ABSTRACT
Epidermal growth factor receptor (EGFR) plays a crucial role in hepatocyte proliferation. Its role in acetaminophen (APAP)-mediated hepatotoxicity and subsequent liver regeneration is completely unknown. Role of EGFR after APAP-overdose in mice was studied using pharmacological inhibition strategy. Rapid, sustained and dose-dependent activation of EGFR was noted after APAP-treatment in mice, which was triggered by glutathione depletion. EGFR-activation was also observed in primary human hepatocytes after APAP-treatment, preceding elevation of toxicity markers. Treatment of mice with an EGFR-inhibitor (EGFRi), Canertinib, 1h post-APAP resulted in robust inhibition of EGFR-activation and a striking reduction in APAP-induced liver injury. Metabolic activation of APAP, formation of APAP-protein adducts, APAP-mediated JNK-activation and its mitochondrial translocation were not altered by EGFRi. Interestingly, EGFR rapidly translocated to mitochondria after APAP-treatment. EGFRi-treatment abolished mitochondrial EGFR activity, prevented APAP-mediated mitochondrial dysfunction/oxidative-stress and release of endonucleases from mitochondria, which are responsible for DNA-damage/necrosis. Treatment with N-acetylcysteine (NAC), 4h post-APAP in mice did not show any protection but treatment of EGFRi in combination with NAC showed decrease in liver injury. Finally, delayed treatment with EGFRi, 12-h post-APAP, did not alter peak injury but caused impairment of liver regeneration resulting in sustained injury and decreased survival after APAP overdose in mice. Impairment of regeneration was due to inhibition of cyclinD1 induction and cell cycle arrest. Our study has revealed a new dual role of EGFR both in initiation of APAP-injury and in stimulation of subsequent compensatory regeneration after APAP-overdose.
Keywords: Hepatotoxicity, mitochondrial dysfunction, N-acetylcysteine, Cyclin D1, hepatocyte proliferation
Epidermal growth factor receptor (EGFR) is a prototypical tyrosine kinase receptor of the ErbB family, which is highly expressed in liver and known to be involved in hepatocytes proliferation, liver regeneration and hepatocellular carcinogenesis (Carver et al., 2002; Michalopoulos and DeFrances, 1997; Michalopoulos and Khan, 2005). EGFR can be activated by variety of extracellular ligands, including EGF, transforming growth factor alpha (TGF-α), heparin-binding EGF (HB-EGF) and amphiregulin (Normanno et al., 2006). Ligand binding leads to dimerization of EGFR and auto-phosphorylation of tyrosine residues in its cytoplasmic domain, which then act as a hub for numerous cell signaling pathways. Apart from hepatocyte growth factor (HGF), EGFR ligands are the only known direct mitogens which can cause hepatocyte proliferation in serum free medium, emphasizing an important role of EGFR in hepatocyte proliferation (Michalopoulos, 2007). In vivo administration of EGFR ligands such as EGF and TGF-α also lead to hepatocyte proliferation along with increased liver size (Bucher et al., 1977). EGFR is activated very rapidly after two-third partial hepatectomy (PH), and is known to play important role in promoting timely liver regeneration (Fausto et al., 2012; Michalopoulos, 2007; Michalopoulos and Khan, 2005). Genetic deletion of EGFR in mouse liver or shRNA-mediated downregulation of EGFR in rats resulted in delayed liver regeneration (Natarajan et al., 2007; Paranjpe et al., 2010). Further, mice deficient in EGFR ligands, amphiregulin and HB-EGF, displayed deficient liver regeneration and overexpression of HB-EGF increased liver regeneration (Fausto et al., 2012; Michalopoulos, 2007; Michalopoulos and Khan, 2005).
Apart from the well-known role of EGFR in cell proliferation, its role in cell death signaling has also been reported, but remained largely ignored (Arany, 2008; Reinehr and Haussinger, 2009). Other than ligand-dependent activation of EGFR, ligand-independent activation of EGFR by hydrophobic bile acids, CD95 ligand and hyperosmolarity has been reported in hepatocytes as well as hepatic stellate cells (HSCs), which was found to be involved in mediating cell death signaling (Reinehr et al., 2003a,b, 2004, 2005a,b; Reinehr and Haussinger, 2009, 2012; Sommerfeld et al., 2009). An unexpected role of EGFR activation in cell death has also been reported in kidney (Arany, 2008). Nephrotoxic insults such as cisplatin and cyclosporine A treatment were shown to activate EGFR in proximal tubular cells, which was involved in toxicity rather than survival (Arany et al., 2004; Sarro et al., 2008).
Acetaminophen (APAP) is the most commonly used analgesic worldwide and its overdose is the major cause of acute liver failure (ALF) in the western world, accounting for almost 50% of all the ALF cases. Current treatment options after APAP overdose are extremely limited with liver transplantation as the final resort (Bernal et al., 2015). APAP toxicity is initiated by metabolism to a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which then binds to cellular proteins (particularly mitochondrial proteins). This mitochondrial distress is exacerbated by a plethora of intracellular signaling events resulting in massive mitochondrial oxidative damage/dysfunction and liver cell necrosis (Jaeschke et al., 2012). Extensive research in the last few decades has revealed that APAP hepatotoxicity involves highly sophisticated pathways, but the exact intracellular signaling events are still not completely known (Jaeschke et al., 2012). Liver toxicity after APAP overdose is also followed by compensatory liver regeneration, which is a critical determinant of final recovery (Bhushan et al., 2014, 2016). Identifying targets to stimulate liver regeneration holds great potential to develop novel therapeutic strategies (Bhushan et al., 2013, 2014; Donahower et al., 2010; Hu and Colletti, 2008). Unfortunately, mechanisms of liver regeneration are mostly studied in PH model, which is considerably different from the APAP hepatotoxicity model.
The role of EGFR in APAP-mediated acute liver injury and subsequent liver regeneration is completely unknown, which was investigated in the current study. We have recently established an incremental dose model in mice to study mechanisms of liver regeneration after APAP-induced ALF (Bhushan et al., 2014). Paradoxically, dose-dependent activation of EGFR was observed after APAP overdose, such that activation of EGFR was greater at higher doses of APAP, where liver regeneration was inhibited. Here we report that EGFR is activated rapidly and translocated to mitochondria after toxic doses of APAP in mice and primary human hepatocytes (PHHs). Whereas, early inhibition of EGFR activation by pharmacological intervention in mice remarkably attenuated APAP hepatotoxicity, delayed inhibition of EGFR activation lead to impaired compensatory liver regeneration, suggesting a dual role of EGFR in both injury initiation and subsequent liver regeneration after APAP overdose.
MATERIALS AND METHODS
Animals, treatments, and tissue harvesting
Eight week old, C57BL/6J mice were purchased from Jackson Laboratories and used in all the studies. All studies were approved by The Institutional Animal Care and Use Committee at the University of Kansas Medical Center. All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities at the University of Kansas Medical Center under a standard 12-h light/dark cycle with access to chow and water ad libitum. For all APAP experiments, mice were fasted overnight and APAP (Sigma, St Louis, Missouri) was dissolved in warm 0.9% saline and administered intraperitoneally (i.p.) next morning. For study with incremental doses of APAP, mice (n = 3–8) were treated with either 300 or 600 mg/kg APAP, and sacrificed at 0, 0.25, 1, 3, 6, 12, 24, 72, and 96 h following APAP treatment. For studies with EGFR inhibitor (EGFRi), canertinib dihydrochloride salt (80 mg/kg, i.p.) (LC Laboratories, Woburn, Massachusetts) was dissolved in warm phosphate-buffered saline (PBS). EGFRi or PBS (vehicle) was administered (1) 1 or (2) 12 h after 300 mg/kg APAP and mice (n = 3–12) were sacrificed at (1) 3, 6, 12, and 24 h or (2) 24 and 48 h after treatment with APAP, respectively (represented by scheme shown in Supplementary Figure S2A and Figure 7A). For mitochondrial respiration analysis, EGFRi or PBS (vehicle) was administered 2 h before or 1 h after 300 mg/kg APAP and mice (n = 3) were sacrificed at 1.5 or 3 h after treatment with APAP, respectively. For N-acetylcysteine (NAC) experiments, NAC (500 mg/kg, dissolved in PBS), EGFRi (80 mg/kg, i.p.) or PBS (vehicle) was administered 1.5 h after APAP (300 mg/kg, i.p.) and mice (n = 3) were sacrificed 6 h after treatment with APAP. Additionally, NAC (500 mg/kg), EGFRi (80 mg/kg, i.p.), NAC + EGFRi or PBS (vehicle) was administered 4 h after APAP (300 mg/kg, i.p.) and mice (n = 5) were sacrificed 24 h after treatment with APAP. For glutathione depletion study, phorone (200 mg/kg, i.p., dissolved in corn oil) was administrated in mice (n = 5) without fasting and mice were sacrificed 2 h later. Euthanasia was performed by cervical dislocation under isoflurane anesthesia, and blood and livers were collected.
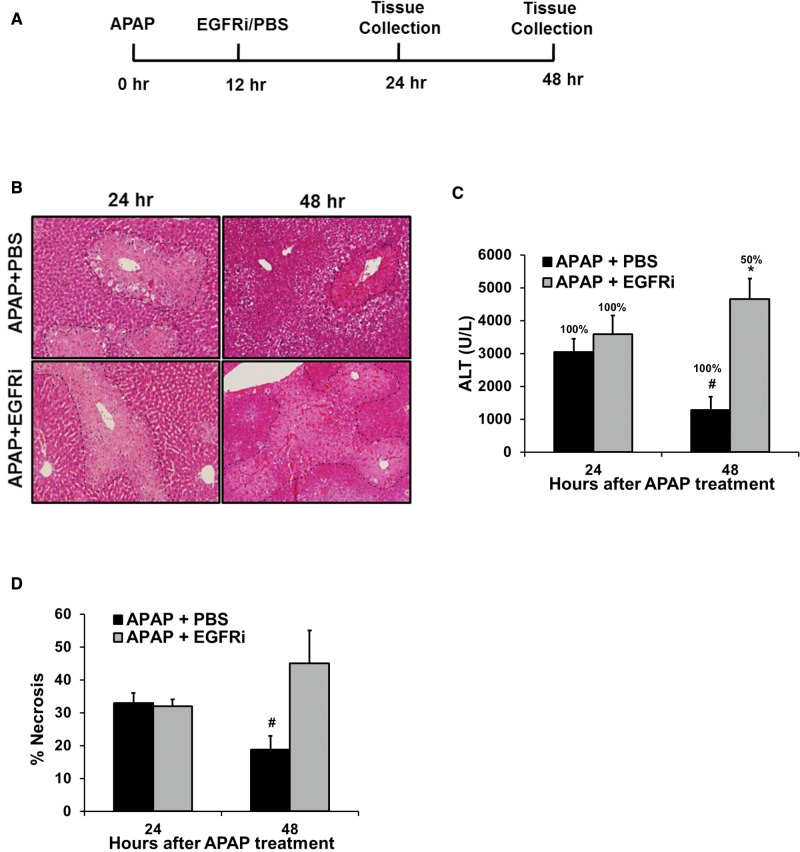
FIG. 7.
Increased progression of injury, impaired recovery and decreased survival after delayed (12 hr post-APAP) treatment with EGFRi. (A) Schematic showing experimental design. (B) Representative photomicrographs of H&E stained liver sections with necrotic area outlined, (C) serum ALT levels with percentage survival specified over bars and (D) percentage necrosis area based on H&E stained liver sections of mice treated with 300 mg/kg APAP followed by treatment with canertinib (80 mg/kg) or PBS, 12 hr post-APAP. All samples were collected and survival was recorded at 24 and 48 hr after APAP treatment (n=5-6). * indicate significant difference between groups at p<0.05. # indicates significant difference w.r.t. 24 hr time point within same treatment group.
PHHs experiments
PHHs were isolated as described previously (Xie et al., 2014), without any modification, after informed consent of patient and study approval by University of Kansas Medical Center Institutional Review Board. All experiments were done in quadruplets with hepatocytes obtained from 4 liver donors. Cells were treated with 10 mM APAP dissolved in serum free Hepatocyte Maintenance Medium (Lonza, Walkersvill, Maryland), supplemented as described previously in (Xie et al. 2014). Cells were harvested at 0, 3, 6, 15, 24, 36, and 48 h after treatment with APAP and used for further downstream analysis as described previously in (Xie et al. 2014).
Respiration analysis in mitochondria isolated from mice liver
Mitochondria were isolated from freshly harvested livers of mice by differential centrifugation method. All the steps of the preparation were performed on ice. Briefly, the liver was rinsed 2× with mitochondria isolation buffer (MSHE + BSA: 70 mM sucrose, 210 mM mannitol, 5 mM HEPES, 1 mM EGTA and 0.5% (w/v) fatty acid-free BSA; pH 7.2) and minced using dounce homogenizer in approximately 10 volumes of MSHE + BSA. Homogenate was centrifuged twice at 1000 g for 10 min at 4 °C. The supernatant was passed through 2 layers of cheesecloth to remove fat/lipid layer. The supernatant was centrifuged at 8000 g for 10 min at 4 °C. The pellet was washed once with MSHE + BSA and resuspended in a minimal volume of MSHE + BSA. Total protein (mg/ml) was determined using BCA protein assay.
All mitochondrial respiration assays were performed using an XF24–3 Extracellular Flux Analyzer by Seahorse Bioscience (North Billerica, Massachusetts). The XF24 sensor cartridge was hydrated with 1 ml calibration buffer per well overnight at 37 °C. Isolated liver mitochondria were first diluted ten times in cold Mitochondrial assay solution (1X MAS: 70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA and 0.2% (w/v) fatty acid-free BSA, pH 7.2 at 37 °C) with substrate (10 mM succinate + 2 µM rotenone), then subsequently diluted to 2 mg/ml and 50 µl of this mitochondrial suspension was plated to each well (except for background correction wells) while the plate was on ice. The plate was transferred to a centrifuge equipped with a swinging bucket and spun at 2000 g for 20 minutes at 4 °C. After centrifugation, 450 µl of 1X MAS + substrate (at room temperature) was added to each well. The mitochondria were viewed briefly under a microscope to ensure consistent adherence to the well, then placed at 37 °C for 10 min to allow the plate to warm. The sensor cartridge was loaded with port A, 40 mM ADP (4 mM final); port B, 25 µg/ml oligomycin (2.5 µg/ml final); port C, 40 µM FCCP (4 µM final); and port D, 40 µM antimycin A (4 µM final) to measure the bioenergetic profile. The plate was then transferred to the XF24 instrument and the respiration was sequentially measured in a coupled state with substrate present (basal respiration), followed by State 3 (phosphorylating respiration, in the presence of ADP and substrate), State 4o (Leak state induced with the addition of oligomycin - inhibitor of ATP synthase), and then maximal uncoupler (FCCP)-stimulated respiration (State 3u). At the end, antimycin A (complex III inhibitor) is added to inhibit mitochondrial respiration completely.
Histological analysis and serum ALT measurement
Liver sections were prepared for histological analysis as described previously Sun et al. 2016). Paraffin-embedded liver sections (4 µm thick) were used for H&E staining and scored for percentage necrotic area. Serum alanine aminotransferase (ALT) was measured using the Infinity ALT kit (ThermoFisher Scientific, Pittsburgh, PA), according to the manufacturer’s protocol.
APAP-protein adducts measurement
APAP-protein adducts levels were measured in liver tissues using HPLC coupled with electrochemical detector as described previously (McGill et al., 2012).
Glutathione (GSH and GSSG) analysis
Total hepatic GSH and GSSG levels were measured in liver homogenates by kinetic assay with a modified method of Tietze and as described previously (Xie et al., 2015).
Statistical analysis
Data presented in the form of bar/line graphs show mean ± SEM. Significant difference between 2 groups was determined using Student’s t-test and between 3 or more groups using 1-way analysis of variance (ANOVA) with Tukey’s post-hoc test. Difference between groups was considered statistically significant at P < .05.
Other details on materials and methods are provided in the Supplementary Materials.
RESULTS
Rapid and Sustained Activation of EGFR in Mice and PHHs after APAP Overdose
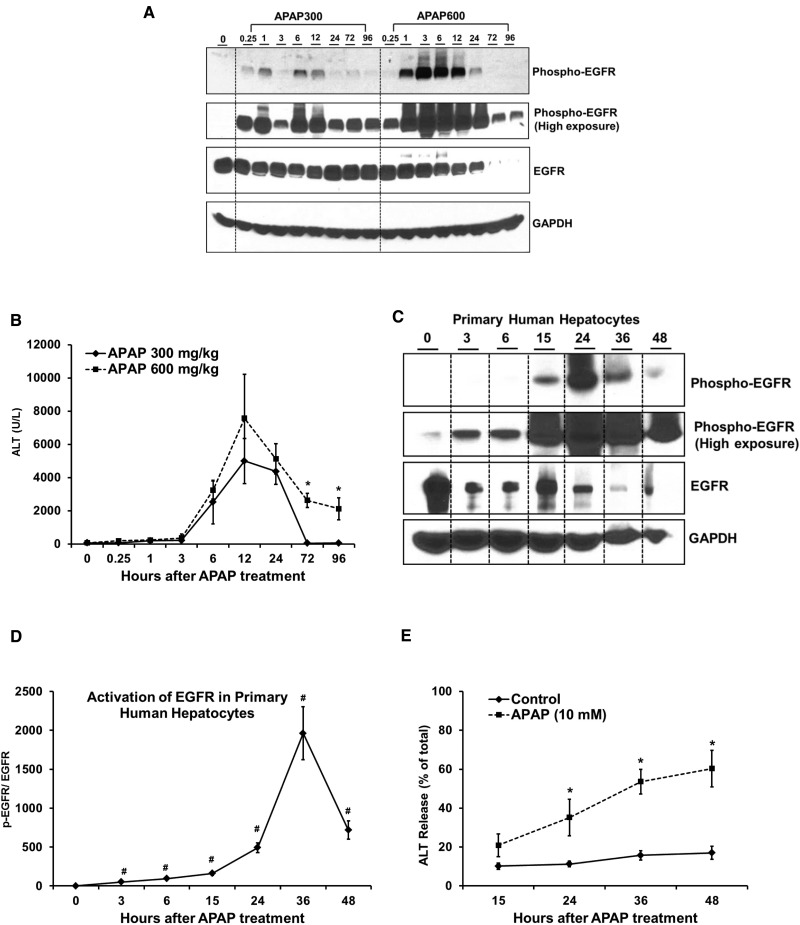
Our previous work established an incremental dose model to study mechanisms of liver regeneration after APAP-induced ALF (Bhushan et al., 2014). Two separate groups of mice were administered either a “regenerating” (300 mg/kg) or a “non-regenerating” (600 mg/kg) dose of APAP, respectively. The lower dose caused extensive liver injury, followed by robust compensatory liver regeneration leading to recovery. In contrast, at higher dose, liver regeneration was inhibited leading to sustained injury and decreased survival (Bhushan et al., 2014). EGFR signaling is considered crucial for hepatocyte proliferation. Paradoxically, activation of EGFR was greater at the higher dose of APAP, where liver regeneration was inhibited (Bhushan et al., 2014). To further follow up, in the current study, activation of EGFR was examined after incremental doses of APAP (300 and 600 mg/kg) in mice over an extended time-course of 15 min to 96 h (Figure 1A). A striking increase in phosphorylation of EGFR at the Tyr-1068 residue (ie, activation) was observed within 15 min after treatment with 300 mg/kg APAP and was sustained up-to 96 h, with peak activation at around 6 h. Overall activation of EGFR was higher after treatment with 600 mg/kg APAP up to 24 h, with peak activation at 3 h, but both EGFR expression and phosphorylation were downregulated from 24 to 96 h at the higher dose. On the contrary, EGFR activation remained sustained at the lower dose and was greater compared with the higher dose after 24 h (Figure 1A). Increase in liver injury, as indicated by elevation of serum ALT levels, was observed beyond 3 h after treatment with both doses of APAP, suggesting activation of EGFR preceded liver injury in mice (Figure 1B). As reported previously (Bhushan et al., 2014), injury was sustained at 72 and 96 h in the higher dose group, but mice treated with lower dose recovered at these time points (Figure 1B).
FIG. 1.
Rapid and sustained activation of EGFR in mice and primary human hepatocytes after APAP-treatment. (A) Western blot analysis of phospho-EGFR (Tyr1068) and EGFR in total cell lysate and (B) ALT levels in serum, at various time points after administration of 300 mg/kg and 600 mg/kg APAP in mice (n=3-8). (C) Western blot analysis of phospho-EGFR and EGFR in total cell lysate and (E) ALT release in medium (represented as percentage of total ALT levels), at various time points after treatment of primary human hepatocytes (PHH) with 10 mM APAP. (D) Densitometric analysis showing activation of EGFR in PHH with data representing mean ± SEM of independent western blot analysis from 4 liver donors. * indicate significant difference between groups at p<0.05. # indicates significant difference w.r.t. 0 hr time point wherever indicated.
We also examined EGFR activation in PHHs following APAP treatment. Similar to mice, significant EGFR activation was observed in PHH starting as early as 3 h (about 20-fold activation at this time point) and activation remained sustained up to 48 h (Figs. 1C and D). Significant toxicity, as indicated by release of ALT in the medium, was observed starting from 24 h after treatment with APAP, compared with control group, as reported previously (Figure 1E). Previous studies have also shown that earliest signs of mitochondrial damage ie, loss of mitochondrial membrane potential occurs between 6 and 15 after APAP treatment (Xie et al., 2014). These data indicate that EGFR activation occurs very rapidly after a toxic dose of APAP, both in mice and PHH, preceding development of injury.
Dramatic Attenuation of APAP Hepatotoxicity by Early Treatment with EGFRi (1 h Post-APAP)
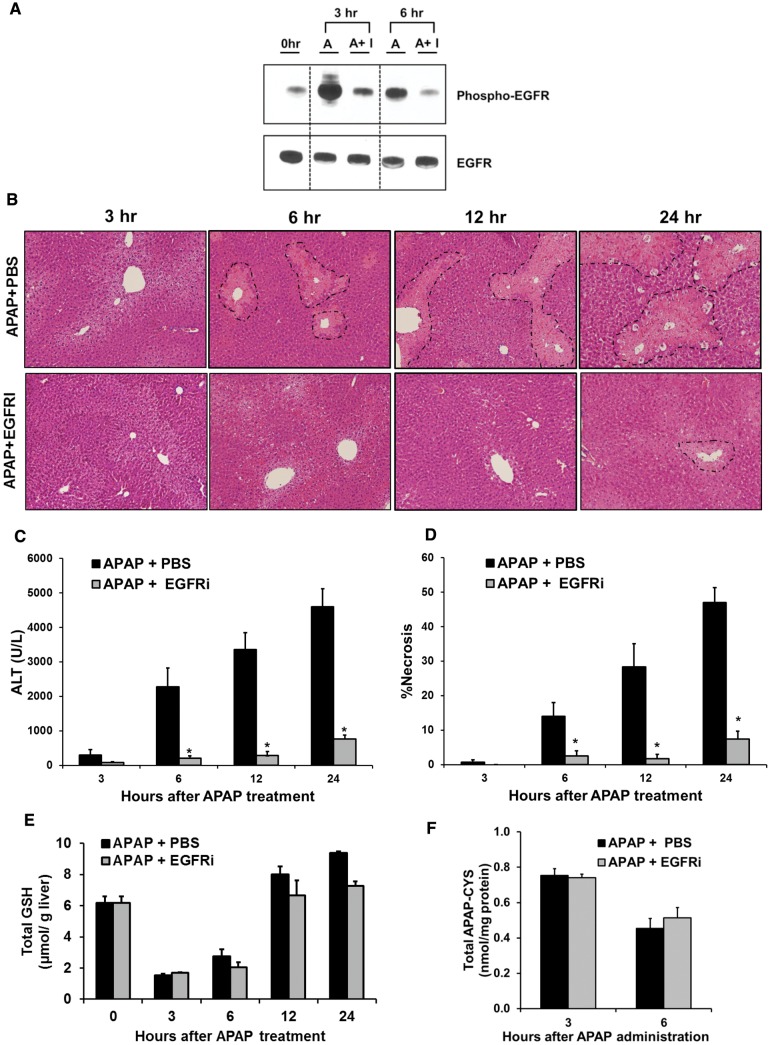
Next, the role of EGFR activation in APAP-induced liver injury was investigated. In a preliminary study, mice were pretreated with the highly potent and selective pan-EGFRi (canertinib, 80 mg/kg) (Slichenmyer et al., 2001), 24 and 4 h before APAP, in order to prevent early EGFR activation after APAP treatment. Pre-treatment with EGFRi resulted in robust decrease in APAP-mediated EGFR activation and dramatic decrease in injury at 6 h after APAP (Supplementary Figure S1A–D). Since pretreatment with an inhibitor is not a clinically relevant treatment approach, subsequently, a post-treatment strategy was utilized. Mice were treated with EGFRi 1 h after administration of APAP, and an extended time-course of 3–24 h after APAP treatment was examined (Supplementary Figure S2A). As expected, APAP treatment caused significant activation of EGFR and treatment with EGFRi decreased EGFR activation to basal levels (Figure 2A). APAP treatment caused significant liver necrosis in the centrilobular region and elevation of serum ALT levels, with injury progressing with time from 3 to 24 h (Figs. 2B–D). Treatment with EGFRi caused a dramatic reduction of the area of necrosis, with almost no observable necrosis up to 12 h and very small zones of necrosis at 24 h (Figs. 2B and D). A similar trend was also observed for serum ALT levels at all the investigated time points (Figure 2C). This dramatic effect with almost complete abolishment of liver injury after pharmacological inhibition of EGFR activation suggested crucial role of EGFR in APAP-induced hepatotoxicity.
FIG. 2.
Early treatment with EGFRi (1-h post-APAP) remarkably attenuated APAP-induced hepatotoxicity without altering APAP bioactivation and APAP-protein adducts formation. A, Western blot analysis of phospho-EGFR and EGFR in liver lysate, B, representative photomicrographs of H&E stained liver sections with necrotic area outlined, C, serum ALT levels, D, percentage necrosis area based on H&E stained liver sections, E, total glutathione levels in liver extract, and F, APAP-protein adducts levels in liver as measured by HPLC-ECD method. For all experiments mice (n = 3–12) were treated with 300 mg/kg APAP followed by canertinib (80 mg/kg) or PBS, 1-h post-APAP. All samples were collected at various time points up-to 24-h after APAP treatment. * indicate significant difference between groups at P < .05.
EGFR Inhibition Does Not Alter APAP-Mediated Glutathione Depletion and APAP-Protein Binding
Next, we investigated several major steps involved in the development of APAP toxicity to determine the mechanisms of EGFRi-mediated reduction in APAP toxicity. The critical initial step is rapid depletion of glutathione due to conjugation with NAPQI, the reactive metabolite of APAP. Excess NAPQI then binds to cellular proteins (especially mitochondrial proteins) leading to formation of APAP-protein adducts (Jaeschke et al., 2012). APAP caused significant depletion of glutathione when measured at 3 h after APAP administration, which was not altered by treatment with EGFRi (1-h post-APAP) (Figure 2E). Similarly, recovery of glutathione levels, which can regulate progression of injury at later time points (6–24 h), was also not increased by EGFRi treatment (Figure 2E). Finally, no significant difference was observed in APAP-protein adduct levels by treatment with EGFRi measured by both western blot analysis (Supplementary Figure S2B) and HPLC based method (Figure 2F). These data indicate that EGFRi treatment does not alter early injury initiation events of APAP toxicity, namely, metabolic activation of APAP to reactive metabolite NAPQI, subsequent glutathione depletion and APAP-protein adducts formation.
JNK Activation, Its Mitochondrial Translocation and Signaling through Other Protein Kinases Not Altered by EGFR Inhibition
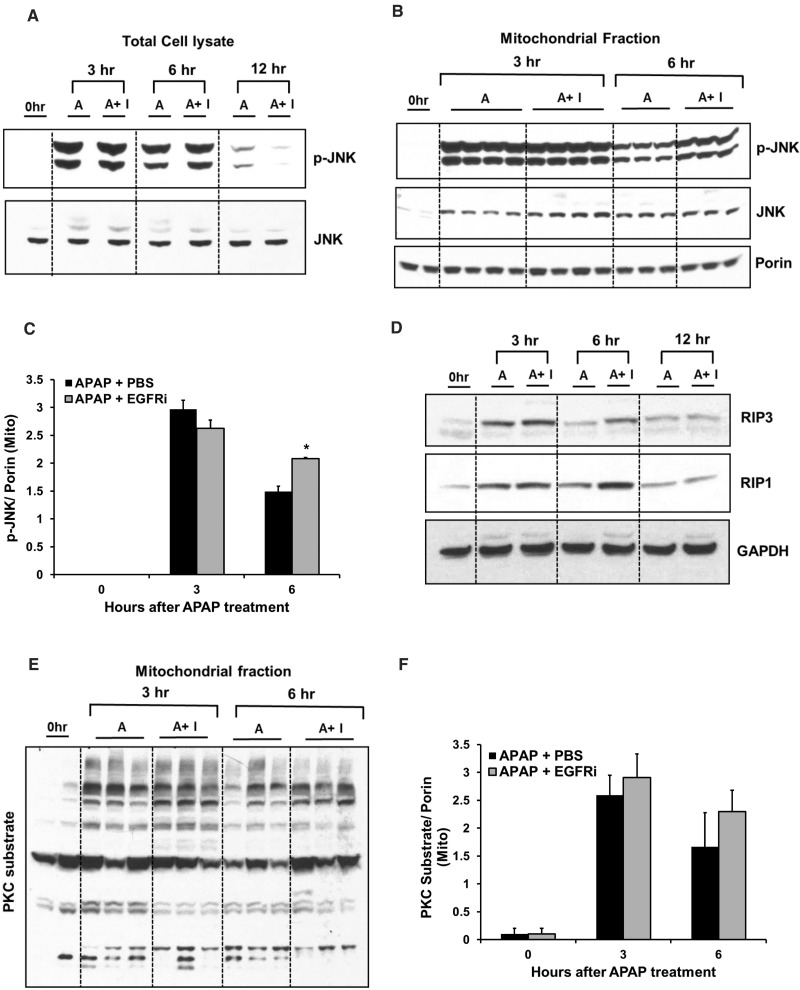
APAP-protein adducts formation is very crucial for initiation of APAP toxicity, but not sufficient to cause hepatocyte death and necrosis (Jaeschke et al., 2012). JNK is considered one of the important mediators of APAP toxicity and can be potentially activated by EGFR signaling. Phosphorylation mediated activation of JNK and its mitochondrial translocation is known to exacerbate mitochondrial oxidative stress during APAP toxicity (Jaeschke et al., 2012). As expected, APAP treatment caused activation (phosphorylation) of JNK at 3 and 6 h followed by rapid decrease in activation at 12 h, when examined using total cell lysate. Interestingly, EGFRi treatment did not alter APAP-mediated JNK activation (Figure 3A). Further, a similar increase in mitochondrial translocation of total and activated JNK was observed at 3 and 6 h after APAP treatment, which was not decreased by treatment with EGFRi. In fact, mitochondrial levels of p-JNK were significantly higher in EGFRi treated group compared with vehicle-treated control at 6 h after APAP (Figs. 3B and C). Induction of RIP1 and RIP3 kinases and their role in activation of JNK during APAP-induced liver injury has been reported recently (Dara et al., 2015; Ramachandran et al., 2013). As reported previously, induction of both RIP1 and RIP3 was observed after APAP-overdose; however, induction was not decreased by EGFRi treatment and was, in fact, higher at 6-h post-APAP administration in EGFRi-treated group compared with vehicle-treated group (Figure 3D and Supplementary Figs. S3A and B). Recently, protein kinase C (PKC) was reported to phosphorylate mitochondrial proteins after APAP overdose and regulate APAP-induced liver injury in JNK-dependent and independent manner (Saberi et al., 2014). As PKC is one of the downstream effectors of EGFR, we investigated if EGFR inhibition can alter APAP-mediated PKC activation. Consistent with the previous report, PKC activation as measured by Western blotting of phosphorylated PKC substrates in mitochondria was evident at 3 and 6 h after APAP administration and was not altered by EGFRi treatment (Figs. 3E and F). Finally, activation of several other protein kinases (p-38, ERK1/2, AKT, and STAT3) were examined (which were, dose-dependently, activated after APAP overdose in our previous study (Bhushan et al., 2014)) and were not remarkably affected by EGFRi treatment after APAP administration (Supplementary Figure S3C).
FIG. 3.
JNK activation, its mitochondrial translocation and signaling through other protein kinases not altered by EGFRi. Western blot analysis of phospho-JNK and JNK in (A) total cell lysate and (B) mitochondrial fraction with densitometric analysis of mitochondrial activated JNK (p-JNK) shown in (C). D, Western blot analysis of RIP3 and RIP1 in total cell lysate. E, Western blot analysis showing PKC activation (studied using antibody against phosphorylated PKC-substrates in mitochondria) with its densitometric analysis shown in (F). All analysis were done on liver samples collected at various time points after treatment with 300 mg/kg APAP + PBS or APAP + canertinib (80 mg/kg, 1-h post-APAP) (n = 3–4). *indicate significant difference between groups at P < .05.
EGFRi Restored APAP-Mediated Early Mitochondrial Dysfunction and Inhibited Mitochondrial Localization of EGFR
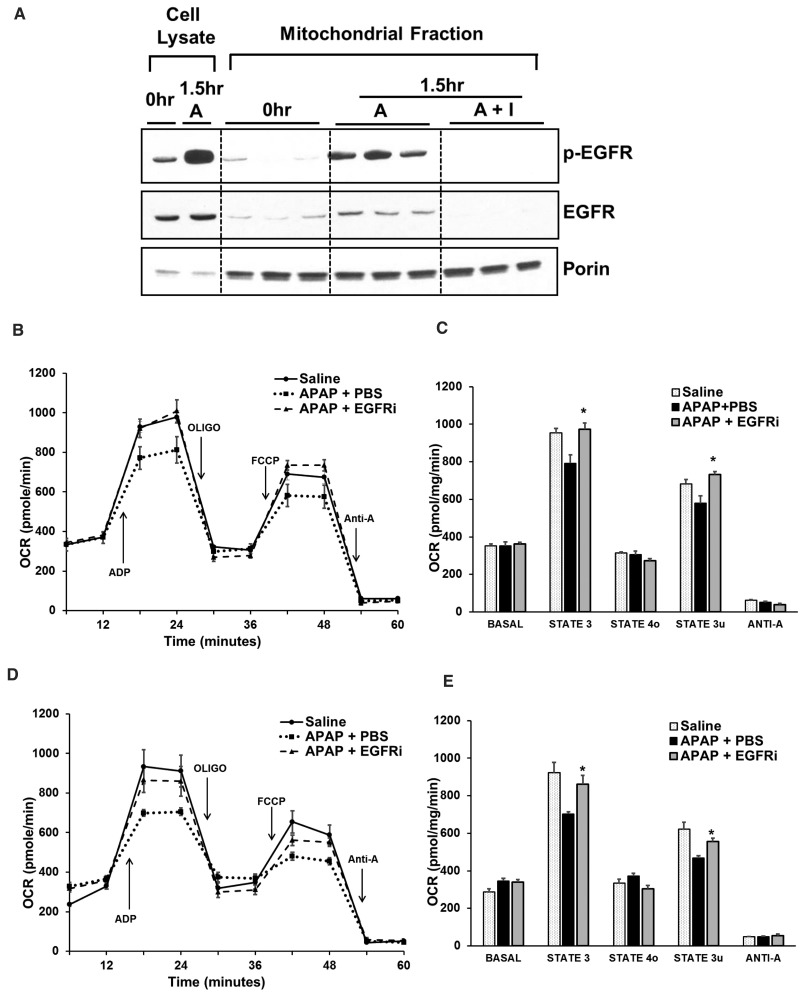
Previous studies have shown that EGFR can translocate to mitochondria in an endocytosis dependent manner. EGFR-mediated phosphorylation was reported to cause alteration of mitochondrial electron transport chain and subsequently mitochondrial function (Boerner et al., 2004; Demory et al., 2009). Interestingly, in our study, EGFR levels increased notably in mitochondria at 1.5 h after treatment with APAP in mice, suggesting APAP-mediated translocation of EGFR to mitochondria (Figure 4A). Similar mitochondrial translocation was also observed for activated EGFR after APAP treatment. Further, mitochondrial translocation of both EGFR and activated EGFR were completely abolished by pretreatment with EGFRi (2 h prior to APAP) (Figure 4A). Similarly in PHH, notable translocation of EGFR into mitochondria was observed at 6 and 15 h after APAP treatment, along with an increase in mitochondrial levels of activated EGFR (Supplementary Figure S4A).
FIG. 4.
APAP caused rapid mitochondrial translocation of EGFR and mitochondrial dysfunction, which was inhibited by EGFRi. A, Western blot analysis of phospho-EGFR and EGFR in mitochondrial fraction at 1.5 h after administration of APAP (300 mg/kg) in mice pretreated (2-h prior to APAP) with canertinib (80 mg/kg). B-E, Oxygen consumption rate analysis in freshly isolated mitochondria from mice (n = 3) treated with APAP (300 mg/kg) + PBS, APAP (300 mg/kg) + canertinib (80 mg/kg) or saline (control), using Seahorse extracellular flux analyzer. B and C, Canertinib was administered 2hr before APAP and analysis was done 1.5 h after APAP treatment. D and E, Canertinib was administered 1-h post-APAP and analysis was done 3 h after APAP treatment. Oxidative phosphorylation was manipulated with injection of ADP, oligomycin (oligo), FCCP, and antimycin A (anti-A). Respiration was sequentially measured in a coupled state with substrate present (basal respiration), followed by State 3 (phosphorylating respiration, in the presence of ADP and substrate), State 4o (leak state induced with the addition of oligomycin - inhibitor of ATP synthase), and then maximal uncoupler (FCCP)-stimulated respiration (State 3u). At the end, antimycin A (complex III inhibitor) was added to inhibit mitochondrial respiration completely * indicate significant difference w.r.t. to APAP + PBS group at P < .05.
Next, we investigated the effect of EGFR inhibition on alteration of APAP-mediated mitochondrial dysfunction using Seahorse extracellular flux analyzer. Oxygen consumption parameters were analyzed in freshly isolated mitochondria 1.5 h after treatment with APAP in mice with or without EGFRi (2 h prior to APAP). Significant reduction in ADP-stimulated phosphorylating mitochondrial respiration and FCCP (uncoupler)-induced maximal mitochondrial respiration was observed within 1.5 h after APAP administration. Interestingly, EGFRi treatment completely restored both these parameters (Figs. 4B and C). Similar restoration of mitochondrial functional parameters was observed at 3 h after APAP treatment in mice when EGFRi was administered 1-h post-APAP (Figs. 4D and E). These data indicate that mitochondrial translocation of EGFR and its activity in mitochondria may be involved in early mitochondrial dysfunction after APAP overdose preceding overt necrosis, which was prevented by treatment with the EGFRi.
Decreased Oxidative Stress, Mitochondrial Protein Nitration and Release of Endonucleases from Mitochondria by EGFRi
Initial mitochondrial dysfunction leads to a vicious circle of mitochondrial oxidative/nitrosative-stress and subsequent mitochondrial damage during APAP-induced liver necrosis (Jaeschke et al., 2012). Percentage of oxidized to total glutathione were measured at various time points after treatment with APAP with and without EGFRi as a marker of oxidative stress. A significant decrease in oxidized glutathione levels (Figure 5A) and the percentage of oxidized glutathione (Supplemental Figuure S5A) was observed in EGFRi-treated group compared with vehicle-treated group at 12 and 24 h after APAP-treatment. Superoxide radicals react with nitric oxide to form the potent oxidant peroxynitrite, which is generated mainly in mitochondria during APAP hepatotoxicity (Jaeschke et al., 2012). Peroxynitrite then reacts with tyrosine residues in proteins to form nitrotyrosine protein adducts, which further results in mitochondrial damage. Staining liver sections using nitrotyrosine antibody revealed significant reduction in APAP-mediated formation of nitrotyrosine adducts at 6 h after treatment with EGFRi (Figure 5B). Further, western blot analysis of mitochondrial fraction revealed that APAP-mediated nitrotyrosine adducts formation was significantly reduced in mitochondria after treatment with EGFRi (Figs. 5C and D). Mitochondrial dysfunction leads to opening of mitochondrial permeability transition pore and release of endonucleases such as apoptosis-inducing factor (AIF) and endonuclease G from mitochondria, which ultimately results in liver necrosis (Jaeschke et al., 2012). As expected, APAP treatment caused release of mitochondrial intermembrane endonucleases, AIF and endonuclease G, as well as SMAC into the cytosol (Figs. 5E and F;Supplementary Figs. S5B and C). EGFR inhibition completely abolished release of AIF at 3 h after APAP-treatment and cytosolic AIF remained very low even at 6 h (Figs. 5E and F). Similarly, significant decrease in early release of endonuclease G was observed after EGFRi treatment (Figure 5E and Supplementary Figure S5B). These data suggest that EGFR plays an important role in development of mitochondrial oxidative stress and mitochondrial damage during pathogenesis of APAP hepatotoxicity.
FIG. 5.
Decreased oxidative stress, mitochondrial protein nitration and release of endonucleases from mitochondria by EGFRi. A, Oxidized glutathione levels in total liver extract. B, Representative photomicrographs of liver sections stained for nitrotyrosine-protein adducts. C, Western blot analysis of nitrotyrosine adducts in mitochondrial fraction with its densitometric analysis shown in (D). E, Western blot analysis of AIF, endonuclease G and SMAC in cytosolic fraction with densitometric analysis of AIF as shown in (F). All analysis were done on liver samples collected at various time points after treatment with APAP (300 mg/kg) + PBS or APAP (300 mg/kg) + canertinib (80 mg/kg, 1-h post-APAP) (n = 3–5). *indicate significant difference between groups at P < .05.
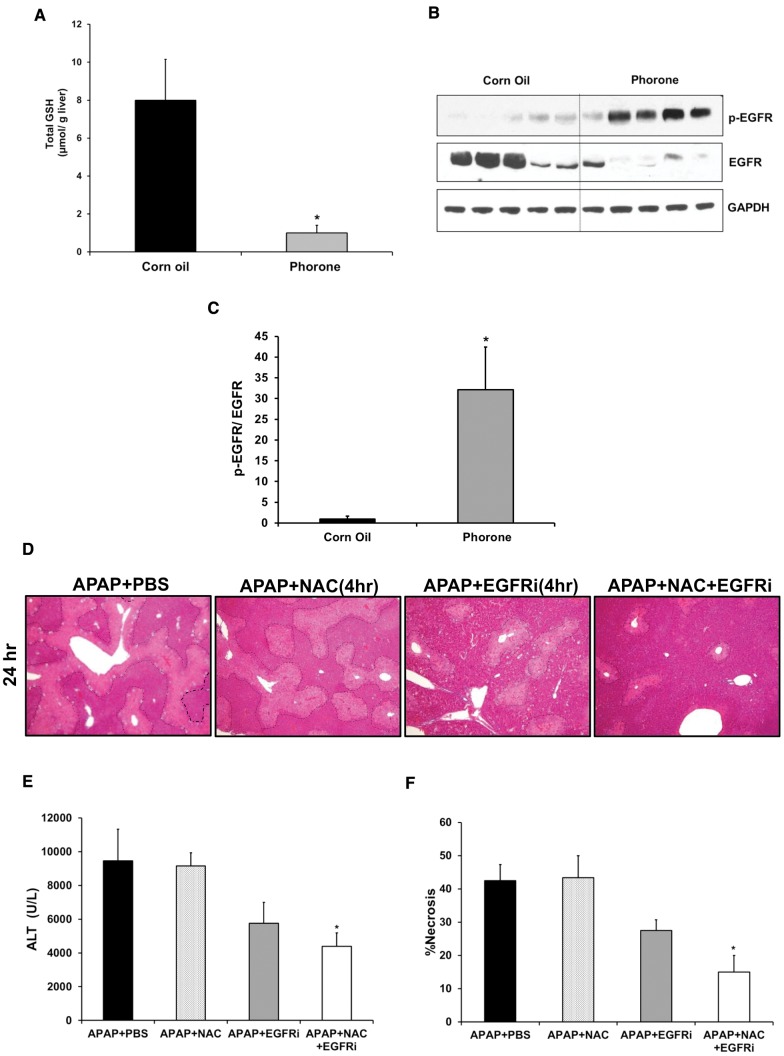
Role of Glutathione Depletion in Rapid Activation of EGFR by Toxic Dose of APAP
Early activation of EGFR was observed after APAP treatment, even in PHHs, when cultured in chemically defined serum free medium with no EGFR ligands or other primary mitogens. This suggested a possible role of ligand-independent intracellular events in triggering of EGFR activation, as reported previously in liver cells after other toxic insults (Reinehr and Haussinger, 2009). Glutathione depletion is one of the earliest events after APAP overdose. In order to investigate if glutathione depletion, per se, can cause activation of EGFR, mice were treated with phorone (200 mg/kg, i.p.), a glutathione depleting agent. As expected, phorone treatment caused significant depletion of glutathione within 2 h (Figure 6A). Interestingly, glutathione depletion by treatment with phorone was accompanied by robust activation of EGFR and downregulation of EGFR (Figs. 6B and C). To further substantiate the role of glutathione depletion in activation of EGFR after APAP overdose, APAP-mediated depleted glutathione was replenished by treatment with NAC (500 mg/kg, i.p.), 1.5 h after APAP (Supplementary Figure S6A). Treatment with NAC caused decrease in APAP-mediated EGFR activation, as analyzed 6 h after APAP treatment, correlating with recovery of glutathione levels (Supplementary Figs. S6B and C). These data suggest that glutathione depletion after APAP overdose may be involved in early activation of EGFR.
FIG. 6.
(A–C) Role of glutathione depletion in rapid activation of EGFR by APAP. (D–F) Enhanced protection against APAP hepatotoxicity by combination of NAC and EGFRi (administered 4-h post-APAP). (A) Total glutathione levels in liver extract and (B) western blot analysis of phospho-EGFR and EGFR in total cell lysate from liver samples obtained 2 h after treatment with Phorone (200 mg/kg in corn oil) or corn oil (control) in mice (n = 5). (C) Densitomertic analysis showing EGFR activation based on western blot image shown in (B). (D) Representative photomicrographs of H&E stained liver sections with necrotic area outlined, (E) serum ALT levels and (F) percentage necrosis area based on H&E stained liver sections of mice treated with 300 mg/kg APAP followed by canertinib (80 mg/kg), NAC (500 mg/kg), combination of canertinib (80 mg/kg) and NAC (500 mg/kg) or PBS (control), 4-h post-APAP (n = 5). Samples were collected 24 h after APAP treatment. *indicate significant difference w.r.t. control group at P < .05.
Enhanced Protection against APAP Hepatotoxicity by Combination of NAC and EGFRi
NAC is the current standard of care for not only APAP overdose patients in the clinic, but for all suspected cases of ALF. However, NAC is effective only when administered at very early stages after APAP overdose (Bernal et al., 2015). We compared effectiveness of EGFRi treatment with NAC treatment after APAP overdose. Both treatment with NAC and EGFRi, 1.5-h post-APAP, caused almost complete abolishment of APAP-mediated liver injury as reflected by reduction in the area of liver necrosis (Supplementary Figs. S6D and F) and serum ALT levels at 6 h (Supplementary Figure S6E). However, treatment with NAC 4 h post-APAP did not cause any decrease in ALT levels or liver necrosis as measured 24 h after APAP treatment (Figs. 6D–F), which is consistent with previous findings (James et al., 2003). In contrast, treatment with EGFRi at 4 h after APAP caused a moderate reduction in APAP-induced liver injury, but could not attain statistical significance (Figs. 6D–F). However, co-treatment of both EGFRi and NAC 4 h post-APAP resulted in significant reduction in APAP-mediated liver injury as indicated by decrease in both serum ALT levels and liver necrosis (Figs. 6D–F). This suggests that combination of EGFRi with NAC can be more beneficial than NAC alone for treatment of APAP-induced ALF.
Increased Progression of Injury, Impaired Recovery and Decreased Survival after Delayed Treatment with EGFRi (12 h Post-APAP)
Several studies using PH model have demonstrated a crucial role of EGFR signaling in liver regeneration after liver re-section (Fausto et al., 2012; Michalopoulos, 2007). Thus, the role of EGFR activation was investigated in compensatory liver regeneration after APAP-induced liver injury. To investigate a direct role of EGFR on liver regeneration, EGFRi was administered 12-h post-APAP, with notion that injury is already established by this time and liver regeneration is just initiated based on previously characterized dynamics of liver injury and regeneration after APAP overdose (Bhushan et al., 2014). Parameters related to liver injury and regeneration were studied at 24 and 48 h after treatment with APAP (as represented by the scheme shown in Figure 7A). As expected, liver injury at 24 h after APAP treatment was not altered by EGFRi-treatment as indicated by percentage necrotic area (Figs. 7B and D) and serum ALT levels (Figure 7C). Similar to our previous report (Bhushan et al., 2014), the injury was significantly resolved at 48 h compared with 24 h in APAP-treated group. However, liver injury did not improve at 48 h compared with 24 h in EGFRi-treated group (Figs. 7B-D) and resulted in 50% decrease in survival at 48 h post-APAP (Figure 7C).
Impaired Liver Regeneration and Cell Cycle Arrest by Delayed Treatment with EGFRi (12-h Post-APAP)
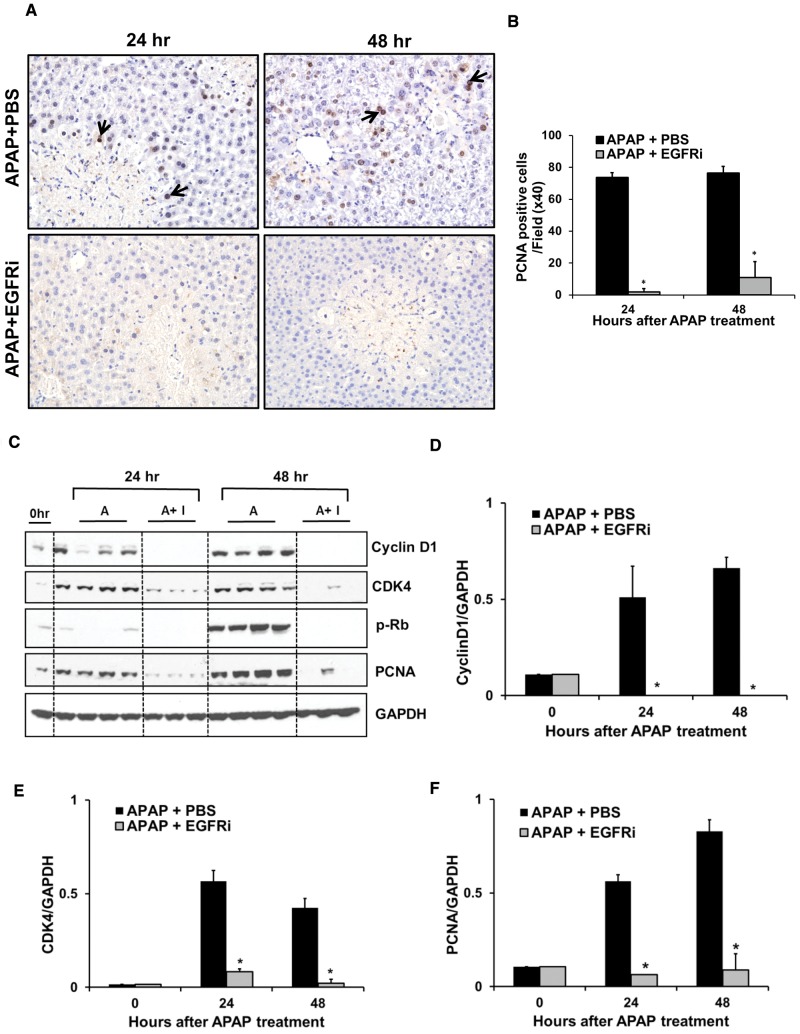
Effect of delayed treatment with EGFRi on liver regeneration was further studied using PCNA staining (Figure 8A), which is a marker of cell proliferation. Consistent with our previous study Bhushan et al. (2014), numerous PCNA-positive cells were observed surrounding the zones of necrosis, at 24 and 48 h after treatment with APAP, suggesting robust liver regeneration. Interestingly, minimal PCNA-positive cells were observed at both time points in the EGFRi group, indicating that the compensatory liver regeneration was almost completely abolished after treatment with EGFRi (Figs. 8A and B). PCNA Western blot analysis corroborated PCNA immunohistochemistry data (Figs. 8C and F).
FIG. 8.
Impaired liver regeneration and cell cycle arrest by delayed (12-h post-APAP) treatment with EGFRi. A, Representative photomicrographs of PCNA stained liver sections with arrows indicating cells in S-phase with nuclear PCNA staining (brown). B, Total number of PCNA-positive cell per high power field (×40). C, Western blot analysis of cyclinD1, CDK4, phospho-Rb, and PCNA using total liver extract. Densitometric analysis of (D) cyclinD1, (E) CDK4, and (F) PCNA. Mice were treated with 300 mg/kg APAP. Canertinib (80 mg/kg) or PBS was administered 12-h post-APAP (n = 3–5). All samples were collected at 24 and 48 h after APAP treatment. * indicate significant difference between groups at P < .05.
Induction of Cyclin D1 is a key initial step that regulates entry into the cell cycle. APAP-treatment caused an increase in the expression of Cyclin D1 at 24 and 48 h. APAP-mediated increase in protein expression of Cyclin D1 was dramatically decreased at both time points after delayed treatment with EGFRi (Figs. 8C and D). Cyclin D1 binds to CDK4 and causes its activation, which then phosphorylates Rb protein, ultimately leading to induction of several key cell cycle genes. CDK4 protein expression followed a pattern similar to Cyclin D1 in both groups (Figs. 8C and E). Further, phosphorylation of Rb protein was observed at 48 h in APAP-treated group, which was completely abolished after treatment with EGFRi (Fig. 8C). These data demonstrate that delayed treatment with EGFRi (12-h post-APAP) prevented cell cycle progression and caused almost complete inhibition of liver regeneration leading to progression of injury, decreased recovery and reduced survival after APAP overdose.
DISCUSSION
Although mechanisms of APAP hepatotoxicity have been extensively studied leading to considerable progress in our knowledge, the exact intracellular signaling events still not completely known (Jaeschke et al., 2012). On the contrary, mechanisms of compensatory liver regeneration that follows liver injury after APAP overdose have just begun to be explored (Bhushan et al., 2014). EGFR signaling is well known to play critical role in hepatocyte proliferation and normal liver regeneration after PH (Fausto et al., 2012; Michalopoulos, 2007), but a few reports also suggest its role in cell death signaling in liver (Arany, 2008; Reinehr and Haussinger, 2009). The role of EGFR in acetaminophen (APAP)-mediated acute liver injury and subsequent liver regeneration is completely unknown, which was investigated in this study.
Similar to our previous report (Bhushan et al., 2014), dose-dependent activation of EGFR was observed after toxic doses of APAP in mice such that activation was greater at a higher non-regenerating dose of APAP. Interestingly, a rapid and consistent EGFR activation was observed in mice and in PHHs after APAP treatment. This dose-dependent early activation of EGFR, preceding injury, with paradoxical inverse correlation with liver regeneration, suggested a possible role of EGFR activation in the development of injury. Indeed, inhibition of EGFR both pre- and post-APAP treatment by using a highly specific pan-EGFRi (Slichenmyer et al., 2001), canertinib, almost completely abolished APAP toxicity consistently and reproducibly, suggesting a crucial role of EGFR activation in APAP hepatotoxicity. This paradoxical role of EGFR activation in cell death is not completely unprecedented. Hydrophobic bile acids and CD95 ligand have been previously reported to activate EGFR in both hepatocytes and HSCs (Reinehr et al., 2003a,b, 2004, 2005a,b; Reinehr and Haussinger, 2009, 2012; Sommerfeld et al., 2009). EGFR activation in these studies was involved in apoptosis through tyrosine phosphorylation of death receptor (CD95) and stabilization of the death-inducing signaling complex and inhibition of EGFR activation resulted in decreased cell death (Reinehr and Haussinger, 2012). Similarly, EGFR was activated after treatment with the nephrotoxic insults cisplatin and cyclosporine A in proximal renal tubular cell lines and inhibition of EGFR activation resulted in diminished cell death (Arany et al., 2004; Sarro et al., 2008).
We further investigated the potential mechanisms underlying role of EGFR activation in mediating APAP hepatotoxicity. Metabolic activation of APAP, reactive metabolite-mediated glutathione depletion and subsequent covalent binding to cellular proteins were not altered by EGFRi treatment indicating that the early events involved in initiation of APAP hepatotoxicity were not changed by EFGRi treatment. Initial mitochondrial dysfunction and its feed-forward accentuation by oxidative stress play a central role in APAP hepatotoxicity with underlying signaling pathways that are not completely known (Jaeschke et al., 2012). Our study revealed, for the first time, that EGFR translocated to mitochondria and its activated form is increased in mitochondria very rapidly after APAP treatment. This was associated with very early decline in mitochondrial respiratory capacity, preceding overt necrosis after APAP overdose. Mitochondrial levels of both total and activated EGFR were drastically decreased by EGFR inhibition, resulting in complete restoration of mitochondrial function. Subsequently, EGFR inhibition also prevented mitochondrial oxidative stress and release of mitochondrial intermembrane proteins, especially AIF, into the cytosol, which is important for nuclear DNA damage and necrosis. These data have revealed a novel role of EGFR in APAP toxicity via modulating mitochondrial dysfunction. The exact mechanisms how mitochondrial EGFR activity can be involved in very early mitochondrial dysfunction remain unknown. EGFR can be involved, directly or indirectly via one of its effectors, in phosphorylation or alteration of mitochondrial proteins involved in APAP-associated mitochondrial dysfunction such as Sab. A previous study reported that activated EGFR can be translocated to mitochondria and phosphorylate mitochondrial cytochrome c oxidase subunit II (CoxII) leading to decrease in its activity and ATP depletion (Demory et al., 2009). Further detailed studies are necessary to determine the exact interacting proteins of EGFR in mitochondria during APAP hepatotoxicity.
The mechanism of rapid activation of EGFR is another interesting aspect, which was investigated in this study. Early activation of EGFR in PHHs in maintenance medium, where no EGFR ligands were present, suggested a possible role of ligand-independent intracellular mechanism in activation of EGFR. Such a mechanism has been demonstrated in liver cells after apoptotic-stimuli and was specifically involved in cell death (Reinehr and Haussinger, 2009). These studies also demonstrated that oxidative stress was responsible for phosphorylation of EGFR via activation of Yes kinase (Reinehr and Haussinger, 2009). In our study, investigations with the glutathione depleting agent (phorone) and glutathione replenishing agent (NAC) indicated a potential role of glutathione depletion in EGFR activation. This is in concordance with the fact that glutathione depletion is one of the very early events that occur after APAP overdose similar to EGFR activation and glutathione is required physiologically to scavenge ROS and limit oxidative stress. The role of GSH depletion in EGFR activation is supported by rapid activation of EGFR in serum free conditions where no EGFR ligands are present. However, our studies do not rule out possibility of ligand-dependent activation of EGFR after APAP overdose, especially at later time points. TGF-α can be produced by hepatocytes and activate EGFR receptor on hepatocytes in an autocrine fashion (Michalopoulos and Khan, 2005). Whether this autocrine loop by de novo TGF-α synthesis may be involved in activation of EGFR remains to be tested.
In order to investigate a direct role of EGFR activation on liver regeneration after APAP overdose, a delayed EGFRi treatment strategy (12-h post-APAP) was utilized, such that APAP hepatotoxicity was already established. Delayed treatment with EGFRi almost completely abolished compensatory hepatocyte proliferation after APAP overdose. Failure of cell cycle progression was due to complete inhibition of cyclin D1 induction. This suggested that apart from the role in liver injury after APAP overdose, EGFR signaling plays a critical function in liver regeneration as well. Thus, activation of EGFR signaling may prove to be beneficial to stimulate liver regeneration at later hours (regenerative phase) post-APAP-induced liver injury. Similarly, a dual role of EGFR was reported previously in HSCs, where activation of EGFR upon stimulation with bile acids caused cell proliferative signaling, which shifted to cell death signaling upon sustained JNK activation-mediated coupling of EGFR with the CD95 death receptor (Reinehr and Haussinger, 2009; Sommerfeld et al., 2009). A similar dual role of EGFR has been proposed in the case of nephrotoxic insults, where EGFR activation can lead to renal injury or recovery depending on degree, duration and type of stress (Arany, 2008). A number of previous studies in the PH model of liver regeneration show that inhibition or elimination of a single pathway (including EGFR signaling) can only delay or diminish liver regeneration but cannot permanently abolish liver regeneration due to upregulation of compensatory pathways (Michalopoulos, 2007). In contrast, in our study, delayed inhibition of EGFR after APAP overdose resulted in almost complete inhibition of liver regeneration, resulting in progression of injury and decreased survival. These data along with our previous study (Bhushan et al., 2014) suggest that the dynamics of liver regeneration is very different in the APAP-induced ALF model (compared with PH), which advances very rapidly and involves progression of injury, such that a timely regenerative response is imminently critical for final recovery. Since most of these studies so far have been focused on studying mechanisms of liver regeneration after PH, there is a critical need to understand mechanisms of liver regeneration in acute liver injury models, which are more relevant for clinical drug-induced ALF.
From a therapeutic standpoint, even after decades of research, pharmacological intervention for patients with APAP-induced ALF is still limited to NAC. Efficacy of NAC is only well demonstrated in early presenting patients, warranting development of novel treatment strategies (Bernal et al., 2015). Although, in our study, protection by EGFRi treatment greatly diminished when treatment was delayed from 1- to 4-h post-APAP, it appears to be more effective than NAC, which completely lost its efficacy at the later time point. Further, combination of EGFRi with NAC produced significant protection even at the later time point (4-h post-APAP) suggesting great promise of the combination treatment, especially considering several EGFR inhibitors are currently used in clinical setting. However, it should be noted that further delayed treatment (12-h post-APAP) caused increased progression of injury due to impaired liver regeneration in mice. Although, it is expected that the potential therapeutic window of EGFRi may be greater in humans considering APAP hepatotoxicity is generally delayed in humans compared with mice (Xie et al., 2014), our data suggest that it will be challenging to develop EGFR inhibition as a therapeutic strategy for APAP toxicity as depending upon the time of inhibition, outcome can be completely opposite. This is particularly important aspect to consider in case of APAP overdose, where the exact time of overdose is not usually known in patients. Further comprehensive studies to investigate the temporal dynamics of signaling pathways affected by EGFR activation during pathogenesis of APAP hepatotoxicity may assist in delineating toxicity effectors from regenerative signaling for targeted development of anti-injury or pro-regenerative therapy.
To conclude, early inhibition of EGFR activation after APAP overdose in mice attenuated liver injury by preventing mitochondrial dysfunction and delayed inhibition of EGFR resulted in impairment of liver regeneration after APAP toxicity by arresting cell cycle activation. Thus our study indicated that EGFR activation can play a dual role in APAP overdose and is involved in both initiation of APAP-induced injury and in stimulating subsequent liver regeneration. Further, our study supports the emerging notion that signaling pathways that regulate liver injury and subsequent tissue repair are intricately linked and similar to death receptors, growth factor receptor such as EGFR can couple to both cell death and proliferation signaling in a context-dependent manner.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by 8P20 GM103549, 5T32ES007079-34, R01 DK102142, and 1R01DK098414
Supplementary Material
REFERENCES
- Arany I. (2008). Dual role of the activated epidermal growth factor receptor in renal tubular cells during stress. Kidney Int. 73, 5–7. [DOI] [PubMed] [Google Scholar]
- Arany I., Megyesi J. K., Kaneto H., Price P. M., Safirstein R. L. (2004). Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol. Renal Physiol. 287, F543–F549. [DOI] [PubMed] [Google Scholar]
- Bernal W., Lee W. M., Wendon J., Larsen F. S., Williams R. (2015). Acute liver failure: A curable disease by 2024?. J. Hepatol. 62(1 Suppl), S112–S120., [DOI] [PubMed] [Google Scholar]
- Bhushan B., Borude P., Edwards G., Walesky C., Cleveland J., Li F., Ma X., Apte U. (2013). Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am. J. Pathol. 183, 1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B., Edwards G., Desai A., Michalopoulos G., Apte U. (2016). Liver-specific deletion of integrin-linked kinase in mice attenuates hepatotoxicity and improves liver regeneration after acetaminophen overdose. Gene Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B., Walesky C., Manley M., Gallagher T., Borude P., Edwards G., Monga S. P., Apte U. (2014). Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol. 184, 3013–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner J. L., Demory M. L., Silva C., Parsons S. J. (2004). Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell Biol. 24, 7059–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N. L., Patel U., Cohen S. (1977). Hormonal factors concerned with liver regeneration. Ciba Found. Symp. (55), 95–107. [DOI] [PubMed] [Google Scholar]
- Carver R. S., Stevenson M. C., Scheving L. A., Russell W. E. (2002). Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology 123, 2017–2027. [DOI] [PubMed] [Google Scholar]
- Dara L., Johnson H., Suda J., Win S., Gaarde W., Han D., Kaplowitz N. (2015). Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 62, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demory M. L., Boerner J. L., Davidson R., Faust W., Miyake T., Lee I., Huttemann M., Douglas R., Haddad G., Parsons S. J. (2009). Epidermal growth factor receptor translocation to the mitochondria: regulation and effect. J. Biol. Chem. 284, 36592–36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahower B. C., McCullough S. S., Hennings L., Simpson P. M., Stowe C. D., Saad A. G., Kurten R. C., Hinson J. A., James L. P. (2010). Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J. Pharmacol. Exp. Ther. 334, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., Campbell J. S., Riehle K. J. (2012). Liver regeneration. J. Hepatol. 57, 692–694. [DOI] [PubMed] [Google Scholar]
- Hu B., Colletti L. M. (2008). Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G45–G53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H., McGill M. R., Ramachandran A. (2012). Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 44, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L. P., McCullough S. S., Lamps L. W., Hinson J. A. (2003). Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 75, 458–467. [DOI] [PubMed] [Google Scholar]
- McGill M. R., Williams C. D., Xie Y., Ramachandran A., Jaeschke H. (2012). Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 264, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. (2007). Liver regeneration. J. Cell Physiol. 213, 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K., DeFrances M. C. (1997). Liver regeneration. Science 276, 60–66. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K., Khan Z. (2005). Liver regeneration, growth factors, and amphiregulin. Gastroenterology 128, 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A., Wagner B., Sibilia M. (2007). The EGF receptor is required for efficient liver regeneration. Proc. Natl. Acad. Sci. U S A 104, 17081–17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N., De Luca A., Bianco C., Strizzi L., Mancino M., Maiello M. R., Carotenuto A., De Feo G., Caponigro F., Salomon D. S. (2006). Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366, 2–16. [DOI] [PubMed] [Google Scholar]
- Paranjpe S., Bowen W. C., Tseng G. C., Luo J. H., Orr A., Michalopoulos G. K. (2010). RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am. J. Pathol. 176, 2669–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A., McGill M. R., Xie Y., Ni H. M., Ding W. X., Jaeschke H. (2013). Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58, 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R., Becker S., Eberle A., Grether-Beck S., Haussinger D. (2005a). Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 280, 27179–27194. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Becker S., Hongen A., Haussinger D. (2004). The Src family kinase Yes triggers hyperosmotic activation of the epidermal growth factor receptor and CD95. J. Biol. Chem. 279, 23977–23987. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Becker S., Keitel V., Eberle A., Grether-Beck S., Haussinger D. (2005b). Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology 129, 2009–2031. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Graf D., Haussinger D. (2003a). Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 125, 839–853. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Haussinger D. (2009). Epidermal growth factor receptor signaling in liver cell proliferation and apoptosis. Biol. Chem. 390, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Haussinger D. (2012). CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch. Biochem. Biophys. 518, 2–7. [DOI] [PubMed] [Google Scholar]
- Reinehr R., Schliess F., Haussinger D. (2003b). Hyperosmolarity and CD95L trigger CD95/EGF receptor association and tyrosine phosphorylation of CD95 as prerequisites for CD95 membrane trafficking and DISC formation. Faseb J. 17, 731–733. [DOI] [PubMed] [Google Scholar]
- Saberi B., Ybanez M. D., Johnson H. S., Gaarde W. A., Han D., Kaplowitz N. (2014). Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology 59, 1543–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro E., Tornavaca O., Plana M., Meseguer A., Itarte E. (2008). Phosphoinositide 3-kinase inhibitors protect mouse kidney cells from cyclosporine-induced cell death. Kidney Int. 73, 77–85. [DOI] [PubMed] [Google Scholar]
- Slichenmyer W. J., Elliott W. L., Fry D. W. (2001). CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin. Oncol. 28(5 Suppl 16), 80–85., [DOI] [PubMed] [Google Scholar]
- Sommerfeld A., Reinehr R., Haussinger D. (2009). Bile acid-induced epidermal growth factor receptor activation in quiescent rat hepatic stellate cells can trigger both proliferation and apoptosis. J. Biol. Chem. 284, 22173–22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Beggs K., Borude P., Edwards G., Bhushan B., Walesky C., Roy N., Manley M. W. Jr., Gunewardena S., O'Neil M. et al. (2016). Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G91–G104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., McGill M. R., Dorko K., Kumer S. C., Schmitt T. M., Forster J., Jaeschke H. (2014). Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 279, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Ramachandran A., Breckenridge D. G., Liles J. T., Lebofsky M., Farhood A., Jaeschke H. (2015). Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 286, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.