Abstract
Vocal dialects have fascinated biologists for over 50 years. This mosaic pattern of geographic variation in learned vocalizations was first described in a songbird, and since that time, most studies investigating dialects have focused on songbird species. Here we examine patterns of geographic variation in the calls of a different group of vocal learning birds, the parrots (Order Psittaciformes). We summarize the growing literature on vocal variation in parrots, and complement this review with a survey of variation in the genus Amazona using calls from sound libraries. We find strikingly similar patterns to those previously found in songbirds. Over 90% of parrots examined in the literature, and 69% of Amazona species surveyed, showed geographic variation consistent with a propensity to share local call types. This trait is evolutionarily labile and widespread; within Amazona most clades contained species with and without geographic variation, and most major lineages of parrots include representatives with dialects. We found little support for the long-standing hypothesis that dialects isolate populations and thus generate genetic differences among populations. Instead, most studies support the idea that dialects are maintained by social benefits of matching local call types, a finding that has implications for the management of captive and endangered populations. Considerable scope remains for studies that experimentally test hypotheses for the exact nature of these benefits, as well as studies that employ comparisons among species, to understand how the interplay between ecology, social dynamics and vocal learning capacities produces different patterns of variation across the parrots.
Keywords: contact calls, cultural evolution, dialect, geographic variation, parrots, Psittaciformes
Introduction
Dialects were first described in animal vocalizations by Marler and Tamura (1962, 1964) in their classic study of the White-crowned Sparrow (Zonotrichia leucophrys nuttalli). They found a distinct pattern of mosaic variation in the territorial songs of males of this species, such that there was relatively minor variation in acoustic structure of songs among males within populations and distinct differences in structure among different populations. Marler and Tamura went on to show in a series of elegant experiments that these songs were acquired by males early in life through social learning of the songs of adult males, a process they termed “cultural transmission” (Marler and Tamura 1964). They inferred that the dialect variation seen among populations was maintained by cultural transmission of different song types within different populations, perhaps coupled with limited dispersal between populations (Marler and Tamura 1962b). Thus the dialects of the White-crowned Sparrow appeared to share many similarities with their namesake dialects in human languages (Cavalli-Sforza 2000).
Since this initial discovery in the White-crowned Sparrow, vocal dialects have been described in many other songbird species (reviewed in Podos and Warren 2007) and in representatives of all taxa with the ability to learn vocalizations, including parrots (Wright 1996), hummingbirds (Wiley 1971), bats (Boughman 1997), and cetaceans (Ford 1991). These studies have revealed diverse patterns of geographic variation, including some species with classic mosaic dialects with sharp transitions in structure, some with graded or clinal variation, and some that lack geographic variation entirely (e.g. in parrots Baker 2000; Bradbury et al. 2001; Guerra et al. 2008; Wright 1996). Even within the subset of species with clear vocal dialects, there is a wide range of spatial scales over which particular variants are shared, ranging from microgeographic variation among neighbourhoods composed of a few territorial males to macrogeographic variation extending over thousands of kilometres (Podos and Warren 2007). This variation has led to considerable interest in determining what underlying processes lead to the formation of vocal dialects, and what determines the form and scale of spatial variation within species (Baker and Cunningham 1985; Handley and Nelson 2005; Podos and Warren 2007). Further questions revolve around the degree of the temporal stability of vocal variation, and whether distinctions in geographic patterns made by humans (e.g. dialects, clinal variation or (apparently) invariant vocalizations) are perceived as meaningful by the animals themselves. Finally, there has been considerable interest in whether geographic variation in learned vocalizations is associated with, and perhaps mutually reinforces, neutral genetic variation among populations (Baker and Cunningham 1985; Baker et al. 1982; Lipshutz et al. 2017; MacDougall-Shackleton and MacDougall-Shackleton 2001; Nottebohm 1972; Soha et al. 2004; Wright et al. 2005; Wright and Wilkinson 2001).
A plethora of hypotheses have been proposed for how dialects are formed and maintained, with overlapping and sometimes confusing terminology (Baker and Cunningham 1985; Handley and Nelson 2005; Nottebohm 1972; Payne 1981; Podos and Warren 2007). Here we have divided hypotheses for the formation and maintenance of vocal dialects into four groups representing discrete underlying processes that lead to the sharing of geographic variants by neighbours; sexual selection, signalling group membership and familiarity, environmental adaptation, and cultural drift. The first two processes are similar in that individuals who adhere to local variants acquire some form of socially advantageous selective advantage. In the sexual selection hypothesis, geographic variation in vocalizations is driven primarily by female preferences for locally specific variants, and individuals with the correct variants are predicted to acquire mates with greater success (Podos and Warren 2007). Geographic variation could result from arbitrary female preferences as females strive to have locally sexy sons or could be driven by females’ desire to mate with males with locally adapted genes, and thus have offspring with genes that are better adapted to the local environment (e.g. the local adaptation hypothesis, Podos and Warren 2007). Note that this contrast echoes the Fisherian runaway/sexy sons versus good genes debate in general sexual selection theory. In the signalling group membership and familiarity hypothesis, the selective advantage is that individuals who converge on the local dialect will be able to integrate into social groups more readily (Sewall et al. 2016). In the environmental adaptation hypothesis, a different form of selective advantage arises from adaptation of specific acoustic variants for better transmission within local environments (Handford and Lougheed 1991; Slabbekoorn 2004). This hypothesis predicts that different acoustic variants would map closely onto environmental variables that might affect signal transmission, such as the amount of vegetation, and that acoustic variants would transmit better in their own habitat than in other habitats.
The fourth and final hypothesis suggests that geographic variants, in and of themselves, provide no selective advantage. The cultural drift hypothesis proposes that geographic variants are simply functionless epiphenomena that are by-products of learning that occurs from local models with some degree of copying errors coupled with limited dispersal between populations (Payne 1981). These processes are predicted to lead to the accumulation of different variants in different regions through a process that has been termed “cultural drift”, in analogy to genetic drift of genetically-transmitted traits. Importantly, cultural drift is not mutually exclusive with hypotheses inferring selective advantages; for example, dialects might originally form due to isolation and copying errors and then persist through benefits associated with sharing vocal variants with neighbours within a certain range (Sewall et al. 2016).
It is also worth noting that discussion about these processes has been muddied by the fact that dialects themselves are not individual-level phenomena that are subject to evolution by natural selection, but instead are population- or species-level phenomena that arise out of a propensity of individuals to share local variants with other individuals. Thus we should not be asking how or why dialects evolve, but rather (i) why individuals evolve the propensity to share local variants of calls, and (ii) what other factors interact with this trait to impact dialect formation such as the propensity for copying errors, the strength of conformation to local types, the degree of dispersal, the timing of learning relative to dispersal and the stability of social group.
Here we examine patterns of geographic variation in the vocal repertoires of the parrots and cockatoos (Order Psittaciformes, hereafter ‘parrots’) with the goal of documenting general patterns of variation, inferring underlying processes, and evaluating their evolutionary consequences. The parrots represent a useful group in which to examine these questions as they are well known for their highly-developed vocal learning abilities and yet differ in social organization, composition of the vocal repertoire, and modes of learning from songbirds, the group in which vocal learning has been most studied (Bradbury and Balsby 2016). Finally, there is a growing literature of natural patterns of geographic variation in this group that has not, to our knowledge, been formally reviewed (but see Bradbury and Balsby 2016) for a general review of vocal learning in parrots). Thus a review of patterns of vocal variation in parrots has the potential to generate novel insights into longstanding questions.
We investigate these issues using two datasets. The first is a review of studies on vocal variation in parrots compiled from papers in the peer-reviewed literature. The second is surveys of geographic variation in the contact calls in a focal taxon, the Amazon parrots (Genus Amazona) conducted using recordings deposited in sound libraries. Previous descriptions of vocal variation in the literature for this group provides a useful benchmark to assess the accuracy of our survey approach, which used recordings collected by many investigators using differing methodologies.
We used these two data sets to address a number of specific questions regarding patterns of geographic variation in the vocal repertoire.
How common is geographic variation and what is its phylogenetic distribution?
What parts of the vocal repertoire show geographic variation?
What patterns are seen in the scale and form of geographic variation?
What evidence is there for temporal stability of either patterns of geographic variation or the acoustic structure of vocalizations?
To what extent does vocal variation correspond to underlying population structure?
We then compare these patterns in parrots to general patterns observed in songbirds and discuss what inferences can be drawn from these patterns about the underlying processes that contribute to the formation and maintenance of vocal dialects. We discuss implications of vocal variation for the conservation of parrots and end by suggesting some promising future directions for better understanding these phenomena.
Literature Review of Vocal Variation in Parrots
Methods
We conducted a literature survey of geographic variation in parrots using the search engines Web of Science and Google Scholar (all years), and also using the citations sections of papers found in these searches. Key words we used in our searches included “birds”, “parrots”, “dialect”, and “geographic variation”. In Web of Science, we also conducted a Cited Reference Search with Wright (1996) as the cited work as this is the first in-depth mapping of vocal variation in a parrot species.
For parrots, we compiled data from 24 studies representing 13 species (Table 1). To be included in the table, the authors must have conducted a formal analysis of variation in call features between geographic regions in wild parrots. Data garnered exclusively from captive studies of parrots, such as the many experiments conducted on budgerigars (Bartlett and Slater 1999; Dahlin et al. 2014; Hile et al. 2000; Hile and Striedter 2000; Striedter et al. 2003), or research that lacked formal comparisons of acoustic variation between geographic regions were excluded (Saunders 1983). In general we followed the approach of Podos & Warren (2007) in their comprehensive review of vocal dialects, which focused on songbirds. For each study we classified aspects of the vocal signals, their patterns of variability, ecological correlates, and degree of support for alternative hypotheses for the formation and maintenance of vocal variation (details on data classification in Supplemental Materials)
Table 1.
Review of geographic variation in parrots from the literature. See details below and the Literature Review Methodology for descriptions of the categories.
| Family | Species | Common Name | Signal: (C)all, (S)ong, (D)uet | Geog. Var: (D)ialect, (G)raded (N)one | Dialect Scale: 0, 1, 2, 3 | Distance between dialects: 1, 2, 3 | Temporal Stability: 1, 2, 3 | Bilingual: (Y)es, (N)o | Territoriality: (Y)es, (N)o | Mobility: (Y)es, (N)o | Associated Genetic Differences (Y)es, (N)o | Devpt. Mech: S, G, E, C | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cacatuidae | Eolophus roseicapillus | Galah | C | D | 1 | 2 | Y | Y | S, G | Baker 2003, Scarl 2009 | |||
| Cacatuidae | Probosciger aterrimus | Australian Palm Cockatoo | C | D | 1 to 2 | 3 | Y | N | G, C | Keighly 2016 | |||
| Psittacidae | Amazona auropalliata | Yellow-Naped amazon | C, D | D | 2 to 3 | 2 | 3 | Y | Y | N | N | G | Wright 1996, Wright and Dorin 2001, Wright and Wilkinson 2001, Wright et al. 2008, Salinas-Melgoza & Wright 2012 |
| Psittacidae | Amazona leucocephala | Cuban Parrot | C | D | 3 | Y | N | G, C | Reynolds et al. 2010 | ||||
| Psittacidae | Amazona versicolor | St. Lucia Parrot | C | G | 2 | Y | N | G, C | Kleeman and Gilardi 2005 | ||||
| Psittacidae | Amazona vittata | Puerto Rican Amazon | C | D | 1 | 1 to 3 | Y | Y | N | G, C | Martinez & Logue, ms in prep. | ||
| Psittacidae | Eupsittula (Aratinga) canicularis | Orange-Fronted Conure | C | G | 2 | 1 | Y | N | G, C | Bradbury et al. 2001, Vehrencamp et. al. 2003, Balsby and Scarl 2008, Balsby et al. 2012 | |||
| Psittacidae | Myiopsitta monachus | Monk Parakeet | C | D | 0 to 3 | 1 to 3 | N | N | G, C | Buhrman-Deever 2007 | |||
| Psittacidae | Rhynchopsitta pachyrhyncha | Thick-billed Parrot | C | N | 3 | N | Y | N | Guerra et al. 2008 | ||||
| Psittaculidae | Barnardius zonarius | Australian Ringneck Parrot | C | D | 3 | 2 to 3 | 3 | N | Y | N | Y & N | S, G, C | Baker 2000, Baker 2008, Baker 2011 |
| Psittaculidae | Barnardius zonarius | Australian Ringneck Parrot | D | N | Y | N | Baker 2011 | ||||||
| Psittaculidae | Platycerus elegans | Crimson Rosella | C | G | Y | N | Y | S, G, C | Ribot et. al. 2012, Ribot et al | ||||
| Psittaculidae | Pezoporus wallicus wallicus | Eastern ground parrot | C | G | 1 | 3 | Y | N | C | Chan and Mudie 2004 | |||
| Strigopidae | Nestor notabilis | Kea | C | G | Y | Y | G | Bond and Diamond 2005 |
Dialects: 0=microgeographic, 1=small, 2=medium, 3=large; Distance between dialects: 1=short, 2=medium, 3=large; Temporal stability over time; 1=short term, 2=moderate term, 3=long term.
Results
Vocal Variation in Parrots
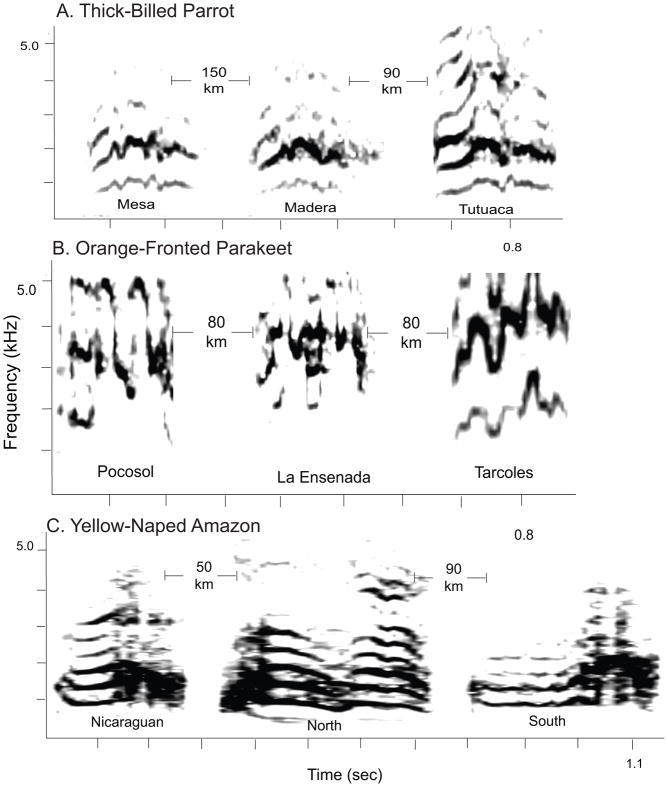
We reviewed data from 23 published studies and one unpublished study provided by the authors (Martinez & Logue, ms in prep.) that examined vocal variation in 13 species of parrots. These studies have focused almost exclusively on variation in short calls given by parrots commonly referred to as ‘contact calls’ or ‘flight calls’. Geographic variation appears common in parrots, with 12 of the 13 species surveyed (92%) showing some form of geographic variation. This variation was found in all four families in Psittaciformes: Psittacidae (6 species), Psittaculidae (3 species), Cacatuidae (2 species) and Strigopidae (1 species). Only one species, the Thick-billed Parrot, Rhynchopsitta pachyrhyncha (Psittacidae), was determined to have calls that were invariant across the sampled range. Examples of invariant, graded variation, and distinct dialects are shown in Figure 1.
Figure 1.
Spectrograms demonstrating the complete spectrum of geographic variation in parrots, including; a) invariant calls in Thick-Billed Parrots, b) graded variation in Orange-Fronted Parakeets, and c) distinct mosaic dialects in Yellow-Naped Amazons. The labels indicate the names of the recording sites (in the case of Thick-Billed Parrots and Orange-Fronted Parakeets) or the dialects (Yellow-Naped Amazons). The distances shown represent the approximate flight distances between recording locations. For all species, the calls are presented from left to right in order from north to south. Orange-Fronted Parakeet calls were provided courtesy of J. Bradbury and S. Vehrencamp (Pocosol) or downloaded from www.Xeno-Canto.org (La Ensenada and Tarcoles).
Geographic variation in note sequences, rather than single-notes, has been examined in two species. Yellow-naped Amazons have distinct dialects in which geographic variation extends across most call types, including not just contact calls but also the notes that compose complex pair duets (Wright and Dorin 2001). In the Australian Ringneck Parrot, Barnardius zonarius, variation extended to calls but not to the ‘tailwag’ duets (Baker 2011).
Distinct dialects were most common, representing 58% of all geographic variation, with graded variation, representing 42%. The Palm Cockatoo, Probosciger aterrimus, and Yellow-naped Amazon have call repertoires with distinct dialectal regions; in both these species several different call types vary at the same boundaries (Dahlin and Wright 2009; Keighley et al. 2017; Wright 1996). In the Eastern Ground Parrot, Pezoporus wallicus wallicus, some call types in the repertoire are shared between geographic areas while others are unique (Chan and Mudie 2004). Orange-fronted Conures, Keas, Nestor notabilis, and Crimson Rosellas, Platycerus elegans, exhibit graded variation in which acoustic variables change across the landscape in a continuous manner (Bond and Diamond 2005; Bradbury et al. 2001; Ribot et al. 2012; Ribot et al. 2013). In Keas, variation within juveniles and adults exhibit different patterns of variation, potentially because juveniles associate in ‘gangs’ that are distinct from adults and use different vocalizations (Bond and Diamond 2005). After excluding the family Strigopidae, which was only represented by one species, we found no association between taxonomic family and the type of geographic variation (X22= 2.96, P=0.23). Both members of Cacatuidae had distinct dialects, but both dialects and graded variation were found in Psittacidae and Psittaculidae; Psittacidae also included the lone representative with invariant calls.
We gathered additional data on dialect scale for seven species that were categorized as having distinct dialects. Scale varied widely among those seven species. Small- and large-scale dialects were equally common at 43% (3 species each), while medium dialects represented 14% of the species (one species). Geographic variation, whether distinct or graded, manifested at all distances, although it was found most commonly at intermediate distances (50%, 5 species), and less commonly at small (20%, 2 species) and large distances (30%, 3 species). We found no relationship between the distance at which geographic variation manifested and the form of variation (distinct vs graded; X22= 1.67, P=0.43). In invasive populations of Monk Parakeets, Myiopsitta monachus, some acoustic variants were found in adjacent neighbourhoods that spanned as few as 3 km (Buhrman-Deever et al. 2007), while dialects in Yellow-Naped Amazons could extend across a 120 km range (Wright 1996).
We found data on temporal stability for only three species: Yellow-naped Amazons, Orange-fronted Conures, and Australian Ringneck Parrots (Baker 2008; Bradbury et al. 2001; Wright 1996; Wright et al. 2008a). The Yellow-naped Amazon has maintained three dialects with fairly stable boundaries within Costa Rica for more than 22 years (Wright 1996; Dahlin and Wright, ms in prep; Wright et al. 2008a). Hybridizing subspecies of Australian Ringneck Parrots have maintained sub-species specific dialects as well as a consistent dialect within the hybrid zone for more than 40 years (Baker 2008). Orange-fronted Conures have evidence of maintaining stability in their graded call variants for short time periods (Bradbury et al. 2001). Thus, although data is lacking in many species, the potential for maintenance of geographic variation across extensive time spans is clearly present in the parrots.
Evidence of bilingualism was noted in two parrot species; Yellow-Naped Amazons and Puerto Rican Amazons, Amazona vittata (Wright 1996; Martinez & Logue, ms in prep.; Wright et al. 2008a). Bilingual birds appear to be strikingly absent in a hybrid zone between two sub-species of Australian Ringneck Parrots, in which hybrid birds, who exhibit variable morphology, have converged onto a shared dialect that appears dissociated from the bird’s phenotypes (Baker 2011).
We were able to correlate genetic differences (or a lack thereof) with geographic variation for only four species. A correlation between genetic and acoustic variation was found in two species. In the Crimson Rosella, a sharp cline in acoustic features occurred at the same areas strong differences in neutral genetic variation occurred as measured by microsatellites. These differences occurred at a contact zone between two phenotypically distinct subspecies, but, interestingly, did not coincide with sharp clines in variation in plumage or mitochondrial DNA variation that occurred at another point in the contact zone (Ribot et al. 2012). Australian Ringneck Parrots also present a complicated genetic picture, because although distinct dialects are associated with phenotypically distinct subspecies, the birds have converged onto a shared dialect in a hybrid zone that contains a mix of phenotypes (Baker 2000; Baker 2008). Thus the dialect in the hybrid zone appears to be culturally determined. Dialect regions in Yellow-naped Amazons in Costa Rica were not found to be genetically distinct, and instead had patterns of genetic variation in both mitochondrial DNA and microsatellites consistent with high gene flow between dialects (Wright et al. 2005; Wright and Wilkinson 2001). Lastly, Thick-billed Parrots that lack geographic variation between populations also appear to be genetically similar across the range (Guerra et al. 2008; D. Acosta and T. Wright unpublished data).
Ecological Correlates
There were no obvious associations between territoriality, seasonal mortality and geographic variation. Of the 12 species with some form of geographic variation in their vocalizations, 11 species are cavity nesters who defend nesting territories. The exceptions are Monk Parakeets, who craft their stick nests and sometimes nest communally in nests with multiple chambers (Navarro et al. 1995). Thick-billed Parrots, who have invariant calls, are cavity nesters that will sometimes nest communally in trees that contain more than one suitable cavity and are not agonistic to their neighbours during the breeding season (Monterrubio-Rico et al. 2006).
Seasonal mobility was almost as rare as a lack of territoriality, and was found in only two species with geographic variation, Galahs and Keas, and the one species with invariant calls, the Thick-billed Parrots. Galahs, which exhibit discrete dialects, are territorial during the wet season, but form large roving flocks in the dry season (Rowley 1990). Keas, with graded variation in adults, have altitudinal movements depending on the climate (Diamond and Bond 1999). Thick-billed Parrots, with invariant calls, are migratory and leave breeding grounds in the northern Sierra Madre of Mexico to move farther south during the winter (Snyder et al. 1999).
Formation & Maintenance of Dialects
The hypotheses for the formation and maintenance of dialects that received the most support among authors were: signalling group membership and familiarity (11 species), cultural drift (9 species), and sexual selection (3 species). The environmental adaptation hypothesis received no support among the parrot species studied. For nine species, multiple hypotheses were proposed as possibilities.
The only hypothesis that has received direct observational or experimental support was signalling group membership and familiarity, which was supported in four species. Three of those species, the Galah, Yellow-naped Amazon, and Puerto Rican Amazon, have distinct dialects, while the Orange-fronted Conures exhibited graded variation. Authors have provided varying support for the signalling group membership hypothesis. In both Galahs and Orange-fronted Conure, birds converged on playback stimuli during the course of playbacks and authors propose that such convergence may have an affiliative function (Baker and Logue 2003; Bradbury et al. 2001; Vehrencamp et al. 2003). Orange-fronted Conures are also less responsive to playbacks from regions that are from a greater distance, and more responsive to calls that more greatly resemble their own (Bradbury et al. 2001; Vehrencamp et al. 2003). Several lines of evidence in Yellow-naped Amazons indicate that knowing the local dialect is socially relevant; nesting pairs are less responsive to playbacks from foreign dialects than a local dialect (Wright and Dorin 2001), and in a translocation experiment, one out of several birds who were translocated to a foreign dialect eventually converged to the new dialect (Salinas-Melgoza and Wright 2012). Most Puerto Rican Amazons translocated to a foreign dialect during reintroductions also converge onto the new dialect (T. Martinez and D. Logue, ms in prep.).
Surveys of Vocal Variation in the Genus Amazona from Sound Libraries
Methods
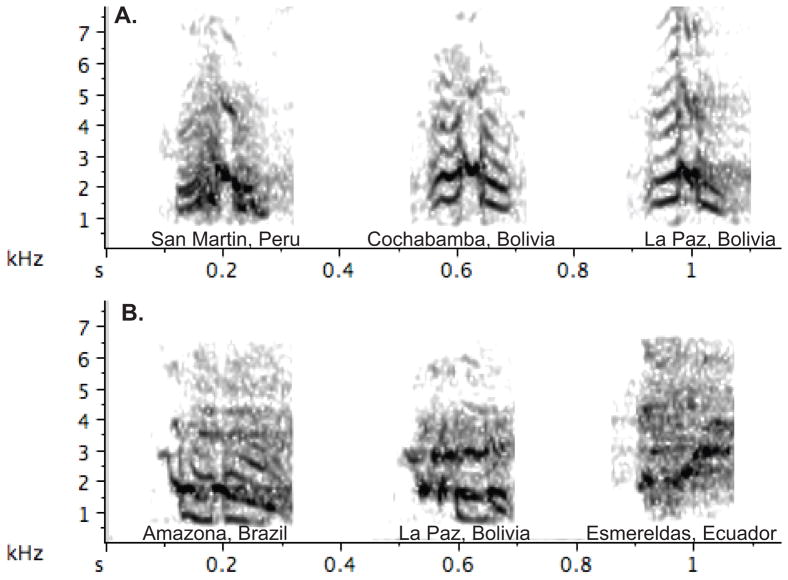
We conducted a species-level survey of vocal variation within a focal taxon using publicly available recordings in sound libraries. The goal was to obtain a finer-grain picture of evolutionary patterns of vocal variation than the current literature would permit. Like most of the published studies reviewed above, we focused on contact calls as their high frequency of use makes it relatively easy to identify homologous calls across different populations and species. We assessed whether there was variation in the acoustic structure (e.g. time and frequency patterning) across different recording sites consistent with the presence of dialects. We focused on the genus Amazona, which is a large group with 31 currently recognized species that occur in a variety of habitats through mainland and insular Neotropics (Forshaw 2010). The genus is thought to have arisen from a common ancestor about 10–15 mya (Schweizer et al. 2011; Wright et al. 2008b). Importantly, geographic variation in vocalizations of Amazona, including vocal dialects, has been described in the literature (Table 1), and the genus is well represented in the publicly accessible sound libraries xeno-canto (http://www.xeno-canto.org) and Macauley Library (Lab of Ornithology, Cornell University, (http://macaulaylibrary.org). Using recordings from these libraries we classified the pattern of variation in each species into one of four categories: i) Invariant Calls that showed no substantial differences in the temporal-frequency patterning of contact calls across the range, ii) Variation Consistent with Vocal Dialects, for species that showed major shifts in the temporal-frequency patterns of contact calls across different sites, iii) Hypervariable, for species who showed such extensive variation in call structure within and among sites such that it was impossible to characterize a single contact call variant at each site, and iv) Insufficient Sampling, for species where fewer than three sites had high quality contact calls that could be used in the comparison (Figure 2). Details on the assessment of geographic variation and associated variables, and for the reconstruction of evolutionary patterns of these forms of variation within Amazona are provided in Supplemental Materials.
Figure 2.
Spectrograms illustrating two of the geographic patterns of vocal variation found a survey of contact calls of Amazona parrots. Panel a) illustrates a pattern of invariant calls in Amazona mercenaria, while b) illustrates variation consistent with the presence of vocal dialects in Amazona farinosa. For each species contact calls from 3 different sites are illustrated. All calls are isolated from recordings downloaded from the online sound library xeno-canto.
Results
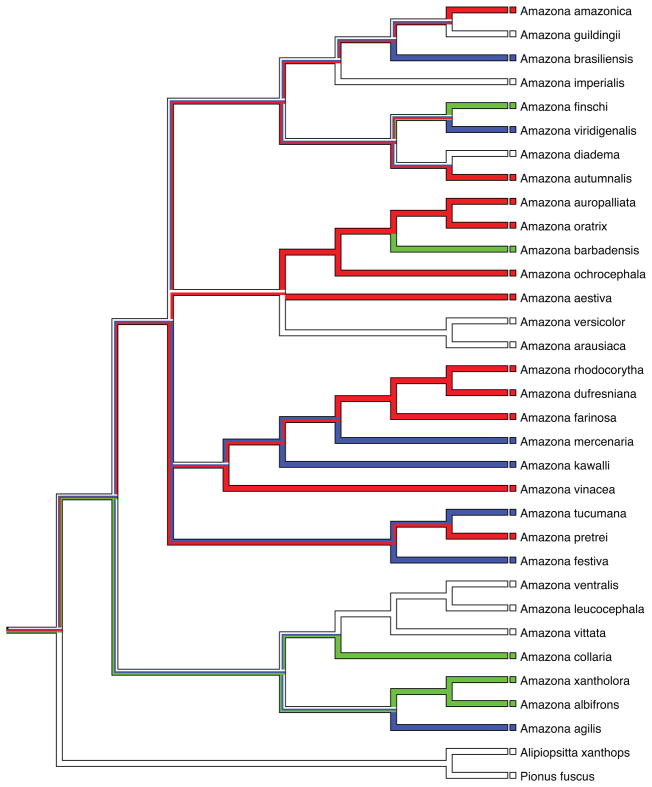
We surveyed vocal variation in all 31 Amazona species listed in xeno-canto. Of these 31 species, 11 (35%) showed variation in contact calls consistent with the presence of vocal dialects, seven (23%) showed invariant contact call structure across the sampled sites, five (16%) had hypervariable calls, and eight (26%) had insufficient sampling to determine patterns of geographic variation. Thus there was diversity of patterns within Amazona similar to that seen in the published studies sampling Order Psittaciformes. When these variation patterns were mapped as character states onto a phylogenetic hypothesis for the 31 Amazona species, the predominant pattern was that three informative character states (invariant, variation consistent with dialects, hypervariable calls) each occur in multiple clades within the tree (Figure 3). If, for example, the genus is divided into five clades corresponding to the four taxa descending from the large polytomy near the base of the tree plus the sister clade that includes Amazona albifrons, then dialects are present in four of those five clades, invariant calls are also found in four clades, and hypervariable calls are found in three clades. This pattern suggests there is considerable evolutionary lability in the propensity of species within Amazona to develop different forms of geographic vocal variation.
Figure 3.
An ancestral state reconstruction of different classifications of geographic variation in the parrot genus Amazona based on a survey of recordings in sound libraries. Red lineages represent species classified as having geographic variation consistent with the presence of vocal dialects, blue lineages were classified as have invariant calls, and green lineages were classified as having hypervariable calls that made unambiguous classification of contact calls difficult. White lineages had insufficient sampling to classify them to a category of vocal variation.
Our survey results were largely consistent with published studies of vocal variation in Amazona. Variation consistent with vocal dialects was readily apparent in the xeno-canto recordings of contact calls of the Yellow-Naped Amazon in which vocal dialects have been previously described (Wright 1996; Wright et al. 2008a). As previously noted (Wright and Wilkinson 2001), similar variation was also apparent in the contact calls of the three other species (A. ochrocephala, A. oratrix, and A. aestiva) that are classified with A. auropalliata in a super-species complex. Geographic variation was also apparent in the calls of the St. Lucia Amazon, A. versicolor, but there were insufficient sites available for this island species to unambiguously classify it to an informative category; Kleeman and Gilardi (2005) documented geographic variation but considered it to be graded rather than distinct dialects. There was also insufficient sampling by our criteria for the island-dwelling Puerto Rican Amazon, A. vitatta, which is highly endangered and only exists at two sites in the wild. A recent study of this species has found evidence of vocal variation between these sites and between different generations of captive-bred birds released into the wild (T. Martinez & D. Logue, ms in prep.). Finally, a third island-dwelling species, the Cuban Amazon, A. leucocephala, was also classified as having insufficient information in our survey but was shown to have dialects in a more in-depth study, although these dialects were confined to populations on the Caymans, the Bahamas, and Cuba that are often classified as different subspecies (Reynolds et al. 2010).
The differences noted between our survey and published studies highlight some shortcomings of relying on archives that vary in sampling effort among species. For example, there was a dramatic difference between island and mainland species in the number of species classified as insufficient sampling, with seven of nine (78%) island species classified with insufficient sampling while only one of 22 (4%) mainland species were so classified. This difference is probably due to both smaller range sizes of island species (mean ± SD of 105 ± 195 km max distance between sampling sites for island species versus 1748 ± 1707 km for mainland species) and to the lower frequency of recording expeditions to the islands of the Caribbean. Sampling intensity may also skew the classification of variation to some degree. Among the three informative categories of geographic variation, species classified with dialects had greater sampling intensity (mean ± SD of 23.5 ± 22.2 sites sampled for species with dialects versus 10.4 ± 8.4 for invariant and 7.6 ± 3.4 for hypervariable species). They also had a larger geographic spread to their sampling (mean ± SD of 2341 ± 1990 km for species with dialects versus 1007 ± 1094 km for invariant and 1190 ± 1349 km for hypervariable species). Nonetheless, the mean number of sampling sites greatly exceeded our minimum of three sites for all three informative categories and the average maximum distance sampled was above 1000 km for each category, suggesting that sufficient sampling was conducted to distinguish between different forms of geographic variation for most species. We have not conducted statistical tests on these differences because of the non-independence of species values due to shared ancestry (Felsenstein 1985).
Comparisons with Songbirds
The patterns of geographic variation in vocalizations we observed in parrots are broadly similar to those described in other taxa. The most comprehensive review of vocal dialects across non-human animal taxa was performed by Podos & Warren (2007). They found robust documentation of the occurrence of mosaic dialects in 42 species, of which most (37 species, or 88%) were from songbird species with the remainders from parrots, hummingbirds, cetaceans and primates. Dialects in songbirds were most commonly described in the Fringillidae, but also occurred in seven other families, suggesting they are a phylogenetically widespread phenomenon in the songbirds, as we found in parrots. Further support for this conclusion comes from a study by Handley and Nelson (2005) of patterns of song sharing within the family Fringillidae. They surveyed published studies of vocal variation for 65 species and subspecies within the family and classified whether or not a species exhibited local song sharing, which should manifest at a larger geographic scale as mosaic dialects of various sizes or potentially graded variation. They found evidence of song sharing in 45 of 65 taxa surveyed (69%). In comparison, we found that 92% of Psittaciformes were reported to have geographic variation consistent with the sharing of local types in the literature, while 61% of Amazona species where call variation could be classified had variation consistent with local call sharing. Phylogenetic reconstructions of the distribution of song sharing in Fringillidae also showed a similar pattern to that seen in Amazona (Figure 3), in which most clades included both sharing and non-sharing taxa, suggesting that the propensity to develop shared geographic variants, is an evolutionarily labile trait.
Another area of similarity between songbird and parrot dialects is their general lack of association with genetic boundaries. Genetic structure has been examined in a number of songbird species, and the general pattern seen is that vocal variation is uncorrelated with genetic variation in most populations (Fleischer and Rothstein 1988; Lougheed and Handford 1992; Payne and Westneat 1988; Soha et al. 2004), and weakly correlated in the remainder (Baker 1982; MacDougall-Shackleton and MacDougall-Shackleton 2001; Zink and Barrowclough 1984). A lack of concordance between dialects and genetic structure has also been observed in two of the three parrot species in which it has been examined, the Port Lincoln Parrot and the Yellow-naped Amazon (Baker 2008; Wright et al. 2005; Wright and Wilkinson 2001). In both taxa the rare exceptions appear to occur at the boundaries between pre-existing subspecies. In songbirds a recent study using a high number of nuclear single-nucleotide polymorphisms as genetic markers found a distinct genetic boundary between the nuttalli and pugetensis subspecies of the White-crowned Sparrow that coincided with differences in song and response to playback (Lipshutz et al. 2017). A similar pattern is seen in the contact zone between subspecies of the Crimson Rosella, in which genetic structure in microsatellites occurs at the boundary between two dialects (Ribot et al. 2012). The fact that, in both these systems, these boundaries occur in a region of secondary contact between two previously isolated populations begs the question of whether dialects contributed to the formation of genetic isolation between the subspecies or developed in isolation before subspecies came into contact.
There are interesting contrasts reported between songbirds and parrots in certain aspects of vocal variation, some of which might arise from differences in the general biology of the two taxa. Bilingualism, in which an individual produces vocalizations characteristic of more than one local dialect, is reported in both taxa, but may occur more commonly in songbirds. Podos & Warren (2007) found evidence for bilingualism in 19 of the 26 songbird species (73%) in which it had been examined directly. In contrast, we only found published evidence in two of seven parrot species (28%) in which dialects were reported. It is not clear whether this difference is due to differences in sampling schemes or is an actual biological difference. Podos & Warren (2007) also found a strong association of dialects with territoriality in songbirds, with 80% of species with dialects exhibiting male defence of territories. In contrast, the majority of the species of parrots examined in both our literature search and our survey of Amazona do not defend resource-based territories and are not considered territorial in the same sense that the typical songbird is, although mated pairs in many species of parrots will vigorously defend the area directly around their nest cavity (Toft and Wright 2015). For example, the Yellow-naped Amazon produces pair duets around its nest site that appear to function in nest defence (Dahlin and Wright 2012a; Dahlin and Wright 2012b); these duets exhibit vocal dialects that are congruent with those seen in contact calls (Wright and Dorin 2001). Finally, both reviews of songbird dialects found an association of vocal dialects with migratory status, such that sedentary species and those with longer breeding seasons were more likely to have dialects, although dialects were also widespread in migratory species (Handley and Nelson 2005; Podos and Warren 2007). In contrast, virtually all parrot species are considered non-migratory, although they may make considerable movements over the landscape on a daily or seasonal basis while foraging (Toft and Wright 2015). Vocal variation has only been examined in one migratory parrot, the Thick-billed Parrot; no evidence of geographic variation was found in this species (Guerra et al. 2008).
The general similarity observed between parrots and songbirds in patterns of vocal variation is all the more striking because of a fundamental difference in the types of vocalizations that are varying in these two groups. The vast majority of studies of geographic vocal variation in songbirds have focused on territorial songs that are produced largely by adult males (at least in temperate regions) and serve to both defend territories and attract potential mates to these territories (Catchpole and Slater 2008). In contrast, most studies in parrots have focused on contact calls that are produced by both sexes and all age groups. Contact calls are thought to function to maintain social contact among specific individuals and group members within the fluid fission-fusion groups exhibited by parrots and some other taxa (Balsby et al. 2012; Sewall et al. 2016). What territorial song and contact calls have in common is a) that they are learned from others, and b) there may be social benefits to matching local types in order to establish territories, attract mates or gain group membership. The co-occurrence of these two factors with dialects in two groups with such different social structure suggest that these factors deserve special scrutiny when considering how dialects form, and what evolutionary implications they might have.
Evolutionary Implications of Vocal Dialects
Much of the initial theoretical and empirical interest in vocal dialects focused on whether such variation in learned cultural traits could serve as markers of local adaptation and this contribute to genetic divergences among populations and ultimately, the formation of new species. Now, some 50 years after the initial description of dialects, the answer to that question appears to be negative (Slabbekoorn and Smith 2002). With rare exceptions, the concordance between vocal and genetic variation that would be predicted by the local adaptation hypothesis has not been observed in either songbirds or parrots, and where it has, it appears to be a by-product of previous isolation rather than been generated de novo by an isolating effect of vocal dialects. This lack of support for the hypothesis of dialects as drivers of speciation does not mean that vocal dialects are a phenomenon of little consequence or interest to evolutionary biologists. On the contrary, further study of dialects and other forms of geographic variation in learned vocalizations has the potential to offer new insights into several related questions of general interest in behavioural and evolutionary biology.
The first is what hypotheses best explain the origin and maintenance of vocal dialects. Numerous hypotheses have been proposed for why geographic variation in vocalizations forms and persists, many of which overlap both conceptually and in their predictions (Baker and Cunningham 1985; Handley and Nelson 2005; Nottebohm 1972; Payne 1981; Podos and Warren 2007). Podos & Warren (2007) argued cogently that evidence from songbirds suggested that vocal dialects could best be explained as population-level epiphenomena resulting as by-products of evolutionary forces acting on the individual level. They suggested that the most important of these forces were likely to be a) natural selection at the local level for physical adaptations that secondarily impacted communication signals, b) social or sexual selection favouring the sharing of local song or call types, and c) cultural or genetic drift in isolated population types. We largely followed this schema in our review of published studies in parrots, in which we recorded whether authors invoked processes of a) sexual selection favouring local types, b) call sharing signalling group membership and familiarity, c) adaptation to local acoustic environments, and d) cultural drift in isolated populations. We found that authors most often cited selection favouring signalling group membership and cultural drift as processes leading to geographic variation in their species. Note that these two processes are not exclusive and may in fact be mutually reinforcing in that any benefit to signalling group membership would be reinforced by the propensity for isolated groups to develop different vocalizations through the accumulation of copy errors. While habitat and environmental differences appear to have promoted geographic variation in the songs of some songbirds, they do not seem to have done so in parrots. This may be due to differences between the two taxa in beak morphology and vocalizations produced; parrots have heavy bills and produce broadband vocalizations that do not require beak movement, while songbirds are more prone to produce trills produced in part by rapid beak movements. Furthermore, the (generally) broadband calls produced by parrots may be less affected by habitat-induced degradation during transmission than the (generally) tonal calls of songbirds. Finally, it is important to note that in most of the studies we reviewed, hypotheses were proposed as explanations for observed patterns, but were rarely tested directly. This pattern is true in songbirds as well, and suggests there is still much scope for carefully designed studies that advance beyond the foundational description of patterns to test the underlying processes that give rise to them.
The second topic is how interactions between social dynamics, movement ecology and communication behaviour affect the temporal and spatial patterns of vocal variation. Patterns of ranging and dispersal vary between species. For parrots in particular, how individuals move across the landscape to exploit food resources, and the size and temporal stability of the groups formed during these movements, appears to vary greatly among species (Toft and Wright 2015). If contact calls and other vocalizations serve to mediate interactions between individuals as generally thought (Bradbury and Balsby 2016; Sewall et al. 2016), then these patterns of movement likely have a strong impact on the degree of local call sharing, whether this sharing results in clinal variation or mosaic dialects, and the temporal stability of these patterns. Groups such as the Amazon parrots that exhibit a diversity of patterns of vocal variation are promising foci for tests of these hypotheses.
The third is the relative importance of genes and learning in the development of behaviour. This topic was a focus of work by Marler and colleagues on song in the White-crowned Sparrow and related species. Their careful experiments that manipulated early life exposure to conspecific and heterospecific song produced iconic examples of how innate templates and social learning both shape the songs of adults (Marler 2004; Marler and Peters 1989; Marler and Tamura 1962a). This work is the foundation of two highly productive areas of biology, namely the behavioural ecology of bird song and the neural basis of song learning (Beecher and Brenowitz 2005; Brenowitz and Beecher 2005; Marler and Slabbekoorn 2004). The latter field in particular has thrived as songbirds, and in particular the Zebra Finch, Taeniopygia guttata, have become the predominant model for understanding the neural and genetic mechanisms underlying human speech acquisition (Bolhuis et al. 2010). Like many songbirds, the Zebra Finch is a close-ended learner that learns its song repertoire early in life and then maintains that song unchanged throughout its life. Such close-ended learning is only one of many forms of learning found across songbirds, and may in fact be rarer than various forms of open-ended learning in which songs can be modified either continuously or at certain times throughout life (Brenowitz and Beecher 2005; Podos and Warren 2007). Solid experimental data on modes of learning are much rarer for parrots (but see (Dahlin et al. 2014; Farabaugh et al. 1994; Hile et al. 2000) for work in budgerigars), but the widespread mimicry of heterospecific sounds by pet parrots suggests that open-ended learning is the norm in this group (Bradbury and Balsby 2016). The extent to which differences in the timing of learning drives differences among species in patterns of geographic variation remains an intriguing question. Adopting a broader comparative approach will provide new insight into how genes and learning interact to produce vocal variation, and why differences in these interactions exist within and between songbirds and parrots.
Finally, further study of dialects, and the process of vocal convergence or song sharing that appears to produce them, has the potential to give new insights into the evolution of vocal learning itself (Sewall et al. 2016). Sewall and colleagues (2016) make the case that the use of learning to develop shared calls in a social group is commonly found in all taxa that exhibit vocal learning, and thus is more taxonomically widespread than the use of elaborate songs as sexually-selected traits, which is largely restricted to songbirds and a few species in other groups. The benefits for such call sharing likely provide a more broadly relevant explanation for the evolution of vocal learning itself than the benefits of elaborate song (Sewall et al. 2016). Understanding the precise nature of both the benefits and the costs associated with call sharing should give important insights into why vocal learning is relatively rare among vertebrate taxa that commonly use vocal communication (i.e. fishes, amphibians, birds and mammals) but has evolved independently in several lineages in the birds and mammals.
Conservation Implications of Vocal Variation
28% of the more than 414 parrot taxa assessed by the IUCN are listed as Vulnerable, Endangered or Critically Endangered (IUCN 2016). Strategies that are increasingly used to help the most-at risk species include captive breeding, reintroduction and translocation (Brightsmith 2005; Derrickson and Snyder 1992; Garcia et al. 2015; Sanz and Grajal 1998). Formal recommendations have been developed to improve the success of these programs that include consideration of the fact that parrots rely heavily on learning to acquire many of their behaviours. For example, many have recommended that prior to release, birds experience socialization, flight training, exposure to local foods and predators, and even food supplementation for extensive periods of time, all of which are intended to help parrots develop appropriate behaviours for life in the wild (Brightsmith 2005; Collazo et al. 2003; Sanz and Grajal 1998; Snyder et al. 2000; White Jr et al. 2012). An additional important consideration is how the presence of acoustic geographic variation in these species may affect the success of reintroduction or translocation. The ability of a bird to integrate into a local flock, acquire a mate and breed successfully may depend on the ability to acquire a local dialect with which they are currently unfamiliar. Translocated Yellow-naped Amazon adults often returned to their original dialect region; while those that remained in the new dialect tended to flock with other translocated individuals and did not learn the local contact calls. The only individual that integrated completely into local flocks and acquired the local dialect was a juvenile bird (Salinas-Melgoza and Wright 2012). While small, this study does suggest that relocating individuals between areas with different call variants, or releasing captive bred individuals with different vocal repertories than wild populations, could negatively impact conservation success. Another consideration for conservation is the potential for parrots to learn their vocalizations from inappropriate models. Green-rumped Parrotlets, Forpus passerinus, and Galahs have both developed heterospecific calls when raised by foster parents in the wild (Berg et al. 2011; Rowley and Chapman 1986). Research on the Puerto Rican Amazon indicates a novel dialect may have developed in a captive breeding facility, possibly due to isolation from wild birds and exposure Hispaniola Amazons, Amazona ventralis, serving as foster parents (T. Martinez and D. Logue, ms in prep.).
More studies are clearly needed to determine the typical rate at which it takes members of different species to converge onto local dialects, and if there are fitness consequences during the learning period. Comparative studies of these factors in species that differ in their patterns of geographic variation would be particularly interesting. One prediction is that species such as the Orange-fronted Conure (Bradbury et al. 2001; Vehrencamp et al. 2003), which have graded variation and readily match the call characteristics of others, might transition more easily into a flock than species with distinct dialects such as the Yellow-naped Amazon (Baker 2003; Wright 1996). Species with little detectable variation among geographic regions, such as the Thick-billed Parrot, might have even less difficulty moving between regions and integrating into new flocks. At present these predictions remain untested, and should not be used as a firm basis for conservation decisions. Instead, we suggest that conservation biologists consider these issues in planning translocations and reintroductions, and, where possible, document such variables as the vocal repertoires and social integration of birds immediately after release and then periodically as they transition into flocks. Doing so may lead to new insights into factors that could affect the success of these programs, which has varied considerably from program to program (White Jr et al. 2012).
If dialects do act as substantial barriers to transition into new populations, then it is worth considering whether dialectal regions should be managed as culturally distinct populations that biologists attempt to avoid mixing in captivity or during release (Reynolds et al. 2010). Alternatively, if birds must be introduced into unfamiliar dialectss, should steps be taken to prepare them for this transition? These might include building breeding facilities where individuals will be exposed to local birds to promote learning of appropriate dialects, maintaining birds in on-site release facilities for longer periods prior to release, and having longer soft release periods (with supplemented food) for individuals who have difficulty integrating. Conducting playbacks of appropriate recordings may also be helpful. Biologists understand that careful consideration must be given to the rearing environment of parrots to ensure they develop appropriate complex learned behaviours, such as those involved with flocking and foraging, prior to release, (Snyder et al. 1996; Snyder et al. 2000). Similar attention may also have to be given to the acoustic environment so that learned vocal behaviours develop appropriately.
A final issue to consider is the value of maintaining vocal cultural diversity within a species. Ethically speaking, is variation that arises and is maintained through learning an important component of biodiversity that should be preserved? Practically speaking, is this variation important to the long-term viability of a population? There is a well-established literature on the value of maintaining genetic diversity within managed populations (Frankham et al. 2010). The precautionary principle might suggest that we treat vocal cultural diversity with a similar regard and do all we can to preserve it as part of our general effort at preserving species threatened with extinction.
Conclusions and Future Directions
When vocal dialects were first described in White-crowned Sparrows over 50 years ago there was much speculation that these cultural features would be found to have substantial impacts on population differentiation and speciation in songbirds and other species with vocal learning. Although dialects now do not appear to be the dominating evolutionary force once hypothesized, they continue to offer a fascinating opportunity for understanding the interplay between genetics and learning in the development of communication repertoires. They also offer the potential for further insights into the role of communication signals in mediating important behavioural processes like dispersal, social group dynamics and mate choice. Our review of published studies and our survey in select taxa give complementary pictures of a diversity of forms of vocal variation that appear to evolve rapidly in parrots. These results suggest there is rich potential for comparative studies across the parrots aimed at understanding the interplay between developmental features like the timing and fidelity of learning, social features like dispersal, mate choice and social group dynamics, and environmental features like seasonality and spatial distribution of resources. There is also continued scope for studies that move beyond the fundamentally important description of geographic patterns of variation to directly testing hypotheses for the formation and maintenance of dialects, as few studies to date have accomplished this (admittedly difficult) task. In addition to further field studies and the comparative approach outlined above, other potentially profitable approaches include laboratory-based studies of groups with experimentally-manipulated membership (Dahlin et al. 2014; Wanker et al. 2005) or modelling studies that incorporate consideration of role of contact calls in mediating social group membership. In combination, these approaches offer the potential for a much richer understanding of parrot vocal behaviour and of the causes and consequences of the vocal learning abilities that have evolved to such a great extent in this fascinating group.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under Grant 5SC1GM112582-06 to TFW and by the University of Pittsburgh under CRDF Award 9011737 to CRD.
We thank undergraduate researchers at the University of Pittsburgh at Johnstown, including Ellen Brady, Kaitlyn Eckley, Emma Gyurisin, and Gwendolyn Illar, for searching the parrot and songbird literature. We also thank the many ornithologists who have deposited their recordings in xeno-canto and the Macauley Library for publicly sharing these recordings.
References
- Baker MC. Vocal dialect recognition and population genetic consequences. American Zoologist. 1982;22(3):561–569. [Google Scholar]
- Baker MC. Cultural diversification in the flight call of the Ringneck Parrot in Western Australia. Condor. 2000;102(4):905–910. [Google Scholar]
- Baker MC. Local similarity and geographic differences in a contact call of the galah (Cacatua roseicapilla assimilis) in Western Australia. Emu. 2003;103:233–237. [Google Scholar]
- Baker MC. Analysis of a cultural trait across an avian hybrid zone: Geographic variation in plumage morphology and vocal traits in the Australian Ringneck Parrot (Platycercus zonarius) Auk. 2008;125(3):651–662. [Google Scholar]
- Baker MC. Geographic variation of three vocal signals in the Australian ringneck (Aves: Psittaciformes): Do functionally similar signals have similar spatial distributions? Behaviour. 2011;148(3):373–402. [Google Scholar]
- Baker MC, Cunningham MA. The biology of bird-song dialects. Behavioral and Brain Sciences. 1985;8:85–100. [Google Scholar]
- Baker MC, Logue DM. Population differentiation in a complex bird sound: a comparison of three bioacoustical analysis procedures. Ethology. 2003;109:223–243. [Google Scholar]
- Baker MC, Thompson DB, Sherman GL, Cunningham MA, Tomback DF. Allozyme frequencies in a linear series of song dialect populations. Evolution. 1982;36:1020–1029. doi: 10.1111/j.1558-5646.1982.tb05470.x. [DOI] [PubMed] [Google Scholar]
- Balsby TJS, Momberg JV, Dabelsteen T. Vocal imitation in parrots allows addressing of specific individuals in a dynamic communication network. PloS One. 2012;7(11):e49747. doi: 10.1371/journal.pone.0049747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P, Slater PJB. The effect of new recruits on the flock specific call of budgerigars (Melopsittacus undulatus) Ethology Ecology and Evolution. 1999;11:139–147. [Google Scholar]
- Beecher MD, Brenowitz EA. Functional aspects of song learning in songbirds. Trends Ecol Evol. 2005;20:143–149. doi: 10.1016/j.tree.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Berg KS, Delgado S, Okawa R, Beissinger SR, Bradbury JW. Contact calls are used for individual mate recognition in free-ranging green-rumped parrotlets, Forpus passerinus. Animal Behaviour. 2011;81:241–248. [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nature Reviews: Neuroscience. 2010;11(11):747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Bond A, Diamond J. Geographic and ontogenetic variation in the contact calls of the kea (Nestor notabilis) Behaviour. 2005;142:1–20. [Google Scholar]
- Boughman JW. Greater spear-nosed bats give group-distinctive calls. Behavioral Ecology and Sociobiology. 1997;40:61–70. [Google Scholar]
- Bradbury JW, Balsby TJS. The functions of vocal learning in parrots. Behavioral Ecology and Sociobiology. 2016;70(3):293–312. [Google Scholar]
- Bradbury JW, Cortopassi KA, Clemmons JR. Geographical variation in the contact calls of orange-fronted parakeets. Auk. 2001;118:958–972. [Google Scholar]
- Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends in Ecology and Evolution. 2005;20:127–132. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Brightsmith DJ. Parrot nesting in southeastern Peru: seasonal patterns and keystone trees. Wilson Bulletin. 2005;117(3):296–305. [Google Scholar]
- Buhrman-Deever SC, Rappaport AR, Bradbury JW. Geographic variation in contact calls of feral North American populations of the monk parakeet. The Condor. 2007;109:389–398. [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. 2. Cambridge University Press; Cambridge: 2008. p. 248. [Google Scholar]
- Cavalli-Sforza LL. Gene, Peoples, and Languages. North Point Press; New York: 2000. p. 388. [Google Scholar]
- Chan K, Mudie D. Variation in vocalisations of the ground parrot at its northern range. Australian Journal of Zoology. 2004;52(2):147–158. [Google Scholar]
- Collazo JA, White TH, Vilella FJ, Guerrero SA. Survival of captive-reared Hispaniolan parrots released in Parque Nacional del Este, Dominican Republic. Condor. 2003;105(2):198–207. [Google Scholar]
- Dahlin CR, Wright TF. Duets in yellow-naped amazons: Variation in syntax, note composition and phonology at different levels of social organization. Ethology. 2009;115:857–871. [Google Scholar]
- Dahlin CR, Wright TF. Does syntax contribute to the function of duets in a parrot, Amazona auropalliata. Animal Cognition. 2012a;15(4):647–656. doi: 10.1007/s10071-012-0493-y. [DOI] [PubMed] [Google Scholar]
- Dahlin CR, Wright TF. Duet function in the yellow-naped amazon, Amazona auropalliata: Evidence from playbacks of duets and solos. Ethology. 2012b;118:95–105. doi: 10.1111/j.1439-0310.2011.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin CR, Young A, Halford D, Cordier B, Wright TF. The function of vocal convergence in female budgerigars Melopsittacus undulatus. Behavioral Ecology and Sociobiology. 2014;68:145–161. doi: 10.1007/s00265-013-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrickson SR, Snyder NFR. Potentials and limits of captive breeding in parrot conservation. In: Beissinger SR, Snyder NFR, editors. New World Parrots in Crisis. Smithsonian Institution Press; Washington: 1992. pp. 133–163. [Google Scholar]
- Diamond J, Bond AB. Kea. Bird of Paradox. University of California Press; Berkeley and Los Angeles: 1999. p. 230. [Google Scholar]
- Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsitticus undulatus): evidence for social factors in the learning of contact calls. J Comp Psychol. 1994;108(1):81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Fleischer RC, Rothstein SI. Known secondary contact and rapid gene flow among subspecies and dialects in the brown-headed cowbird. Evolution. 1988;42(6):1146–1158. doi: 10.1111/j.1558-5646.1988.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Ford JKB. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Canadian Journal of Zoology. 1991;69:1454–1483. [Google Scholar]
- Forshaw JM. Parrots of the World. Princeton University Press; Princeton: 2010. [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. 2. Cambridge University Press; Cambridge, UK: 2010. [Google Scholar]
- Garcia NC, Arrieta RS, Kopuchian C, Tubaro PL. Stability and change through time in the dialects of a Neotropical songbird, the Rufous-collared Sparrow. Emu. 2015;115(4):309–316. [Google Scholar]
- Guerra JE, Cruz-Nieto J, Ortiz-Maciel SG, Wright TF. Limited geographic variation in the contact calls of the endangered thick-billed parrot: implications for conservation strategies. Condor. 2008;110(4):639–647. doi: 10.1525/cond.2008.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford P, Lougheed SC. Variation in duration and frequency characteristics in the song of the rufous-collared sparrow, Zonotrichia capensis, with respect to habitat, trill dialect and body size. Condor. 1991;93:644–658. [Google Scholar]
- Handley HG, Nelson DA. Ecological and phylogenetic effects on song sharing in songbirds. Ethology. 2005;111(2):221–238. [Google Scholar]
- Hile AG, Plummer TK, Striedter GF. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim Behav. 2000;59:1209–1218. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Hile AG, Striedter GF. Call convergence within groups of female budgerigars (Melopsittacus undulatus) Ethology. 2000;106:1105–1114. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2016-2. 2016;2016 [Google Scholar]
- Keighley MV, Langmore NE, Zdenek CN, Heinsohn R. Geographic variation in the vocalizations of Australian palm cockatoos (Probosciger aterrimus) Bioacoustics. 2017;26(1):91–108. [Google Scholar]
- Lipshutz SE, Overcast IA, Hickerson MJ, Brumfield RT, Derryberry EP. Behavioural response to song and genetic divergence in two subspecies of white-crowned sparrows (Zonotrichia leucophrys) Molecular Ecology. 2017 doi: 10.1111/mec.14002. Epubv ahead of print. [DOI] [PubMed] [Google Scholar]
- Lougheed SC, Handford P. Cultural transmission, gene flow, population genetic structure, song dialects, Zonotrichia leucophrys oriantha. Evolution. 1992;46:1443–1456. doi: 10.1111/j.1558-5646.1992.tb01135.x. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton EA, MacDougall-Shackleton SA. Cultural and genetic evolution in mountain white-crowned sparrows: song dialects are associated with population structure. Evolution. 2001;55:2568–2575. doi: 10.1111/j.0014-3820.2001.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Marler P. Science and birdsong: the good old days. In: Marler P, Slabbekoorn H, editors. ‘Nature’s Music: the Science of Birdsong. Elsevier; San Diego: 2004. pp. 1–38. [Google Scholar]
- Marler P, Peters S. Species differences in auditory responsiveness in early vocal learning. In: Dooling RJ, Hulse S, editors. The Comparative Psychology of Audition: Perceiving Complex Sounds. Lawrence Erlbaum; Hillsdale: 1989. pp. 243–273. [Google Scholar]
- Marler P, Slabbekoorn H, editors. Nature’s Music: the Science of Birdsong. Elsevier; San Diego: 2004. [Google Scholar]
- Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1962a;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- Marler P, Tamura M. Song “dialects” in three populations of white-crowned sparrows. Condor. 1962b;64:368–377. [Google Scholar]
- Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- Monterrubio-Rico TC, Cruz-Nieto J, Enkerlin-Hoeflich E, Venegas-Holguin D, Tellez-Garcia L, Marin-Togo C. Gregarious nesting behavior of Thick-Billed Parrots, (Rhynchopsitta pachyrhyncha) in aspen stands. Wilson Journal of Ornithology. 2006;118(2):237–243. [Google Scholar]
- Navarro JL, Martella MB, Bucher EH. Effects of laying date, clutch size, and communal nest size on the reproductive success of Monk parakeets. Wilson Bulletin. 1995;107(4):742–746. [Google Scholar]
- Nottebohm F. The origins of vocal learning. American Naturalist. 1972;106:116–140. [Google Scholar]
- Payne RB. Population structure and social behavior: Models for testing the ecological significance of song dialects in birds. In: Alexander RD, Tinkle DW, editors. Natural Selection and Social Behavior: Recent research and new theory. Chiron Press; New York: 1981. pp. 108–120. [Google Scholar]
- Payne RB, Westneat DF. A genetic and behavioral analysis of mate choice and song neighborhoods in indigo buntings. Evolution. 1988;42(5):935–947. doi: 10.1111/j.1558-5646.1988.tb02512.x. [DOI] [PubMed] [Google Scholar]
- Podos J, Warren PS. Advances in the Study of Behavior. Vol. 37. Academic Press; 2007. The Evolution of Geographic Variation in Birdsong; pp. 403–458. [Google Scholar]
- Reynolds MBJ, Hayes WK, Wiley JW. Geograhic variation in the flight call of the Cuban parrot (Amazona leucocephala) and its taxonomic relevance. Journal of Caribbean Ornithology. 2010;23(1):4–18. [Google Scholar]
- Ribot RF, Buchanan KL, Endler JA, Joseph L, Bennett AT, Berg ML. Learned vocal variation is associated with abrupt cryptic genetic change in a parrot species complex. Plos One. 2012;7(12):e50484. doi: 10.1371/journal.pone.0050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot RFH, Berg ML, Buchanan KL, Bennett ATD. Is there variation in the response to contact call playbacks across the hybrid zone of the parrot Platycercus elegans? Journal of Avian Biology. 2013;44(4):399–407. [Google Scholar]
- Rowley I. Behavioural Ecology of the GalahEolophus roseicapillus.’. Surrey Beatty & Sons; Chipping Norton, Australia: 1990. p. 200. [Google Scholar]
- Rowley I, Chapman G. Cross-fostering, imprinting and learning in two sympatric species of cockatoo. Behaviour. 1986;96:1–16. [Google Scholar]
- Salinas-Melgoza A, Wright TF. Evidence for Vocal Learning and Limited Dispersal as Dual Mechanisms for Dialect Maintenance in a Parrot. Plos One. 2012;7(11):7. doi: 10.1371/journal.pone.0048667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz V, Grajal A. Successful reintroduction of captive-raised Yellow-Shouldered Amazon Parrots on Margarita Island, Venezuela. Conservation Biology. 1998;12(2):430–441. [Google Scholar]
- Saunders DA. Vocal repertoire and individual vocal recognition in the Short-Billed White-Tailed Black Cockatoo, Calyptorhynchus funereus latirostris Carnaby. Australian Wildlife Research. 1983;10:527–536. [Google Scholar]
- Schweizer M, OS, Hertwig ST. Macroevolutionary patterns in the diversification of parrots: effects of climate change, geological events and key innovations. Journal of Biogeography. 2011;38:2176–2194. [Google Scholar]
- Sewall KB, Young AM, Wright TF. Social calls provide novel insights into the evolution of vocal learning. Animal Behaviour. 2016;120:163–172. doi: 10.1016/j.anbehav.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H. Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H, editors. ‘Nature’s Music. Elsevier Academic Press; Amsterdam: 2004. pp. 178–205. [Google Scholar]
- Slabbekoorn H, Smith TB. Bird song, speciation and ecology. Philos Trans R Soc Lond B Biol Sci. 2002;357(1420):493–503. doi: 10.1098/rstb.2001.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder NF, Enkerlin-Hoeflich EC, Cruz-Nieto MA. The Birds of North America. Cornell Lab of Ornithology; Ithaca: 1999. Thick-billed Parrot (Rhynchopsitta pachyrhyncha) [Google Scholar]
- Snyder NFR, Derrickson SR, Beissinger SR, Wiley JW, Smith TB, Toone WD, Miller B. Limitations of captive breeding in endangered species recovery. Conservation Biology. 1996;10(2):338–348. [Google Scholar]
- Snyder NFR, McGowan P, Gilardi J, Grajal A. Parrots: Status Survey and Conservation Action Plan 2000–2004. IUCN; Gland, Switzerland and Cambridge, UK: 2000. [Google Scholar]
- Soha JA, Nelson DA, Parker PG. Genetic analysis of song dialect populations in Puget Sound white-crowned sparrows. Behavavioral Ecology. 2004;15:636–646. [Google Scholar]
- Striedter GF, Freibott L, JHile AG, Burley NT. For whom the male calls: an effect of audience on contact call rate and repertoire in budgerigars, Melopsittacus undulatus. Animal Behaviour. 2003;65(5):875–882. [Google Scholar]
- Toft CA, Wright TF. Parrots of the Wild: A Natural History of the World’s Most Captivating Birds. University of California Press; Berkeley, CA: 2015. [Google Scholar]
- Vehrencamp SL, Ritter AF, Keever M, Bradbury JW. Responses to playback of local vs. distant contact calls in the orange-fronted conure, Aratinga canicularis. Ethology. 2003;109(1):37–54. [Google Scholar]
- Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Animal Behaviour. 2005;70(Part 1):111–118. [Google Scholar]
- White TH, Jr, Collar NJ, Moorhouse RJ, Sanz V, Stolen ED, Brightsmith DJ. Psittacine reintroductions: Common denominators of success. Biological Conservation. 2012;148(1):106–115. [Google Scholar]
- Wiley RH. Song groups in a singing assembly of Little Hermits. Condor. 1971;73:28–35. [Google Scholar]
- Wright TF. Regional dialects in the contact call of a parrot. Proceedings of the Royal Society of London B. 1996;263:867–872. [Google Scholar]
- Wright TF, Dahlin CR, Salinas-Melgoza A. Stability and change in vocal dialects of the yellow-naped amazon. Animal Behaviour. 2008a;76:1017–1027. doi: 10.1016/j.anbehav.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TF, Dorin M. Pair duets in the yellow-naped amazon (Psittaciformes: Amazona auropalliata): reponses to playbacks of different dialects. Ethology. 2001;107:111–124. [Google Scholar]
- Wright TF, Rodriguez AM, Fleischer RC. Vocal dialect, sex-biased dispersal and microsatellite population structure in the parrot Amazona auropalliata. Molecular Ecology. 2005;14:1197–1205. doi: 10.1111/j.1365-294X.2005.02466.x. [DOI] [PubMed] [Google Scholar]
- Wright TF, Schirtzinger EE, Matsumoto T, Eberhard JR, Graves GR, Sanchez JJ, Capelli S, Mueller H, Scharpegge J, Chambers GK, Fleischer RC. A multilocus molecular phylogeny of the parrots (Psittaciformes): Support for a Gondwanan origin during the Cretaceous. Molecular Biology and Evolution. 2008b;25(10):2141–2156. doi: 10.1093/molbev/msn160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TF, Wilkinson GS. Population genetic structure and vocal dialects in an amazon parrot. Proceedings of the Royal Society of London B. 2001;268:609–616. doi: 10.1098/rspb.2000.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink RM, Barrowclough GF. Allozymes and song dialects: a reassessment. Evolution. 1984;38:444–48. doi: 10.1111/j.1558-5646.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.