Abstract
Background
Type 2 diabetes mellitus (DM) is a major risk factor for development of tuberculosis (TB), however the underlying molecular foundations are unclear. Since lipids play a central role in the development of both DM and TB, lipid metabolism may be important for TB-DM pathophysiology.
Methods
A 1H NMR spectroscopy-based platform was used to determine 225 lipid and other metabolic intermediates in plasma samples of healthy controls (n = 50) and patients with TB (n = 50), DM (n = 50) or TB-DM (n = 27).
Results
TB patients presented with wasting disease, represented by decreased amino acid levels including histidine and alanine. Conversely, DM patients were dyslipidemic as evidenced by high levels of very low-density lipoprotein triglycerides and low high-density lipoprotein cholesterol. TB-DM patients displayed metabolic characteristics of both wasting and dyslipidemia combined with disease interaction-specific increases in phospholipid metabolites (e.g. sphingomyelins) and atherogenic remnant-like lipoprotein particles. Biomarker analysis identified the ratios of phenylalanine/histidine and esterified cholesterol/sphingomyelin as markers for TB classification regardless of DM-status.
Conclusions
TB-DM patients possess a distinctive plasma lipid profile with pro-atherogenic properties. These findings support further research on the benefits of improved blood lipid control in the treatment of TB-DM.
Keywords: Diabetes, Tuberculosis, Lipids, NMR, Biomarkers
Highlights
-
•
Patients with concurrent tuberculosis and type 2 diabetes display metabolic characteristics of wasting and dyslipidemia.
-
•
Disease interaction leads to specific increases in phospholipids (e.g. sphingomyelins) and remnant-like lipoproteins.
-
•
In conclusion, concurrent tuberculosis and type 2 diabetes results in a pro-atherogenic plasma lipid profile.
Epidemiological studies have demonstrated that type 2 diabetes triples the risk of developing active tuberculosis, however the mechanisms underlying this association are unknown. Lipids play an important role in the pathology of both diseases, and the use of statins as part of the tuberculosis treatment regimen has shown promise. This cross-sectional study was conceived to comprehensively dissect the relative impact of concurrent tuberculosis and type 2 diabetes on patient plasma lipid profiles. The results of this study constitute an important foundation for future studies on the benefits of lipid-lowering drugs for treatment of comorbid tuberculosis and type 2 diabetes.
1. Introduction
Type 2 diabetes mellitus (DM) is a major risk factor for tuberculosis (TB) and triples the risk of developing active TB disease [1]. At present approximately 15% of global TB cases can be attributed to DM comorbidity [2]. Clinically, DM increases TB severity and impairs TB treatment [3], while conversely TB hampers glycemic control [4]. DM impacts both susceptibility to infection and progression towards active disease [1,5], however the immunological processes involved are unclear [6]. The number of DM patients in TB-endemic regions of Africa and Asia is predicted to rise significantly during the coming decades [7], and TB-DM comorbidity is estimated to seriously affect TB and consequently general global health. Therefore the TANDEM project seeks to optimize treatment and diagnosis of comorbid TB-DM and to understand its causal mechanisms [8].
While DM is primarily characterized by hyperglycemia and insulin resistance, it is often also associated with severe dyslipidemia as a result of high dietary fat intake and deregulated hepatic lipid metabolism [9]. DM-associated high insulin levels stimulate de novo lipogenesis in hepatocytes while failing to suppress lipolysis in insulin-resistant adipocytes of DM patients, leading to increased free fatty acid flux to the liver and overproduction of large triglyceride-rich very low-density lipoprotein (VLDL) particles [10]. Diabetic dyslipidemia is defined as having high levels of plasma triglycerides and/or cholesterol in combination with low levels of high-density lipoprotein (HDL) cholesterol and is a major risk factor for cardiovascular disease and atherosclerosis, which often complicate DM.
In contrast to DM, TB is often associated with malnutrition and wasting syndrome [11], and a low bodyweight is a risk factor for TB disease [12,13]. Additionally, TB leads to decreased body fat mass and levels of the adipocyte hormone leptin [14]. Interestingly, the causative agent of TB, Mycobacterium tuberculosis (Mtb), has been shown to rely heavily on host-derived lipids for its survival [[15], [16], [17]]. Mtb induces the formation of lipid-loaded foamy macrophages, similar to the ones found in atherosclerotic lesions, and exploits these cells as its primary niche for replication. Several studies have identified high cholesterol levels as risk factor for TB [[18], [19], [20]], and reducing cholesterol levels using statins was beneficial in Mtb-infected macrophages, mice and patients through enhancing the bactericidal effect of first-line antibiotics and phagosome maturation [[21], [22], [23], [24], [25]].
To identify potential differences in lipid metabolism, we compared plasma lipid profiles of patients with TB-DM to those of patients with TB or DM. To this end we determined plasma metabolic profiles [26] in healthy controls (HC) and patients with TB, DM or TB-DM. We hypothesized that the combination of these two diseases on seemingly opposite sides of the metabolic spectrum would result in distinctive plasma lipid profiles, as well as novel biomarkers.
2. Materials & Methods
2.1. Ethics Statement/Patient Inclusion
This study was undertaken as part of a EU-funded collaborative project (TANDEM) [8]. Patients (18–70 years) were enrolled in Cape Town, South-Africa from six public health care clinics around Tygerberg Academic Hospital (Elsies River, Ravensmead, Uitsig, Adriaanse, Durbanville, Fisantekraal). In total, 177 participants were included: 50 healthy community controls, 50 DM patients, 50 TB patients and 27 TB-DM patients. DM patients without TB were recruited from community health centers/day hospitals in Elsies River and Durbanville and previously diagnosed with DM according to WHO-criteria [27]. TB patients were screened for DM and classification was based on hyperglycaemia (random plasma glucose ≥ 200 mg/dL), HbA1c ≥ 6.5% and/or self-reported DM, in which case previous determination of random plasma glucose levels was not repeated. From the patients included in this study there is clinical evidence suggesting that one participant has type 1 diabetes, whereas all other patients suffered from type 2 diabetes. TB patients were identified based on positive Xpert Mtb/RIF assay (Cephaid Inc., Sunnyvale, CA, USA), MGIT culture and Mtb confirmation. Participants were excluded if they were HIV-positive, pregnant, on steroid therapy (in the last 6 months), had a hemoglobin <10 g/L, presented with emphysema, chronic bronchitis, asthma, steroid-induced DM, cancer or known alcohol abuse. The study was approved by the Health Research Ethics Committee of the University of Stellenbosch, and conducted according to the Helsinki Declaration and International Conference of Harmonization guidelines. Written informed consent was obtained from all participants.
2.2. Metabolic Profile Quantification by 1H-Nuclear Magnetic Resonance (NMR) Spectroscopy
A high-throughput 1H NMR spectroscopy platform was used to determine plasma metabolic profiles consisting of 225 parameters (Nightingale Health, Helsinki, Finland), including detailed concentrations and compositions of 14 lipoprotein subclasses, fatty acids & glycerides, amino acids and glycolytic molecules [28]. Methods regarding sample preparation and measurement procedures were described previously [26].
2.3. Statistical Analysis
For multivariate analysis, metabolite ratios were excluded. Metabolites were log-transformed to correct for skewed distributions, with the exception of lipoprotein particle concentrations for regression analysis due to a substantial amount of zero measurements. Partial least squares discriminant analysis (PLS-DA) modelling and hierarchical clustering was used to visualize metabolic differences between the groups. Only samples and measurements with ≤10% missing or zero values were considered for PLS-DA modelling. The optimal number of components was determined based on estimated classification error rates calculated by fivefold cross validation, which was repeated ten times. To illustrate the differences between our individual groups (HC, TB, DM and TB-DM patients), separate linear regression models were fitted for each pairwise combination of groups while adjusting for age and sex. 98 measures of specific particle concentrations and compositions of 14 lipoprotein subclasses were analyzed distinctly from the remaining parameters (44 metabolite subset, Supplementary Table S1). Next, interaction-specific effects of TB-DM comorbidity were investigated by fitting the following linear model:
where TB = TB-status (true/false), DM = DM-status (true/false), TB*DM = disease interaction effect, Age = age (years) and Sex = sex (male/female).
Univariate biomarker analysis was performed to identify metabolic measures with potential for TB diagnosis. Analysis was stratified by DM-status (HC vs. TB, DM vs. TB-DM). Log-transformed data of the 44 metabolite subset was mean-centred, scaled to standard deviation (SD) units and top 20 metabolite ratios based on p-values were calculated and added to the analysis. For each biomarker receiver operating characteristic (ROC) curves were plotted and area under the curve (AUC) values with 95% confidence interval (CI) determined. Biomarker analysis was performed using the online tool MetaboAnalyst 3.5 and methodological details were published previously [29].
Statistical analysis of clinical characteristics was performed in SPSS 23 (IBM) by one-way ANOVA (reported p-values are the outcome of the F-test), independent samples t-test or chi-squared test. Univariate analysis of absolute metabolite concentrations was done in Graphpad Prism 7 by Kruskal-Wallis test with post-hoc Dunn's test. PLS-DA and multiple linear regression analysis were performed using R version 3.3.2. including the following packages: mixOmics [30] version 6.3.0, limma [31] version 3.30.13 and phenotypicForest [32] version 0.3.
3. Results
3.1. Clinical and Metabolic Characteristics of the Study Population
Patient characteristics are shown in Table 1. On average, DM patients were older and had a higher BMI compared to the other groups. TB patients had a relatively low BMI, while this was comparable for TB-DM patients and HC. All (non-TB) DM patients were on anti-diabetic drugs, while this was the case for 55.6% of TB-DM patients. TB-DM patients not on treatment were newly diagnosed DM cases. In total, 177 participants were included in the study and their plasma metabolic profiles were determined using 1H NMR spectroscopy. Metabolite ratios were excluded in the multivariate analysis to limit parameter interdependence, resulting in a total of 142 variables.
Table 1.
Patient clinical characteristics according to disease group (n = 177).
| HC |
DM |
TB |
TB-DM |
p-Value | |
|---|---|---|---|---|---|
| n = 50 | n = 50 | n = 50 | n = 27 | ||
| Ethnicity: colored | 48/50 (96%) | 50/50 (100%) | 47/50 (94%) | 26/27 (96.3%) | 0.414 |
| Sex (male/female) | 25/25 | 23/27 | 33/17 | 13/14 | 0.186 |
| Age (years) | 37.7 ± 9.2 | 51.6 ± 11.2 | 46.3 ± 9.3 | 44.4 ± 9.5 | <0.001 |
| BMI (kg/m2) | 24.2 ± 6.4 | 29.1 ± 5.8 | 19.1 ± 2.6 | 22.2 ± 5.2 | <0.001 |
| HbA1c (%) | 5.3 ± 0.4a | 10.1 ± 2.6a, c | 5.5 ± 0.4b | 9.5 ± 2.5b | <0.001 |
| Random blood glucose (mmol/L) | 5.1 ± 1.2 | 13.3 ± 5.1 | 6.1 ± 1.6 | 8.6 ± 4.9d | <0.001 |
| Previous TB (>1 year ago) | na | 13/50 (26%) | 26/50 (52%) | 7/27 (25.9%) | 0.012 |
| Smoking (currently) | na | 20/50 (40%) | 47/50 (94%) | 18/27 (66.7%) | <0.001 |
| Quantiferon positive | 35/46 (76%) | 41/48 (85%) | na | na | 0.250 |
| Time to positivity (days) | na | na | 7.3 ± 4.5e | 7.1 ± 4.4f | 0.612 |
| DM medication | na | 50/50 (100%) | na | 15/27 (55.6%) | <0.001 |
| Insulin | 28/50 (56%) | 5/27 (18.5%) | 0.002 | ||
| Metformin | 45/50 (90%) | 11/27 (40.7%) | <0.001 | ||
| Other | 12/50 (24%) | 5/27 (18.5%) | 0.580 | ||
| Statins | 18/50 (36%) | 4/27 (14.8%) | 0.050 | ||
| Years since DM diagnosis | na | na | |||
| <1 | 0/49 (0%) | 14/27 (51.2%) | <0.001 | ||
| 1–5 | 16/49 (32.7%) | 3/27 (11.1%) | 0.038 | ||
| 6–15 | 19/49 (38.3%) | 7/27 (25.9%) | 0.258 | ||
| >15 | 14/49 (28.6%) | 3/27 (11.1%) | 0.080 |
Data is presented as percentage of total (%) or mean ± SD, na = not available.
Point-of-care measurements.
Lab measurements.
Data available from 48/50 patients.
Data available from 12/27 patients.
Data available from 35/50 patients.
Data available from 21/27 patients.
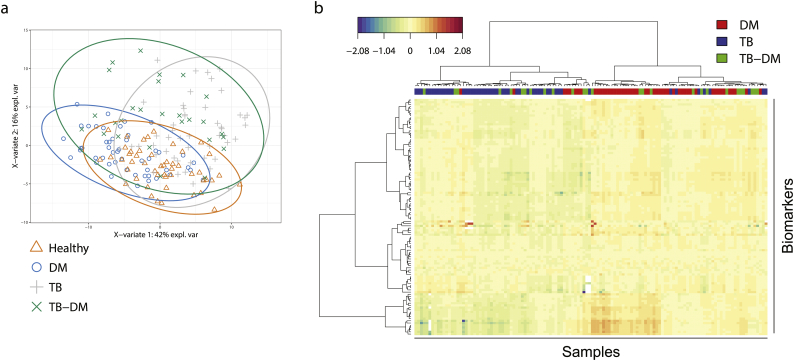
Partial least square discriminant analysis (PLS-DA) (Fig. 1a) was performed to visualize the metabolic differences between the four groups based on the complete metabolic signature. The score plot of the first two principal components (explaining 42% and 16% of total variance, respectively) is depicted in Fig. 1a. TB-DM patients appeared largely scattered over both single disease groups, implying significant metabolic heterogeneity. To explore this further we compared TB, DM and TB-DM patients by hierarchical clustering analysis (Fig. 1b). While the majority of TB and DM patients each clustered together, TB-DM patients were again dispersed throughout the two single disease groups, further illustrating high inter-individual variation.
Fig. 1.
Discrimination of patient groups based on metabolic profiles. PLS-DA was used for discrimination of patient groups based on 1H NMR-spectroscopy plasma metabolites. Only samples and variables with ≤10% missing or zero values were included, resulting in 107 parameters and 175 individuals (healthy = 49, DM = 49, TB = 50, TB-DM = 27). (a) Score plot of the first two components of a PLS-DA model obtained from healthy controls (orange triangles), DM-only (blue circles), TB-only (grey plusses) and TB-DM patients (green crosses). (b) Two-way hierarchical clustering analysis using Euclidean distance and Ward's method of DM-only (red), TB-only (blue) and TB-DM (green) patients.
3.2. DM is Associated with Dyslipidemia while TB is Associated with Wasting
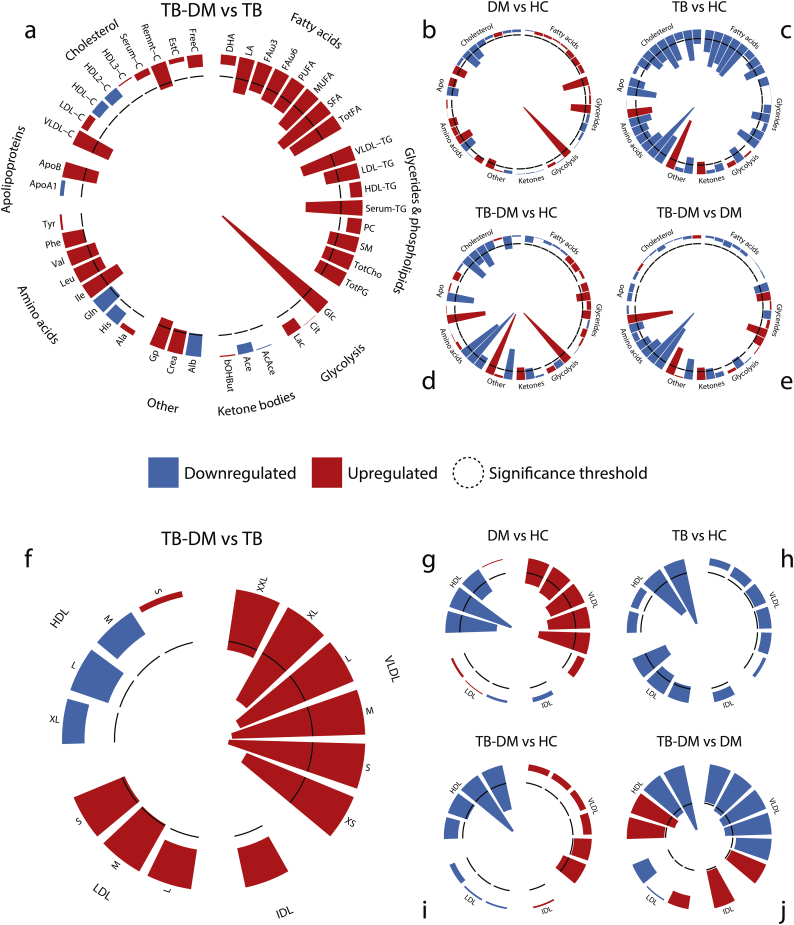
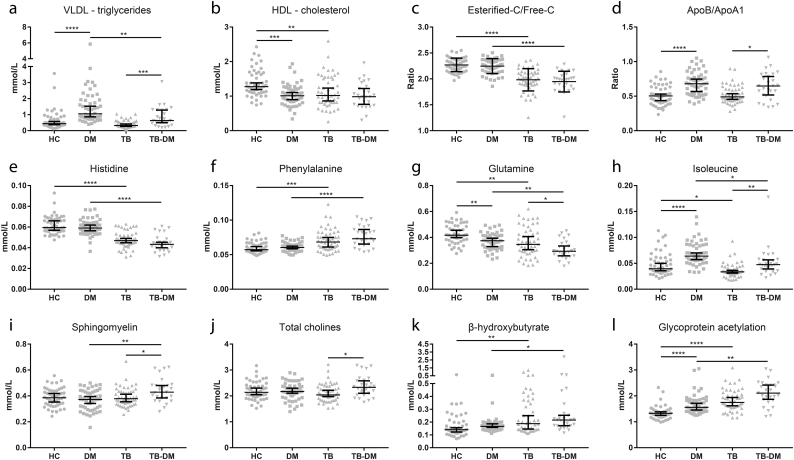
We performed pairwise comparisons of all groups by multiple linear regression analysis adjusting for age and sex. For each comparison the FDR-corrected–log(p) values of 44 metabolic parameters are plotted in circular histograms (Fig. 2a–e). Measures of size-specific (XS, S, M, L, XL) lipoprotein particle concentrations (Fig. 2f–j), lipid composition (Fig. S1b) and average lipoprotein diameter (Fig. S1a) were analyzed separately. Absolute concentrations or ratios of a subset of metabolites are plotted in Fig. 3 (a-l). Metabolite means, standard deviations and numbers of successful measurements are presented in Supplementary Table S1, and raw measurement data can be found in Supplementary Table S2.
Fig. 2.
Pairwise comparisons of metabolic profiles and specific lipoprotein particle concentrations. 1H NMR-spectroscopy plasma metabolites were analyzed by multiple linear regression and the resulting –log-transformed FDR-corrected p-values (t-test) are displayed as circular histograms for a subset of 44 metabolic parameters (a-e) and for the particle concentrations of 14 lipoprotein subclasses (f-j). Metabolites are grouped by metabolite family and lipoproteins by density. The following comparisons are plotted: TB-DM vs. TB (a & f), DM vs. healthy controls (b & g), TB vs. healthy controls (c & h), TB-DM vs. healthy controls (d & i) and TB-DM vs. DM (e & j). Detailed legends given in the larger plots also apply for the corresponding smaller plots. Up- and downregulated metabolites are indicated by red and blue bars, respectively, and the significance threshold (p = 0.05) is indicated by a dashed line.
Fig. 3.
Absolute values for a subset of metabolites. (A-L) Concentrations (mmol/L), or ratios of 12 selected important metabolites. Data are displayed as scatter plots with median and 95% CI, with each dot representing one individual. HC (n = 50) and TB-DM (n = 27) were compared to TB-only (n = 50) and DM-only (n = 50) by Kruskal-Wallis test with post-hoc Dunn's test. * p = 0.05, ** p = 0.01, *** p = 0.001, **** p = 0.0001.
First, we wanted to define the metabolic effects of DM and TB vs. HC (Fig. 2b and c). As expected, the primary parameter associated with DM was increased plasma glucose (p = 1.83E−16; p-values reported here are from multivariate analyses) (Fig. 2b). DM patients showed major hallmarks of dyslipidemia, namely high levels of VLDL-triglycerides (VLDL-TG) (p = 5.72E−7) (Fig. 3a), VLDL-cholesterol (VLDL-C) (p = 6.66E−4) and ApoB (p = 0.017), with low levels of HDL-C (p = 4.34E−5) (Fig. 3b) and ApoA1 (p = 1.82E−3), resulting in an increased ApoB/ApoA1 ratio (Fig. 3d). Correspondingly, DM patients displayed elevated plasma concentrations of VLDL particles (XXL to S) and lower numbers of HDL particles (XL to M) (Fig. 2g). Furthermore, DM patients had increased amounts of branched-chain amino acids (valine (p = 0.011), leucine (p = 0.093), isoleucine (p = 1.29E−4) (Fig. 3h)), a subclass of amino acids associated with insulin resistance [33], while glutamine levels were lower (p = 5.50E−4) (Fig. 3g).
In contrast, TB patients presented with signs of wasting disease as the majority of metabolites were decreased compared to HC (Fig. 2c). Most notably, TB patients had low levels of amino acids (e.g. histidine (p = 5.36E−10) (Fig. 3e), glutamine (p = 7.38E−4) (Fig. 3g), alanine (p = 1.29E−4)), serum cholesterol (p = 1.72E−4) and total fatty acids (p = 1.32E−3), including a prominent reduction in polyunsaturated fatty acids (p = 3.36E−6), as well as decreased amounts of phospholipid metabolites. Except for inflammation marker Gp (glycoprotein acetylation) (p = 4.12E−8) (Fig. 3l), TB patients only displayed elevated levels of two metabolites: phenylalanine (p = 3.07E−4) (Fig. 3f), an aromatic amino acid, and β-hydroxybutyrate (p = 0.017) (Fig. 3k), a ketone body. Intriguingly, the average low-density lipoprotein (LDL) particle diameter was very significantly increased as a result of TB (Fig. S1a) through a relative decrease in smaller (sizes M to S) LDL and HDL particles (Fig. 2h).
3.3. TB-DM Patients Display the Most Prominent Metabolic Characteristics of Both Diseases
As DM and TB displayed divergent effects on plasma metabolite concentrations, we next compared the effect of TB-DM comorbidity with the single disease states. Compared to DM (Fig. 2e), TB-DM patients showed a similar metabolic signature as TB vs. HC, i.e. reduced levels of amino acids (e.g. histidine (p = 8.87E−11) (Fig. 3e), alanine (p = 6.02E−8)) combined with increased concentrations of phenylalanine (p = 8.57E−8) (Fig. 3f) and β-hydroxybutyrate (p = 0.013) (Fig. 3k). Although serum cholesterol and total fatty acids were unaffected (p = 0.738 and 0.692 respectively), VLDL-TG levels were lower in TB-DM compared to DM patients (p = 6.40E−3) (Fig. 3a), which is consistent with a decreased amount of VLDL particles (XL to M) (Fig. 2j). Similar to the TB group, the average LDL diameter was significantly larger (Fig. S1a), which was also the case for HDL due to a relative increase in larger (XL to L) vs. smaller (M to S) particles (Fig. 2j).
Importantly, TB-DM patients displayed major hallmarks of DM when compared to the TB group (Fig. 2a), namely elevated plasma concentrations of glucose (p = 6.21E−9), VLDL-TG (p = 5.37E−4) (Fig. 3a), ApoB (p = 8.06E−3), VLDL-C (p = 4.43E−4), total fatty acids (p = 5.37E−4) and branched-chain amino acids (valine (p = 0.016), leucine (p = 0.016), isoleucine (p = 3.41E−3) (Fig. 3h)). Moreover, TB-DM comorbidity reduced glutamine levels even further compared to either TB or DM alone (p = 0.036 and 7.19E−4 respectively) (Fig. 3g). Similar to DM, VLDL particle levels were elevated, accompanied by an additional increase in LDL particles (M to S) (Fig. 2f).
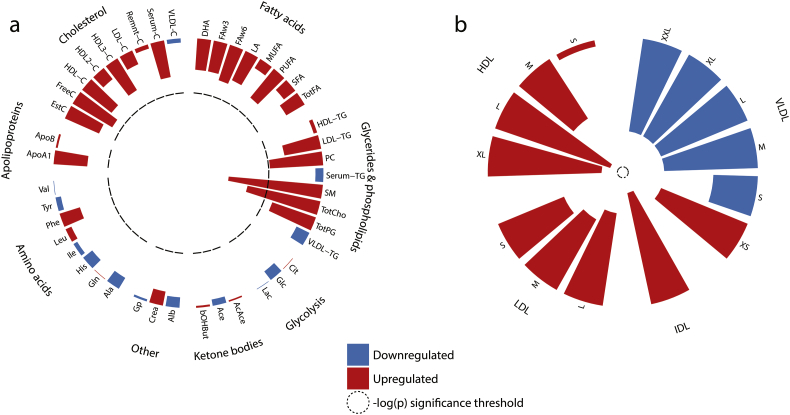
3.4. TB-DM Interaction Increased Levels of Phospholipid Metabolites and Atherogenic Lipoprotein Remnants
In addition to the pairwise comparison of all disease groups, we investigated whether TB-DM comorbidity resulted in disease interaction-specific effects by adding an interaction term (TB*DM) to the multiple linear regression model. Resulting FDR-corrected p-values (Fig. 4) reflect changes in metabolite levels which were not explained by the effects of TB or DM alone. We identified a TB-DM interaction-specific increase in phospholipid metabolite levels (Fig. 4a), i.e. sphingomyelins (p = 5.57E−3) (Fig. 3i), phosphatidylcholine (p = 0.054), total cholines (p = 0.017) (Fig. 3j) and phosphoglycerides (p = 0.079). Furthermore, TB-DM interaction trended towards increased atherogenic lipoprotein remnants, XS VLDL (p = 0.115) and IDL (p = 0.072), while decreasing the concentrations of larger VLDL particles (Fig. 4b). This relative increase in remnant-like particles is also apparent from our pairwise comparisons (Fig. 2f: TB-DM vs TB–XS VLDL: p = 1.34E−3, IDL: p = 0.101; Fig. 2j: TB-DM vs DM–XS VLDL: p = 0.055, IDL: p = 0.055). Finally, the data suggest TB-DM is associated with elevated LDL-TG concentrations when compared to HC, TB or DM patients (Fig. 2d, a, e: p = 7.80E−3, 8.40E−3 and 7.18E−3, respectively), which could be driven by a relative enrichment of LDL particle TG content (Fig. S1c) due to diminished LDL lipolysis.
Fig. 4.
TB-DM interaction-specific effect on metabolic profiles. 1H NMR-spectroscopy plasma metabolites were analyzed by multiple linear regression and the resulting –log-transformed FDR-corrected p-values (t-test) are displayed as circular histograms for a subset of 44 metabolic parameters (a) and for the particle concentrations of 14 lipoprotein subclasses (b). Metabolites are grouped by metabolite family and lipoproteins by density. Up- and downregulated metabolites are indicated by red and blue bars respectively and the significance threshold (p = 0.05) is indicated by a dashed line.
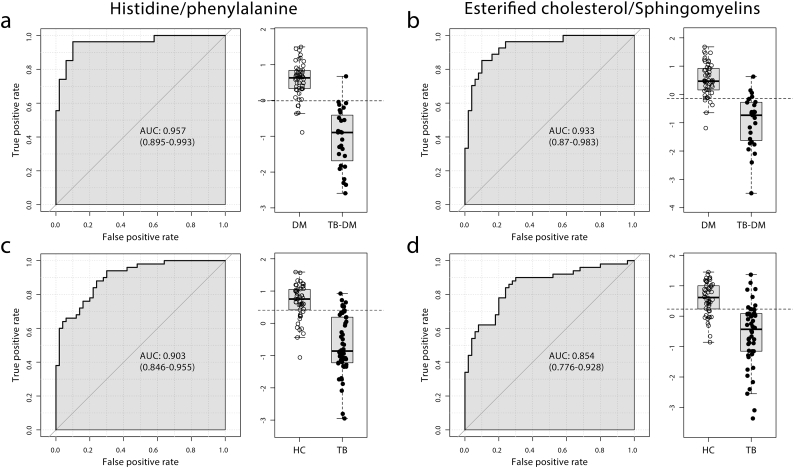
3.5. Histidine/Phenylalanine Ratio is a Potential Biomarker for TB Regardless of DM Status
We next performed univariate analyses using MetaboAnalyst 3.5 to identify metabolic markers with potential to identify active TB regardless of DM-status. The top 20 metabolite ratios with the highest individual p-values were tested in conjunction with our 44 parameter set. To compensate for potential overfitting, the analysis was stratified by DM-status and only biomarkers with AUC values of >0.8 in both analyses were considered (Table 2).
Table 2.
Univariate biomarker analysis.
| TB vs HC |
TB-DM vs DM |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | AUC | t-test | 95% CI | Biomarker | AUC | t-test | 95% CI |
| His/Gp | 0.934 | 2.97E−17 | 0.888–0.971 | His/Phe | 0.957 | 3.88E−17 | 0.895–0.993 |
| His/Phe | 0.903 | 2.81E−14 | 0.846–0.955 | EstC/SM | 0.933 | 1.44E−12 | 0.870–0.983 |
| His | 0.888 | 8.52E−14 | 0.805–0.941 | Serum-C/FreeC | 0.903 | 2.10E−10 | 0.832–0.964 |
| Serum-C/EstC | 0.885 | 8.15E−11 | 0.821–0.943 | EstC/FreeC | 0.902 | 4.13E−10 | 0.828–0.963 |
| Serum-C/FreeC | 0.885 | 5.96E−12 | 0.809–0.942 | Serum-C/EstC | 0.902 | 1.82E−09 | 0.830–0.961 |
| EstC/FreeC | 0.885 | 1.38E−11 | 0.815–0.938 | His | 0.901 | 1.22E−12 | 0.823–0.971 |
| Ala/Gp | 0.876 | 9.02E−13 | 0.811–0.944 | ApoB/Gp | 0.897 | 2.79E−11 | 0.819–0.958 |
| ApoB/Gp | 0.871 | 1.20E−12 | 0.788–0.933 | Ala/Gp | 0.896 | 2.43E−12 | 0.803–0.967 |
| EstC/SM | 0.854 | 2.34E−10 | 0.776–0.928 | His/Gp | 0.895 | 3.97E−12 | 0.800–0.973 |
| Ile/Gp | 0.850 | 2.14E−10 | 0.771–0.922 | Ile/Gp | 0.894 | 1.42E−10 | 0.782–0.975 |
The histidine/phenylalanine ratio was the biomarker with the highest accuracy for classifying TB-DM vs. DM (AUC: 0.957, 95% CI: 0.895–0.993, Fig. 5a) and the second highest for TB vs. HC (AUC: 0.903, 95%CI: 0.846–0.955, Fig. 5c). Interestingly, a decreased ratio of esterified to free cholesterol (Fig. 3c) also showed strong predictive power for TB in both groups, with AUCs of 0.885 (95% CI: 0.815–0.938) and 0.902 (95% CI: 0.828–0.963) for TB and TB-DM respectively. The ratio of esterified cholesterol/sphingomyelin was especially predictive of TB in comorbidity patients (AUC: 0.933, 95% CI: 0.870–0.983, Fig. 5b), but also apparent without DM (AUC: 0.854, 95%CI: 0.776–0.928, Fig. 5d). Many of the remaining overlapping markers involved ratios to the non-specific inflammatory marker Gp, which was strongly elevated in TB patients.
Fig. 5.
TB biomarker analysis. Biomarkers and ratios from the 44 metabolite set were used to identify TB status in DM-only (n = 50) vs. TB-DM (n = 27) (a, b) and in HC (n = 50) vs. TB-only (n = 50) (c,d) patients. ROC curves for the histidine/phenylalanine (a, c) and esterified cholesterol/sphingomyelins (b, d) ratios are plotted. Individual patients are shown as dots in accompanying boxplots with cut-off (dashed line).
Finally, we investigated possible correlations between individual metabolites and TB severity as signified by sputum culture time to positivity (TTP), a measure which reflects mycobacterial load. Univariate analysis showed a trending inverse correlation between TTP and levels of individual and total branched-chain amino acids, as well as the ketone bodies acetoacetate and β-hydroxybutyrate (Fig. S2a–f). However, these findings need to be corroborated in follow-up studies with greater power.
4. Discussion
Although accumulating epidemiological evidence from recent and older studies indicates a link between TB and DM, the underlying pathophysiological mechanisms remain elusive. Since lipids play important roles in both diseases, we studied the impact of TB disease on host lipid metabolism and analyzed how this relates to TB-DM comorbidity. Our results show that plasma from patients with TB displayed signatures of extensive wasting, represented by lower levels of amino acids, cholesterol, fatty acids and phospholipid metabolites, while conversely DM was associated with dyslipidemia. Plasma from TB-DM patients showed the most prominent metabolic characteristics of both diseases, i.e. wasting, exemplified by reduced concentrations of amino acids (histidine, alanine, glutamine), and dyslipidemia in the form of high levels of VLDL-TG and low HDL cholesterol. Although TB-DM-associated dyslipidemia was less severe than that of DM, the inter-individual heterogeneity was high. This could suggest that there are TB-DM patients in whom the effects of wasting dominate over the DM-associated dyslipidemia, as well as patients with the opposite metabolic phenotype. Alternatively, it is possible that this inter-individual variation is the result of differences in DM-duration, as the TB-DM group comprised a mix of long-term diabetics and patients who were recently diagnosed with DM (Table 1).
In addition to its overlapping effects with either TB or DM metabolic profiles, we find that TB-DM comorbidity leads to a relative increase in remnant-like plasma lipoprotein particles (XS-VLDL, IDL) which are strongly associated with cardiovascular disease and atherosclerosis [34]. The formation of remnant-like particles depends on the relative contributions of lipoprotein lipase (LPL) and hepatic triglyceride lipase (HTGL) to lipoprotein hydrolysis: the former initiates the cascade through lipolysis of chylomicrons and nascent VLDL while the latter preferentially converts smaller VLDL and IDL particles to LDL [35]. Furthermore, both play roles in HDL metabolism and have been shown to have opposing effects on HDL size [36]. The elevated amount of remnant-like particles and LDL TG-enrichment indicate relatively decreased HTGL/LPL activity in TB-DM patients. This is further supported by a reduction in smaller HDL particles (sizes M to S) in plasma from both TB and TB-DM patients and the relative increase in LDL diameter in both groups as a result of TB. Additionally, TB-DM interaction leads to a specific increase in sphingomyelins and related phospholipid metabolites. It has been demonstrated that hepatic sphingolipid synthesis is increased under inflammatory conditions [37], specifically through increased levels of the rate-limiting enzyme serine palmitoyltransferase (SPT). High levels of circulating sphingomyelin are associated with coronary artery disease as they increase the atherogenic potential of lipoproteins [38] and also function as a physiological inhibitor of HTGL activity [39].
The ratio of histidine/phenylalanine in plasma was a potential biomarker for TB irrespective of DM-status. This result is congruent with earlier metabolomic profiling of TB which reported changes in histidine and/or phenylalanine metabolism in plasma [40] and urine [41], and will require further validation in independent cohorts with larger sample sizes. It is possible that a decreased histidine/phenylalanine ratio reflects non-specific oxidative stress and/or inflammation as similar changes were demonstrated in other inflammatory conditions, including rheumatoid arthritis [42], sepsis [43], obesity [44] and cancer [45]. Furthermore, TB disease status was associated with a decreased ratio of esterified to free cholesterol in plasma. Cholesterol esterification is regulated by lecithin–cholesterol acyltransferase (LCAT), a liver-produced enzyme which is bound to HDL particles in plasma. A possible explanation for this shift is the ability of sphingomyelin to inhibit LCAT activity [46,47] as the esterified cholesterol/sphingomyelin ratio also showed strong predictive power, particularly in TB-DM patients. Furthermore, LCAT and HTGL levels were shown to be decreased during the acute phase response, the early reaction of the body to infection or inflammation, strengthening the notion that these enzymes could be deregulated during TB. Taken together, our results support a model in which the unique lipid profile of TB-DM patients is the outcome of the interaction between DM-induced dyslipidemia and TB-induced changes in lipoprotein metabolism.
We postulate that the pro-atherogenic phenotype of TB-DM patients might contribute to TB susceptibility or reactivation. Some striking similarities exist between the progression of TB and atherosclerosis. Pivotal in both pathologies is the formation of lipid-loaded foamy macrophages [15]. Mtb has been demonstrated to reprogram macrophage lipid metabolism for its own benefit as it requires host-derived lipids as nutrient source for survival and replication [[48], [49], [50], [51]]. Interestingly, we find that TB leads to increased plasma levels of β-hydroxybutyrate, a ketone body which has been implicated in Mtb-induced intracellular lipid droplet formation [50]. A recent study showed that DM-associated dyslipidemia exacerbates the severity of caseous lung necrosis in TB patients [52], supporting the hypothesis that aberrant lipid levels negatively affect TB outcome. Paradoxically, others reported a DM-independent protective effect of high BMI on the risk of TB [13,53], however it has been suggested that this association depends on the local TB incidence [54]. Regardless, it would be of great interest to compare lipid profiles in obese TB patients with or without DM.
Some inherent weaknesses in study design will have to be addressed in future follow-up studies as the overall statistical power was inadequate to control for all possible confounding factors. Firstly, differences in both the DM and TB-DM populations could be related to the use of anti-diabetic medication such as insulin, metformin or statins, all of which affect glucose and lipid metabolism. Secondly, some of the observed effects of TB and/or DM could have been driven by differences in BMI and not by disease state as such. However, correcting for this could obscure genuine metabolic effects induced by TB, DM or both which are (partially) mediated through changes in energy expenditure or storage and therefore reflected by the patients' BMI. Thirdly, it was not possible to correctly control for differences in smoking habits as this information was not available for the HC group. Finally, the studied patient population was ethnically uniform, the majority being from the Colored population of South Africa. It will be important to explore how the results of this study translate to patient populations with different ethnic backgrounds.
In conclusion, TB-DM comorbidity results in a distinctive lipid profile with pro-atherogenic properties, including significantly elevated levels of sphingomyelins and remnant-like lipoprotein particles. These results will have to be validated in independent cohort studies, and simultaneous investigation of HTGL, LPL, SPT and LCAT activity is warranted. Our findings may have therapeutic implications and encourage more extensive studies into the possible beneficial effects of lipid-lowering drugs on TB outcome in TB-DM patients. We suggest that after initiation of antibiotic treatment TB-DM patients should receive life-style change counseling, and it may be valuable to determine blood lipid profiles. Statin or other lipid-lowering treatments could be started in case of aberrant lipid levels or existing cardiovascular malconditions. Furthermore, systematically determining TG and/or cholesterol levels at TB diagnosis could help identifying patients at risk.
The following are the supplementary data related to this article.
Mean concentrations, standard deviations and numbers of successful measurements of the 44 metabolite subset and size-specific lipoprotein particles.
Raw measurement results of 225 metabolic parameters determined by NMR spectroscopy.
Supplementary Figures S1 and S2.
Funding Sources
This study was supported by the TANDEM (Tuberculosis and Diabetes Mellitus) Grant of the ECFP7 (European Union's Seventh Framework Programme) under Grant Agreement No. 305279 for patient recruitment, data collection and analysis; and by TBVAC2020 Grant of EC HOR2020 (Grant Agreement No. 643381) for analysis.
Conflicts of Interest
All authors: no reported conflicts.
Author Contributions
Conceived and designed the study: SAJ THMO GW. Supervised sample collection and selection: KR, LK, GW. Analyzed the data: FV EvdA SAJ. Wrote the paper: FV SAJ THMO.
Acknowledgements
We thank the members of the TANDEM consortium for discussion of the data. We are grateful to all study participants, to the clinical team Stephanus T. Malherbe, Elizna Maasdorp, Charmaine Abrahams and Shriley McAnda as well as to the database manager Kim Stanley.
References
- 1.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5(7) doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2016. Diabetes & TB - Fact sheet. [Geneva, Switzerland] [Google Scholar]
- 3.Baker M.A., Harries A.D., Jeon C.Y., Hart J.E., Kapur A., Lonnroth K. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niazi A.K., Kalra S. Diabetes and tuberculosis: A review of the role of optimal glycemic control. J Diabetes Metab Disord. 2012;11(1):28. doi: 10.1186/2251-6581-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensel R.L., Kempker R.R., Tapia J., Oladele A., Blumberg H.M., Magee M.J. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int J Tuberc Lung Dis. 2016;20(1):71–78. doi: 10.5588/ijtld.15.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar Nathella P., Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152(1):13–24. doi: 10.1111/imm.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation . 8th ed. 2013. Diabetes atlas. Brussels, Belgium 2017. [Report No] [Google Scholar]
- 8.van Crevel R., Dockrell H.M., Consortium T TANDEM: Understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol. 2014;2(4):270–272. doi: 10.1016/S2213-8587(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 9.Taskinen M.R., Boren J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239(2):483–495. doi: 10.1016/j.atherosclerosis.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Haas M.E., Attie A.D., Biddinger S.B. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab. 2013;24(8):391–397. doi: 10.1016/j.tem.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwenk A., Macallan D.C. Tuberculosis, malnutrition and wasting. Curr Opin Clin Nutr Metab Care. 2000;3(4):285–291. doi: 10.1097/00075197-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Khan A., Sterling T.R., Reves R., Vernon A., Horsburgh C.R. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174(3):344–348. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 13.Lonnroth K., Williams B.G., Cegielski P., Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 14.van Crevel R., Karyadi E., Netea M.G., Verhoef H., Nelwan R.H., West C.E. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87(2):758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 15.Russell D.G., Cardona P.J., Kim M.J., Allain S., Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee W., VanderVen B.C., Fahey R.J., Russell D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem. 2013;288(10):6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M.J., Wainwright H.C., Locketz M., Bekker L.G., Walther G.B., Dittrich C. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2(7):258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soh A.Z., Chee C.B., Wang Y.T., Yuan J.M., Koh W.P. Dietary cholesterol increases the risk whereas PUFAs reduce the risk of active tuberculosis in Singapore Chinese. J Nutr. 2016;146(5):1093–1100. doi: 10.3945/jn.115.228049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martens G.W., Arikan M.C., Lee J., Ren F., Vallerskog T., Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76(8):3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martens G.W., Vallerskog T., Kornfeld H. Hypercholesterolemic LDL receptor-deficient mice mount a neutrophilic response to tuberculosis despite the timely expression of protective immunity. J Leukoc Biol. 2012;91(6):849–857. doi: 10.1189/jlb.0311164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parihar S.P., Guler R., Khutlang R., Lang D.M., Hurdayal R., Mhlanga M.M. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209(5):754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 22.Lai C.C., Lee M.T., Lee S.H., Hsu W.T., Chang S.S., Chen S.C. Statin treatment is associated with a decreased risk of active tuberculosis: An analysis of a nationally representative cohort. Thorax. 2016;71(7):646–651. doi: 10.1136/thoraxjnl-2015-207052. [DOI] [PubMed] [Google Scholar]
- 23.Dutta N.K., Bruiners N., Pinn M.L., Zimmerman M.D., Prideaux B., Dartois V. Statin adjunctive therapy shortens the duration of TB treatment in mice. J Antimicrob Chemother. 2016;71(6):1570–1577. doi: 10.1093/jac/dkw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su V.Y., Su W.J., Yen Y.F., Pan S.W., Chuang P.H., Feng J.Y. Statin use is associated with a lower risk of TB. Chest. 2017;152(3):598–606. doi: 10.1016/j.chest.2017.04.170. [DOI] [PubMed] [Google Scholar]
- 25.Lobato L.S., Rosa P.S., Ferreira Jda S., Neumann Ada S., da Silva M.G., do Nascimento D.C. Statins increase rifampin mycobactericidal effect. Antimicrob Agents Chemother. 2014;58(10):5766–5774. doi: 10.1128/AAC.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soininen P., Kangas A.J., Wurtz P., Suna T., Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192–206. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 27.Definition and diagnosis of diabetes mellitus and intermediate Hyperglycemia: Report of a WHO/IDF consultation. Geneva. 2006. [Google Scholar]
- 28.Wurtz P., Kangas A.J., Soininen P., Lawlor D.A., Davey Smith G., Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -Omic technologies. Am J Epidemiol. 2017;186(9):1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J., Wishart D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics. 2016;55:14 0 1–0 91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 30.Lê Cao K.A., Rohart F., Gonzalez I., Déjean S., Gautier B., Bartolo F. MixOmics: Omics data integration project. 2017. https://CRAN.R-project.org/package=mixOmics Available from.
- 31.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7) doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladroue C. PhenotypicForest. R package version 0.3 2015. https://github.com/chrislad/phenotypicForest Available from.
- 33.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varbo A., Benn M., Nordestgaard B.G. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141(3):358–367. doi: 10.1016/j.pharmthera.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg I.J., Le N.A., Paterniti J.R., Jr., Ginsberg H.N., Lindgren F.T., Brown W.V. Lipoprotein metabolism during acute inhibition of hepatic triglyceride lipase in the cynomolgus monkey. J Clin Invest. 1982;70(6):1184–1192. doi: 10.1172/JCI110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tani M., Horvath K.V., Lamarche B., Couture P., Burnett J.R., Schaefer E.J. High-density lipoprotein subpopulation profiles in lipoprotein lipase and hepatic lipase deficiency. Atherosclerosis. 2016;253:7–14. doi: 10.1016/j.atherosclerosis.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Memon R.A., Holleran W.M., Moser A.H., Seki T., Uchida Y., Fuller J. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18(8):1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 38.Dong J., Liu J., Lou B., Li Z., Ye X., Wu M. Adenovirus-mediated overexpression of sphingomyelin synthases 1 and 2 increases the atherogenic potential in mice. J Lipid Res. 2006;47(6):1307–1314. doi: 10.1194/jlr.M600040-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Yang P., Subbaiah P.V. Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins. Biochim Biophys Acta. 2015;1851(10):1327–1336. doi: 10.1016/j.bbalip.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner J., 3rd, Parida S.K., Maertzdorf J., Black G.F., Repsilber D., Telaar A. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luies L., Loots D. Tuberculosis metabolomics reveals adaptations of man and microbe in order to outcompete and survive (vol 12, 40, 2016) Metabolomics. 2016;12(3) [Google Scholar]
- 42.Gerber D.A. Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. J Clin Invest. 1975;55(6):1164–1173. doi: 10.1172/JCI108033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su L., Li H., Xie A., Liu D., Rao W., Lan L. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0121933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu Y.C., Feng R.N., Hou Y., Li K., Kang Z., Wang J. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br J Nutr. 2012;108(1):57–61. doi: 10.1017/S0007114511005289. [DOI] [PubMed] [Google Scholar]
- 45.Neurauter G., Grahmann A.V., Klieber M., Zeimet A., Ledochowski M., Sperner-Unterweger B. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. 2008;272(1):141–147. doi: 10.1016/j.canlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Subbaiah P.V., Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin-cholesterol acyltransferase reaction. J Biol Chem. 1993;268(27):20156–20163. [PubMed] [Google Scholar]
- 47.Subbaiah P.V., Jiang X.C., Belikova N.A., Aizezi B., Huang Z.H., Reardon C.A. Regulation of plasma cholesterol esterification by sphingomyelin: Effect of physiological variations of plasma sphingomyelin on lecithin-cholesterol acyltransferase activity. Biochim Biophys Acta. 2012;1821(6):908–913. doi: 10.1016/j.bbalip.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyron P., Vaubourgeix J., Poquet Y., Levillain F., Botanch C., Bardou F. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4(11) doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahajan S., Dkhar H.K., Chandra V., Dave S., Nanduri R., Janmeja A.K. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188(11):5593–5603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 50.Singh V., Jamwal S., Jain R., Verma P., Gokhale R., Rao K.V. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12(5):669–681. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Ouimet M., Koster S., Sakowski E., Ramkhelawon B., van Solingen C., Oldebeken S. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol. 2016;17(6):677–686. doi: 10.1038/ni.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong Z., Shi J., Dorhoi A, Zhang J., Soodeen-Lalloo A.K., Chen W. Hemostasis and lipoprotein indices signify exacerbated lung injury in tuberculosis with diabetes comorbidity. Chest. 2018;153(5):1187–1200. doi: 10.1016/j.chest.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Lin H.H., Wu C.Y., Wang C.H., Fu H., Lonnroth K., Chang Y.C. Association of obesity, diabetes, and risk of tuberculosis: Two population-based cohorts. Clin Infect Dis. 2018;66(5):699–705. doi: 10.1093/cid/cix852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Li X., Xin H., Li H., Li M., Lu W. Association of Body Mass Index with the tuberculosis infection: A population-based study among 17796 adults in Rural China. Sci Rep. 2017;7 doi: 10.1038/srep41933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean concentrations, standard deviations and numbers of successful measurements of the 44 metabolite subset and size-specific lipoprotein particles.
Raw measurement results of 225 metabolic parameters determined by NMR spectroscopy.
Supplementary Figures S1 and S2.