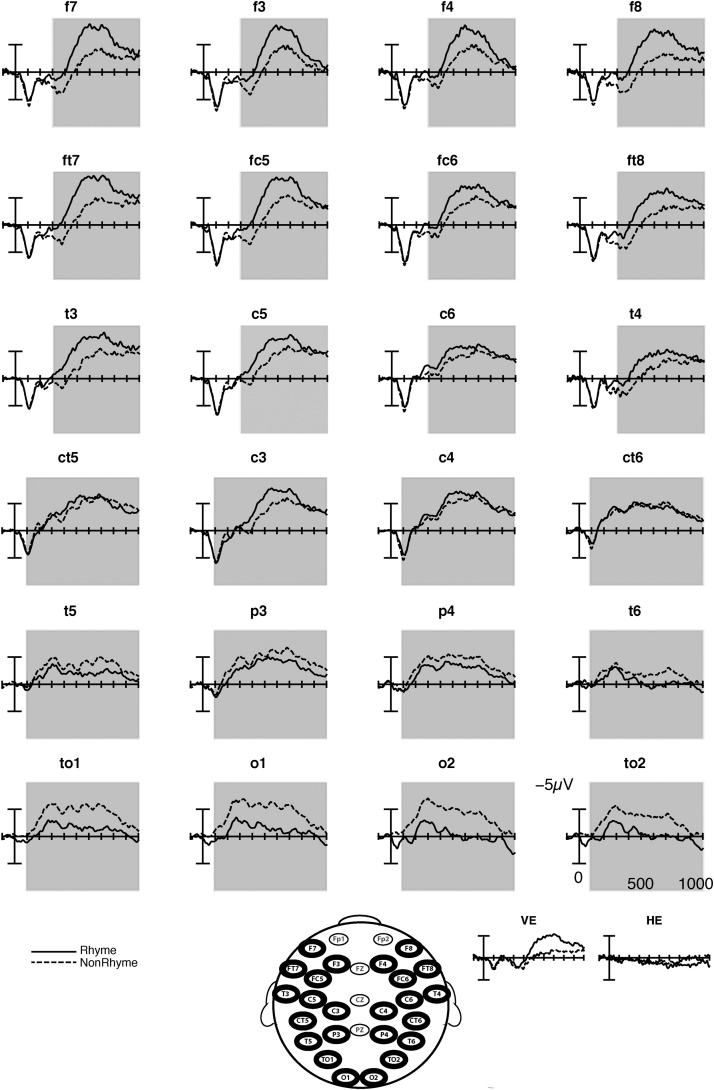
As predicted and consistent with previous results from older children and adults (Coch et al., 2002), nonrhyming targets elicited a larger posterior negativity than rhyming targets in 3- to 5-year-old children. This difference, evident in the earliest time window of measurement and continuing through all time windows (i.e., from 100 to1000 ms), was largest medially and at occipital sites (see Fig. 2 and Table 3). Follow-up analyses corrected for multiple comparisons (Bonferroni-corrected ps < .017) over central, parietal, and occipital sites separately showed that this effect was significant across all sites in the 500–700 ms epoch, but restricted to occipital sites in the other time windows (100–300 ms, 300–500 ms, and 700–1000 ms).
Fig. 2.
Grand average ERPs at all analyzed sites. The response to rhyming targets is shown as solid lines and the response to nonrhyming targets is shown as dashed lines. Time windows with significant main effects of Rhyme (see Table 3) are shaded in grey.
Table 3.
Analyses of effects of rhyming over anterior and posterior electrode sites in 3- to 5-year-olds.
| 100–300 ms |
300–500 ms |
500–700 ms |
700–1000 ms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrode sites: Variables | df | F | ηp2 | F | ηp2 | F | ηp2 | F | ηp2 |
| F FT T: | |||||||||
| Rhyme | 1, 61 | 23.54*** | 0.28 | 26.68*** | 0.30 | 5.52* | 0.08 | ||
| Rhyme x Lateral | 1, 61 | 4.55* | 0.07 | 6.90* | 0.10 | 6.02* | 0.09 | ||
| Rhyme x Ant/post | 2, 122 | 8.62** | 0.12 | ||||||
| PA-grp x Rhyme x Hem x Lateral | 1, 60 | 6.41* | 0.10 | ||||||
| PA-grp x Rhyme x Ant/post | 2, 120 | 5.25* | 0.08 | ||||||
| Lateral F FT T: | |||||||||
| Rhyme | 1, 61 | 31.02*** | 0.34 | 38.10*** | 0.38 | 9.57** | 0.14 | ||
| Rhyme x Ant/post | 2, 122 | 8.88** | 0.13 | 3.57* | 0.06 | ||||
| Rhyme x Hem x Ant/post | 2, 122 | 3.47* | 0.05 | ||||||
| Medial F FT T: | |||||||||
| Rhyme | 1, 61 | 14.32*** | 0.14 | 16.16*** | 0.21 | ||||
| Rhyme x Ant/post | 2, 122 | 5.92* | 0.09 | ||||||
| C P O: | |||||||||
| Rhyme | 1, 61 | 7.53** | 0.11 | 4.68** | 0.07 | 6.33* | 0.09 | 4.77* | 0.07 |
| Rhyme x Ant/post | 2, 122 | 10.88** | 0.15 | 25.45*** | 0.29 | 29.92*** | 0.33 | 11.94*** | 0.16 |
| Rhyme x Lateral x Ant/post | 2, 122 | 7.45** | 0.11 | 9.06*** | 0.13 | ||||
| PA x Rhyme x Hem x Ant/post | 2, 120 | 7.01** | 0.11 | 5.92** | 0.09 | 3.24# | 0.05 | ||
| Left C P O: | |||||||||
| PA x Rhyme | 1, 60 | 5.83* | 0.09 | 5.43* | 0.08 | 4.64* | 0.07 | ||
| C: | |||||||||
| Rhyme x Lateral | 1, 61 | 5.92* | 0.09 | 16.11*** | 0.21 | 5.38* | 0.08 | ||
| P: | |||||||||
| Rhyme | 1, 61 | 5.90* | 0.09 | 4.23* | 0.07 | 6.30* | 0.09 | 4.29* | 0.07 |
| O: | |||||||||
| Rhyme | 1, 61 | 13.09** | 0.18 | 16.44*** | 0.21 | 23.13*** | 0.28 | 11.56** | 0.16 |
| Rhyme x Lateral | 1, 61 | 6.73* | 0.10 | 10.65** | 0.15 | 5.92* | 0.09 | ||
Note. Rhyme (condition effect, rhyme/nonrhyme), Lateral (lateral/medial), Hem (left/right hemisphere sites), Ant/post (anterior/posterior channels, up to 3 levels), PA-grp (Higher/Lower Phonological Awareness group). Only significant and no more than 4-level interactions are reported. F: frontal, FT: fronto-temporal, T: temporal, C: central, P: parietal, and O: occipital.
p = .055.
p < .05.
p < .01.
p < .001.
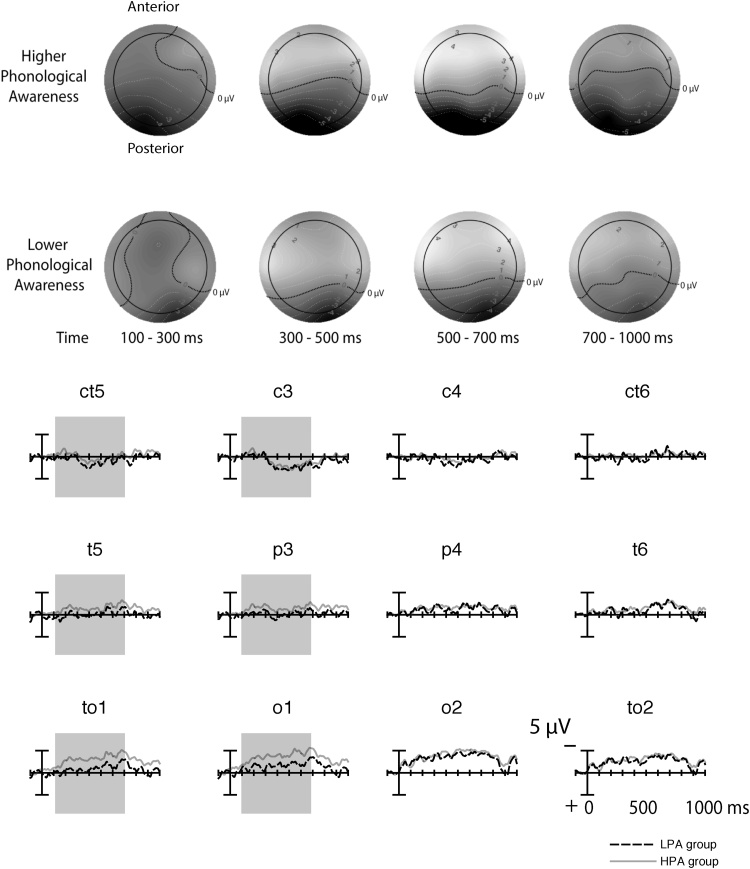
A significant interaction between the phonological awareness group, rhyme condition, and electrode position factors for mean amplitude in the time windows 100–300, 300–500, and 500–700 ms (see Fig. 3, Table 3) suggested differences in the posterior rhyming effect by group. All such differences were in the direction of a larger rhyming effect in the HPA group compared to the LPA group, especially over the left hemisphere. However, phonological awareness group by rhyme condition interactions at subsets of electrodes did not survive Bonferroni correction. Analyses investigating ERPs elicited by rhyming and nonrhyming targets separately also did not show any significant interactions with group.
Fig. 3.
Topographic maps for each of the four time windows analyzed (100–300 ms, 300–500 ms, 500–700 ms, and 700–1000 ms). For each time window, the top row displays maps for the higher phonological awareness group (HPA) and the bottom row shows maps for the lower phonological awareness group (LPA). Maps were created based on difference waves: the subtraction of ERPs to rhyming targets from ERPs to nonrhyming targets. For the corresponding difference wave plots, time windows with main effects of Group (at p < .05, but above Bonferroni-corrected p = .025) at central, parietal, and occipital electrode sites are shaded in grey (see Table 3). The difference wave for the LPA group is shown as dashed lines and the difference wave for the HPA group is shown as dotted lines.
Consistent with the Group interaction, results for the HPA and LPA groups alone differed. In the HPA group, there was a main effect of rhyme condition across posterior sites in all four time windows of measurement (spanning 100–1000 ms). Follow-up analyses over central, parietal, and occipital sites separately showed the effect was restricted to parietal and occipital sites. Over parietal sites, the rhyming effect (p = .040) did not survive Bonferroni correction (p = .017) in the final time window (700–1000 ms). Over occipital sites, the effect was robust in all time windows (100–300, 300–500, 500–700, and 700–1000 ms). In contrast, in the LPA group, there was no main effect of rhyme condition across posterior sites in any time window (see Table 4). Further, follow-up analyses to explore the rhyme condition by electrode position interactions revealed no typical posterior rhyming effect that survived Bonferroni correction.
Table 4.
Analyses of effects of rhyming at posterior sites for the HPA and LPA groups.
| 100–300 ms |
300–500 ms |
500–700 ms |
700–1000 ms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | ηp2 | F | ηp2 | F | ηp2 | F | ηp2 | |
| a) HPA | |||||||||
| C P O: | |||||||||
| Rhyme | 1, 30 | 9.11** | 0.23 | 8.32** | 0.22 | 7.19* | 0.19 | 5.05* | 0.14 |
| Rhyme x Ant/post | 2, 60 | 8.91** | 0.23 | 13.23** | 0.31 | 17.74*** | 0.37 | 7.63** | 0.20 |
| Rhyme x Hemisphere x Ant/post | 2, 60 | 5.71* | 0.16 | 4.28* | 0.13 | ||||
| Rhyme x Lateral x Ant/post | 2, 60 | 4.76* | 0.14 | ||||||
| C: | |||||||||
| Rhyme x Lateral | 1, 30 | 5.99* | 0.17 | ||||||
| P: | |||||||||
| Rhyme | 1, 30 | 8.89** | 0.23 | 7.49* | 0.20 | 6.68* | 0.18 | 4.59* | 0.13 |
| O: | |||||||||
| Rhyme | 1, 30 | 15.76*** | 0.34 | 15.47*** | 0.34 | 19.17*** | 0.39 | 9.87** | 0.25 |
| Rhyme x Lateral | 1, 30 | 6.01* | 0.17 | ||||||
| b) LPA | |||||||||
| C P O: | |||||||||
| Rhyme | 1, 30 | ||||||||
| Rhyme x Lateral | 1, 30 | 4.81* | 0.14 | ||||||
| Rhyme x Ant/post | 2, 60 | 11.93** | 0.28 | 12.40** | 0.29 | 4.28* | 0.13 | ||
| Rhyme x Hemisphere x Ant/post | 2, 60 | 8.52** | 0.22 | ||||||
| Rhyme x Lateral x Ant/post | 2, 60 | 4.36* | 0.13 | 4.29* | 0.13 | ||||
| C: | |||||||||
| Rhyme | 1, 30 | 4.67* | 0.13 | ||||||
| Rhyme x Lateral | 1, 30 | 10.42** | 0.26 | ||||||
| O: | |||||||||
| Rhyme | 1, 30 | 5.87* | 0.16 | ||||||
| Rhyme x Lateral | 1, 30 | 4.34* | 0.13 | 6.58* | 0.18 | ||||
| Rhyme x Hemisphere | 1, 30 | 6.44* | 0.18 | ||||||
Note. Rhyme (condition effect, rhyme/nonrhyme), Lateral (lateral/medial), Ant/post (anterior/posterior channels, 3 levels). Only significant and no more than 3-level interactions are reported. C: central, P: parietal, O: occipital. LPA = Lower Phonological Awareness group, HPA = Higher Phonological Awareness group. Analyzes over central, parietal, and occipital sites with licensed follow-ups are included for each group.
p < .05.
p < .01.
p < .001.
Overall, the amplitude of the posterior rhyming effect over central, parietal, and occipital sites, measured as the difference amplitude (nonrhyme-rhyme), was not related to age or SES, but was correlated with the ability to segment sentences (scores on the CTOPP subtest; 300–500 ms: r = −0.32; 500–700 ms: r = −0.26; both ps < .05), with better receptive vocabulary (PPVT scores, 300–500 ms: r = −0.29; 700–1000 ms: r = −0.27; both ps < .05), and with the ability to produce rhymes (scores on the CTOPP subtest, 500–700 ms: r = −0.27, p < 0.05).
3.2.2. Anterior rhyming effect
As predicted and consistent with previous findings with older children (Coch et al., 2005), rhyming targets elicited a larger anterior negativity than nonrhyming targets in 3- to 5-year-old children. This effect was evident in the three time windows between 300 and 1000 ms and was largest over lateral sites in the latest (700–1000 ms) time window (see Table 3, Fig. 2). Follow-up analyses of the interaction with laterality showed a significant effect over lateral sites in the three time windows spanning 300–1000 ms and over medial sites in the two time windows spanning 300–700 ms (Bonferroni-corrected ps .025).
The significant interaction among the phonological awareness group, rhyme condition, and electrode position factors for mean amplitude reflected small group differences in the distribution of the anterior rhyming effect in the 300–500 ms window, suggesting a more frontal and lateral effect in the HPA group and a more temporal and medial effect in the LPA group. However, there were no significant differences in the amplitude of the anterior rhyming effect between the LPA and HPA groups at any subset of electrodes (ps > .15). As was true for the posterior rhyming effect, ERPs elicited by rhyming and nonrhyming targets separately did not show any significant interactions with group.
Neither the amplitude of the rhyming effect in the time windows 300–500 or 500–700 ms over frontal, fronto-temporal, and temporal sites nor the amplitude of the effect in the 700–1000 ms time window over lateral frontal, fronto-temporal, and temporal sites correlated with age, SES, or any of the language proficiency scores.
3.2.3. Relations between the posterior and anterior rhyming effects
The amplitude of the anterior rhyming effect measured over frontal, fronto-temporal, and temporal sites correlated with the amplitude of the posterior rhyming effect measured over central, parietal, and occipital sites in the 500–700 ms (r = 0.38, p < .01) and 700–1000 ms (r = 0.53 p < .001) time windows, such that a larger posterior rhyming effect was associated with a smaller anterior rhyming effect.
4. Discussion
Here, we have established that preliterate 3- to 5-year-old children show both posterior and anterior ERP rhyming effects in an auditory nonword rhyming paradigm. Overall, the posterior effect, such that nonrhyming targets elicited more negative waveforms than rhyming targets, was clearly present and largest over occipital sites across an extended time window (within each of our 100–300, 300–500, 500–700, and 700–1000 ms measurement epochs). The reversed anterior effect, such that rhyming targets elicited more negative waveforms than nonrhyming targets, was evident as early as 300 ms, but was largest later (700–1000 ms) and at lateral sites. Thus, as predicted, pre-school aged children demonstrated ERP rhyming effects that were similar to those reported for older children (6- to 8-year-olds) and adults (e.g., Coch et al., 2002; Coch et al., 2005; Coch et al., 2011; Grossi et al., 2001). Further, level of phonological awareness in preschoolers was related to one, but not the other, ERP rhyming effect. Specifically, only children with higher phonological awareness showed a posterior rhyming effect, while the anterior effect, apparent in both the higher and lower phonological awareness groups, seemed unrelated to phonological awareness skill. These findings indicate that neural networks for rhyme processing are established early during the acquisition of rhyming, but that the networks indexed by the anterior and posterior ERP rhyming effects have different developmental time courses and different relationships to phonological awareness skill during the preschool years.
4.1. Posterior and anterior ERP rhyming effects in 3- to 5-year-olds
To our knowledge, this is the first report of posterior and anterior ERP rhyming effects in children aged 3–5, a time period during which rhyming skills are typically developing. Overall, the two rhyming effects were similar to ERP effects observed in previous studies with older children and adults, implying that neurocognitive systems for processing rhyme are already in place by this young age (Coch et al., 2002; Coch et al., 2005; Coch et al., 2011; Grossi et al., 2001). Although ERPs have poor spatial resolution and we did not conduct localization analyses, it is possible that the anterior effect is related to anterior activity in the inferior frontal gyrus (IFG) and the posterior effect is related to posterior activity in the IFG, the supramarginal gyrus, and the angular gyrus. fMRI effects related to phonological processing have been reported in these regions in adults (Poldrack et al., 2001) and children as young as 5 years of age (Powers et al., 2016; Raizada et al., 2008).
Despite the overall similarities with previous ERP findings, there were some apparent differences. One difference is that, although qualitatively similar, the rhyming effects appeared broader or more temporally extended in preschoolers than in older participants in previous studies (cf. Coch et al., 2002; Coch et al., 2005). Speculatively, this temporal extension could be characteristic of nascent rhyme processing networks; for example, it could be related to more variance in the timing of processing across individual trials in these younger participants (see Lukie et al., 2014, for similar results). Because we anticipated more variation in the ERPs in younger children than adults (e.g., Luck, 2014; Lukie et al., 2014), we were careful to use an adequate sample size. Including 62 preschoolers likely contributed to our ability to reliably identify the rhyming effects despite increased variability in the timing of effects within and across subjects.
Whereas most previous studies have reported a similar posterior rhyming effect (e.g., Coch et al., 2002; Davids et al., 2011; Rugg, 1984), some have not reported an anterior effect (e.g., Coch et al., 2008a; Coch et al., 2011; Perrin and García-Larrea, 2003). Synthesizing across these reports, it seems that the anterior effect may be more prominent in younger participants, consistent with the findings of a developmental ERP study of visual rhyming indicating that the amplitude and latency of the anterior rhyming effect decreased with increasing age (Grossi et al., 2001). Although we were not able to conduct direct statistical comparisons with previous data collected from older participants in different versions of rhyming paradigms, we return to this suggestion of a developmental pattern below in our discussion of the relationship between the ERP rhyming effects and phonological awareness skills.
Our findings indicate that young children do not necessarily have to engage in an overt rhyming task to process rhymes or show evidence of ERP rhyming effects. However, the pattern of rhyming effects could be influenced by methodological differences, such as the use of attention-reinforcing movies, nonword stimuli, or the lack of an overt response on every trial in the present paradigm. In studies in which adults listened to rhyming word pairs while asked to focus on images on the screen (passive processing) or make rhyme judgments (active processing), the anterior rhyming effect appeared to be more prominent during passive listening whereas the posterior effect was more pronounced during active listening (Davids et al., 2011; Perrin and García-Larrea, 2003). Thus, the use of a mostly passive task (only 18% of trials involved a response) may have affected the distribution of the effects here. Perhaps relatedly, fMRI studies of phonological processing have reported task differences, with greater activity in left IFG for semantic tasks, but more activity in posterior parts of the IFG (Burton et al., 2003; Poldrack et al., 2001), the superior temporal gyrus (Poldrack et al., 2001), and occipito-parietal regions (Seghier et al., 2004) for tasks focused on phonology. Here, performance was not significantly above chance when children were asked (eight times) if just-presented nonword pairs sounded the same or not. Poor performance on the task highlights the sensitivity of ERPs as a measurement tool for investigating rhyming ability in the early stages of development, when overt rhyming decisions may be difficult to produce and not reflect nascent phonological abilities (Wood and Terrell, 1998).
Another difference from previous ERP studies with older children and adults is that the amplitudes of the posterior and anterior rhyming effects were correlated in 3- to 5-year-olds. This finding suggests that the rhyme processing networks indexed by these separable ERP effects work in concert in early rhyming, perhaps in a way that they do not in later, fluent rhyming. As described above, previous studies with older participants have more consistently reported a posterior rhyming effect (e.g., Coch et al., 2008a; Weber-Fox et al., 2008) and have not reported a relation between the two effects. As such, the current finding of a smaller anterior effect that is associated with a larger posterior effect may reflect increased reliance on the phonological processing indexed by the posterior effect for simple rhyming over the course of development.
4.2. ERP rhyming effects and phonological awareness in 3- to 5-year-olds
Whereas the amplitudes of the anterior and posterior ERP rhyming effects were positively correlated with each other, only the amplitude of the posterior effect was associated with phonological awareness (measured by the CTOPP subtests); there were no significant correlations between the size of the anterior ERP rhyming effect and any of the included phonological awareness or language proficiency measures. This pattern of results is consistent with previous studies reporting relationships between the posterior ERP rhyming effect and scores on the phonological awareness composite of the CTOPP (e.g., Coch et al., 2008b) or performance on the ERP rhyme judgment task (Coch et al., 2011).
Here, in preliterate preschoolers, of the two neurophysiological indexes of rhyme processing, it is the posterior ERP rhyming effect that is most closely related to standardized behavioral measures of phonological awareness. Thus, the pattern of correlations suggests that the anterior effect is indexing processing separable from that required by phonological awareness tasks such as rhyme production. This is consistent with the lack of previous reports of a relationship between any phonological awareness measure and the amplitude of the anterior rhyming effect. That being said, the relatively weak correlations between the scores on phonological awareness measures and the size of the posterior rhyming effect indicate that these electrophysiological and behavioral measures, while drawing on some overlapping resources, mostly provide nonoverlapping indices of rhyming ability.
Considering phonological awareness as a categorical variable, rather than a continuous variable as in the correlation analyses, revealed striking developmental differences. Our higher and lower phonological awareness groups were matched for SES; although our measure of SES (maternal education level as a proxy, Bornstein et al., 2003) was not significantly correlated with any of our behavioral measures (perhaps due to little variability in maternal education here), previous language-based neurocognitive studies with older children and adults have found effects of SES (e.g., Hackman et al., 2010; Noble et al., 2007; Pakulak and Neville, 2010). The two groups were also matched on age, controlling for chronological development and allowing us to interpret the findings in terms of behavioral proficiency. Whereas we interpret the ‘behavioral proficiency’ findings here in terms of phonological awareness skill, the significant (although relatively weak, r = 0.26 to 0.32) correlations among phonological awareness composite, receptive language, and fluid reasoning scores do not allow us to rule out the contribution of receptive language and fluid reasoning skills to our pattern of results. In general, though, this pattern is consistent with other reports of rhyming ability being related to both receptive and expressive language skills, as noted above (Tsao et al., 2004).
Nevertheless, in analyses by group, we discovered that only those preschoolers with higher phonological awareness scores showed a broadly distributed posterior effect and an anterior rhyming effect. In contrast, children with lower phonological awareness evidenced only the anterior effect; the weaker, temporarily and spatially restricted posterior effect was not significant with conservative alpha correction. Moreover, the amplitude of the anterior rhyming effect did not differ between the two phonological awareness groups. This pattern suggests that the processes involved in rhyming indexed by the anterior effect may be developmental precursors to those indexed by the posterior effect.
The group differences that we observed were limited to the rhyming effects: In separate analyses, responses to the rhyming targets and the nonrhyming targets were not different between groups; rather, it was the differentiation between rhyming and nonrhyming target processing, particularly across left hemisphere posterior recording sites, that distinguished the higher and lower phonological awareness groups. In a previous study with adults using source analysis, rhyming targets elicited activity in left frontal and temporal areas, whereas nonrhyming targets elicited activity in bilateral temporal and parietal regions (Khateb et al., 2007). Given the lack of difference between groups here for both rhyming and nonrhyming targets, but the presence of a between-groups processing difference for the posterior effect, this complex interplay of anterior and posterior systems and rhyming and nonrhyming target processing does not appear to be developed yet in preschoolers passively listening to nonword stimuli. Speculatively, given that the anterior effect has been related more to passive processing and the posterior effect more to active processing (Davids et al., 2011; Perrin and García-Larrea, 2003), the children in the higher phonological awareness group may have been more actively listening to both the rhyming and the nonrhyming target stimuli than the children in the lower phonological awareness group; their better phonological awareness skills may have ‘tuned them into’ the rhyming nature of the paradigm more so than for the children with weaker phonological awareness skills. This would be consistent with other ERP findings (in terms of the Nd) suggesting that language proficiency interacts with attention to speech sounds (Shafer et al., 2007).
Taken together, the pattern of findings here consistently suggests a developmental shift from almost exclusive use of an anterior phonological processing system (or, more specifically, a network indexed by the anterior ERP rhyming effect) to growing, additional use of a posterior phonological processing system (or, more specifically, a network indexed by the posterior ERP rhyming effect) with improving phonological awareness skills across the preschool years. To our knowledge, as the first ERP study of rhyme processing in preliterate preschoolers, this is the first report of such a developmental shift with burgeoning phonological awareness skills. Future studies with this and younger populations will be needed to replicate and extend this developmental pattern.
Conflict of Interest
None.
Acknowledgements
We would like to thank Jessica Fanning and Kathryn Ravitch and their team for collecting and scoring behavioral data. Thanks especially to David Paulsen and Jeff Currin, and also to Theodore Bell, Petya Ilcheva, and Brittni Lauinger for help with collecting ERP data. Paul Compton programmed the paradigm. Yoshiko Yamada consulted on developing the movies and Courtney Stevens provided the voice that asked questions during the movie. Finally, we thank Miriam Munoz for providing the acoustic analysis of auditory stimuli. We would also like to thank the anonymous reviewers for helpful comments and suggestions.
Footnotes
Financial support for data collection was provided by the National Institutes of Health [NIDC RO1 DC000481]; for the preparation of this manuscript was given by the Knut & Alice Wallenberg Foundation [grant KAW 2010.0178 ‘Culture, brain, learning’].
Contributor Information
Annika Andersson, Email: annika.andersson@humlab.lu.se, annika.andersson@lnu.se.
Lisa D. Sanders, Email: lsanders@psych.umass.edu.
Donna Coch, Email: donna.j.coch@dartmouth.edu.
Christina M. Karns, Email: ckarns@uoregon.edu.
Helen J. Neville, Email: neville@uoregon.edu.
Appendix A
| List 1 | |||
|---|---|---|---|
| Rhyming pairs | Nonrhyming pairs | ||
| bly | gry | blane | vox |
| chole | thole | blauer | flam |
| chuz | luz | blore | plo |
| crail | lale | blug | kroar |
| crute | doot | bome | slines |
| daip | laip | bro | slore |
| dat | lat | bry | pag |
| demp | semp | clate | pline |
| doan | pone | dabe | lum |
| dorde | morde | daf | coom |
| drere | vair | doode | keer |
| fam | cham | dreat | ged |
| feap | neap | drig | stug |
| frield | geeled | floos | cho |
| gite | clite | foo | breet |
| glir | flir | fum | zi |
| gox | brocks | gee | blail |
| grize | yise | gines | rabe |
| grood | bood | gour | druze |
| jate | yate | ji | claid |
| kile | spile | jite | fauer |
| maft | yaft | ked | voo |
| moce | boce | kow | deeb |
| mun | lun | krobe | zite |
| murze | thurze | kun | gree |
| nake | dake | ky | tate |
| nef | gef | mag | yare |
| nilled | dilled | mide | gome |
| nin | rin | neeb | stide |
| nobe | drobe | poom | dite |
| nool | shull | pooze | lauer |
| pake | spake | prail | stobe |
| plew | snew | rine | clum |
| plol | groll | sarp | cly |
| poat | hoat | shum | hane |
| poe | trow | slair | jun |
| quo | zow | stam | glig |
| sare | nare | taid | chy |
| siff | piff | throre | slin |
| stee | kwee | trin | phy |
| trum | pum | vite | balf |
| vease | meeze | yi | marp |
| vore | jore | yocks | toos |
| zare | jare | zeer | pud |
| List 2 | |||
|---|---|---|---|
| Rhyming pairs | Nonrhyming pairs | ||
| blane | hane | bly | shull |
| blauer | fauer | chole | pum |
| blore | slore | chuz | semp |
| blug | stug | crail | thole |
| bome | gome | crute | lale |
| bro | plo | daip | piff |
| bry | chy | dat | clite |
| clate | tate | demp | geeled |
| dabe | rabe | doan | lat |
| daf | balf | dorde | gry |
| doode | pud | drere | yate |
| dreat | breet | fam | zow |
| drig | glig | feap | vair |
| floos | toos | frield | bood |
| foo | voo | gite | thurze |
| fum | clum | glir | meeze |
| gee | gree | gox | luz |
| gines | slines | grize | morde |
| gour | lauer | grood | nare |
| ji | zi | jate | yise |
| jite | zite | kile | pone |
| ked | ged | maft | snew |
| kow | cho | moce | rin |
| krobe | stobe | mun | gef |
| kun | jun | murze | hoat |
| ky | phy | nake | trow |
| mag | pag | nef | doot |
| mide | stide | nilled | groll |
| neeb | deeb | nin | laip |
| poom | coom | nobe | kwee |
| pooze | druze | nool | drobe |
| prail | blail | pake | brocks |
| rine | pline | plew | dilled |
| sarp | marp | plol | neap |
| shum | lum | poat | spake |
| slair | yare | poe | flir |
| stam | flam | quo | jare |
| taid | claid | sare | cham |
| throre | kroar | siff | jore |
| trin | slin | stee | spile |
| vite | dite | trum | dake |
| yi | cly | vease | boce |
| yocks | vox | vore | lun |
| zeer | keer | zare | yaft |
References
- Ackerman P.T., Dykman R.A., Oglesby D. Visual event-related potentials of dyslexic children to rhyming and nonrhyming stimuli. J. Clin. Exp. Neuropsychol. 1994;16(1):138–154. doi: 10.1080/01688639408402624. [DOI] [PubMed] [Google Scholar]
- Adams M.J. MIT Press; Cambridge: 1990. Beginning to Read: Thinking and Learning About Print. [Google Scholar]
- Bornstein M.H., Hahn C.-S., Suwalsky J.T.D., Haynes O.M. Socioeconomic status, parenting, and child development: the Hollingshead four-factor index of social status and the socioeconomic index of occupations. In: Bornstein M.H., Bradley R.H., editors. Socioeconomic Status, Parenting, and Child Development. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US: 2003. pp. 29–82. [Google Scholar]
- Bradley L., Bryant P. Categorizing sounds and learning to read: a causal connection. Nature. 1983;301(5899):419–421. [Google Scholar]
- Bryant P., MacLean M., Bradley L., Crossland J. Rhyme and alliteration, phoneme detection, and learning to read. Dev. Psychol. 1990;26(3):429–438. [Google Scholar]
- Burton H., Diamond J.B., McDermott K.B. Dissociating cortical regions activated by semantic and phonological tasks: a fMRI study in blind and sighted people. J. Neurophysiol. 2003;90(3):1965. doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisero C.A., Royer J.M. The development and cross-language transfer of phonological awareness. Contemp. Educ. Psychol. 1995;20(3):275–303. [Google Scholar]
- Coch D., Grossi G., Coffey-Corina S., Holcomb P.J., Neville H.J. A developmental investigation of ERP auditory rhyming effects. Dev. Sci. 2002;5(4):467–489. [Google Scholar]
- Coch D., Grossi G., Skendzel W., Neville H. ERP nonword rhyming effects in children and adults. J. Cogn. Neurosci. 2005;17(1):168–182. doi: 10.1162/0898929052880020. [DOI] [PubMed] [Google Scholar]
- Coch D., George E., Berger N. The case of letter rhyming: an ERP study. Psychophysiology. 2008;45(6):949–956. doi: 10.1111/j.1469-8986.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Coch D., Hart T., Mitra P. Three kinds of rhymes: an ERP study. Brain Lang. 2008;104(3):230–243. doi: 10.1016/j.bandl.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Coch D., Mitra P., George E., Berger N. Letters rhyme: electrophysiological evidence from children and adults. Dev. Neuropsychol. 2011;36(3):302–318. doi: 10.1080/87565641.2010.549985. [DOI] [PubMed] [Google Scholar]
- Davids N., van den Brink D., van Turennout M., Verhoeven L. Task-related influences on neurophysiological assessment of auditory rhyme: implications for clinical studies. Clin. Neurophysiol. 2011;122(8):1629–1636. doi: 10.1016/j.clinph.2010.12.058. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynanmics. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dumay N., Benraiss A., Barriol B., Colin C., Radeau M., Besson M. Behavioral and electrophysiological study of phonological priming between bisyllabic spoken words. J. Cogn. Neurosci. 2001;13(1):121–143. doi: 10.1162/089892901564117. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn L.M. 3rd ed. American Guidance Services; Circle Pines, MN: 1997. Peabody Picture Vocabulary Test. [Google Scholar]
- Ellis N.C., Large B. The development of reading: as you seek so shall you find. Br. J. Psychol. 1987;78(1):1–28. doi: 10.1111/j.2044-8295.1987.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Willis C., Baddeley A.D. Differentiating phonological memory and awareness of rhyme: reading and vocabulary development in children. Br. J. Psychol. 1991;82(3):387–406. [Google Scholar]
- Goswami U. Orthographic analogies and reading development. Q. J. Exp. Psychol. A: Hum. Exp. Psychol. 1988;40A(2):239–268. [Google Scholar]
- Goswami U. Reading by analogy: theoretical and practical perspectives. In: Hulme C., Snowling M., editors. Reading Development and Dyslexia. Whurr Publishers; Philadelphia, PA, US: 1994. pp. 18–33. [Google Scholar]
- Grossi G., Coch D., Coffey-Corina S., Holcomb P.J., Neville H.J. Phonological processing in visual rhyming: a developmental ERP study. J. Cogn. Neurosci. 2001;13(5):610–625. doi: 10.1162/089892901750363190. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R.A., Slater A., Brown E. Infants' ability to categorise on the basis of rhyme. Cognit. Dev. 2000;15(4):405–419. [Google Scholar]
- Hayes R.A., Slater A.M., Longmore C.A. Rhyming abilities in 9-month-olds: the role of the vowel and coda explored. Cognit. Dev. 2009;24(2):106–112. [Google Scholar]
- Hollingshead A.B. 1975. Four Factor Index of Social Status. manuscript. [Google Scholar]
- Janssen C., Segers E., McQueen J.M., Verhoeven L. Transfer from implicit to explicit phonological abilities in first and second language learners. Bilingualism: Lang. Cognit. 2016;20(4):795–812. [Google Scholar]
- Khateb A., Pegna A.J., Landis T., Michel C.M., Brunet D., Seghier M.L., Annoni J.-M. Rhyme processing in the brain: an ERP mapping study. Int. J. Psychophysiol. 2007;63(3):240–250. doi: 10.1016/j.ijpsycho.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Lovio R., Pakarinen S., Huotilainen M., Alku P., Silvennoinen S., Näätänen R., Kujala T. Auditory discrimination profiles of speech sound changes in 6-year-old children as determined with the multi-feature MMN paradigm. Clin. Neurophysiol. 2009;120(5):916–921. doi: 10.1016/j.clinph.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Lovrich D., Cheng J.C., Velting D.M. Late cognitive brain potentials, phonological and semantic classification of spoken words, and reading ability in children. J. Clin. Exp. Neuropsychol. 1996;18(2):161–177. doi: 10.1080/01688639608408272. [DOI] [PubMed] [Google Scholar]
- Lovrich D., Cheng J.C., Velting D.M. ERP correlates of form and rhyme letter tasks in impaired reading children: a critical evaluation. Child Neuropsychol. 2003;9(3):159–174. doi: 10.1076/chin.9.3.159.16458. [DOI] [PubMed] [Google Scholar]
- Luck S.J. 2nd ed. MIT Press books; USA: 2014. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- Lukie C.N., Montazer-Hojat S., Holroyd C.B. Developmental changes in the reward positivity: an electrophysiological trajectory of reward processing. Dev. Cognit. Neurosci. 2014;9:191–199. doi: 10.1016/j.dcn.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean M., Bryant P., Bradley L. Rhymes, nursery rhymes, and reading in early childhood. Merrill-Palmer Q. 1987;33(3):255–281. [Google Scholar]
- Mohan R., Weber C. Neural systems mediating processing of sound units of language distinguish recovery versus persistence in stuttering. Neurodev. Disord. 2015;7(28):1–13. doi: 10.1186/s11689-015-9124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pakulak E., Neville H.J. Proficiency differences in syntactic processing in monolingual native speakers indexed by event-related brain potentials. J. Cogn. Neurosci. 2010;22(12):2728–2744. doi: 10.1162/jocn.2009.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perre L., Midgley K., Ziegler J.C. When beef primes reef more than leaf: orthographic information affects phonological priming in spoken word recognition. Psychophysiology. 2009;46(4):739–746. doi: 10.1111/j.1469-8986.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- Perrin F., García-Larrea L. Modulation of the N400 potential during auditory phonological/semantic interaction. Cognit. Brain Res. 2003;17(1):36–47. doi: 10.1016/s0926-6410(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Pihko E., Kujala T., Mickos A., Alku P., Byring R., Korkman M. Language impairment is reflected in auditory evoked fields. Int. J. Psychophysiol. 2008;68(2):161–169. doi: 10.1016/j.ijpsycho.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Temple E., Protopapas A., Nagarajan S., Tallal P., Merzenich M., Gabrieli J.D.E. Relations between the neural bases of dynamic auditory processing and phonological Processing: evidence from fMRI. J. Cogn. Neurosci. 2001;13(5):687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Powers S.J., Wang Y., Beach S.D., Sideridis G.D., Gaab N. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Ann. Dyslexia. 2016;66(3):337–360. doi: 10.1007/s11881-016-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P., Stegeman D.F. Phonological effects on the auditory N400 event-related brain potential. Cognit. Brain Res. 1993;1(2):73–86. doi: 10.1016/0926-6410(93)90013-u. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Kujala T., Shtyrov Y., Simola J., Tiitinen H., Alku P.…Näätänen R. Memory traces for words as revealed by the Mismatch Negativity. Neuroimage. 2001;14(3):607–616. doi: 10.1006/nimg.2001.0864. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Shtyrov Y., Kujala T., Näätanen R. Word-specific cortical activity as revealed by the mismatch negativity. Psychophysiology. 2004;41(1):106–112. doi: 10.1111/j.1469-8986.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Raizada R.D.S., Richards T.L., Meltzoff A.N., Kuhl P.K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G.H. 5th ed. Riverside Publishing; Itasca, IL: 2003. Stanford-Binet Intelligence Scales. [Google Scholar]
- Rugg M.D. Event-related potentials and the phonological processing of words and non-words. Neuropsychologia. 1984;22(4):435–443. doi: 10.1016/0028-3932(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Seghier M.L., Lazeyras F., Pegna A.J., Annoni J.-M., Zimine I., Mayer E.…Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum. Brain Mapp. 2004;23(3):140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer V.L., Ponton C., Datta H., Morr M.L., Schwartz R.G. Neurophysiological indices of attention to speech in children with specific language impairment. Clin. Neurophysiol. 2007;118(6):1230–1243. doi: 10.1016/j.clinph.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao F.-M., Liu H.-M., Kuhl P.K. Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev. 2004;75(4):1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- van Goch M.M., McQueen J.M., Verhoeven L. Learning phonologically specific new words fosters rhyme awareness in dutch preliterate children. Sci. Stud. Read. 2014;18(3):155–172. [Google Scholar]
- Wagensveld B., Segers E., Alphen P., Hagoort P., Verhoeven L. A neurocognitive perspective on rhyme awareness: the N450 rhyme effect. Brain Res. 2012;1483:63–70. doi: 10.1016/j.brainres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Wagensveld B., Segers E., van Alphen P., Verhoeven L. The role of lexical representations and phonological overlap in rhyme judgments of beginning, intermediate and advanced readers. Learn. Individual Diff. 2012 (No Pagination Specified) [Google Scholar]
- Wagensveld B., van Alphen P., Segers E., Verhoeven L. The nature of rhyme processing in preliterate children. Br. J. Educ. Psychol. 2012;82(4):672–689. doi: 10.1111/j.2044-8279.2011.02055.x. [DOI] [PubMed] [Google Scholar]
- Wagner R.K., Torgesen J.K., Rashotte C.A. Austin, TX; Pro-Ed: 1999. The Comprehensive Test of Phonological Processing. [Google Scholar]
- Weber-Fox C., Davis L.J., Cuadrado E. Event-related brain potential markers of high-language proficiency in adults. Brain Lang. 2003;85(2):231–244. doi: 10.1016/s0093-934x(02)00587-4. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Spruill J.E., III, Spencer R., Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Dev. Sci. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig E.H., Secord W.A., Semel E. The Psychological Corperation: Harcourt Assessment, Inc; San Antonio, TX: 2004. The Clinical Evaluation of Language Fundamentals (Preschool 2nd Edition Ed.) [Google Scholar]
- Wood C., Farrington-Flint L. Orthographic analogy use and phonological priming effects in non-word reading. Cognit. Dev. 2001;16(4):951–963. [Google Scholar]
- Wood C., Terrell C. Pre-school phonological awareness and subsequent literacy development. Educ. Psychol. 1998;18(3):253–274. [Google Scholar]