Abstract
Vascular disease is a major cause of death worldwide, and the growing need for replacement vessels is not fully met by autologous grafts or completely synthetic alternatives. Tissue engineering has emerged as a compelling strategy for the creation of blood vessels for reconstructive surgeries.
One promising method to obtain a suitable vessel scaffold is decellularization of donor vascular tissue followed by recellularization with autologous cells. To prevent thrombosis of vascular grafts, a confluent and functional autologous endothelium is required, and researchers are still looking for the optimal cell source and recellularization procedure.
Recellularization of a decellularized scaffold with only a small volume of whole blood was recently put forward as a feasible option. Here we show that, in contrast to the published results, this method fails to re-endothelialize decellularized veins. Only occasional nucleated cells were seen on the luminal surface of the scaffolds. Instead, we saw fibrin threads, platelets and scattered erythrocytes. Molecular remnants of the endothelial cells were still attached to the scaffold, which explains in part why earlier results were misinterpreted.
Decellularized vascular tissues may still be the best scaffolds available for vascular tissue engineering. However, for the establishment of an adequate autologous endothelial lining, methods other than exposure to autologous whole blood need to be developed.
Keywords: Tissue engineering, Decellularization, Recellularization, Endothelial progenitor cells, Personalized tissue engineered vein
Highlights
-
•
Recellularization with peripheral blood was recently put forward as a method to create tissue engineered blood vessels.
-
•
We show that this method fails to re-cellularize decellularized vein scaffolds.
-
•
Fibrin, platelets and erythrocytes are deposited on the luminal side of the scaffolds instead of a functional endothelium.
Vascular disease is a major cause of death worldwide, and the growing need for replacement vessels is not fully met by autologous grafts or synthetic alternatives. Tissue engineering has emerged as a compelling strategy for the creation of blood vessels for reconstructive surgeries. One promising method to obtain a suitable vessel scaffold is decellularization of donor vascular tissue followed by recellularization with autologous cells.
Recellularization of a decellularized scaffold with only a small volume of whole blood was recently presented as a feasible option. We show that, in contrast to the published results, this method fails to re-endothelialize decellularized veins.
1. Introduction
Cardiovascular disease is the leading cause of death globally [1,2]. A common treatment for patients with advanced vascular disease is the use of vascular grafts to replace or bypass damaged or obstructed vessels [3]. Autologous vessel grafts remain the gold standard, but not all patients have sufficient or healthy autologous veins for vascular grafting [4]. Synthetic alternatives like Dacron and polytetrafluorethylene are being used with relative success for some applications needing large diameter (>6 mm) grafts, but for smaller diameter applications, synthetic grafts tend to suffer unacceptably high failure rates [5, 6, 7]. In addition, for cases requiring more than just a new conduit, like reconstructive vein surgery where valve function is essential, these alternatives are not optimal [8,9].
One technique utilized to make a vascular scaffold is to decellularize allogeneic vascular tissues. Decellularization refers to the removal of antigenic cellular material from tissue [10]. The starting point can be native tissue, or extracellular matrix (ECM) produced from cells ex vivo [11]. By using decellularized natural matrices, one can take advantage of the intrinsic properties of the tissue, including ECM composition, biocompatibility, shape and mechanical properties [12,13]. The decellularization process may involve a variety of chemical agents, solvents and enzymes, and must balance the task of removing all cellular material with the aim of preserving composition, biological activity and mechanical integrity of the remaining ECM [12]. Inadequate decellularization could potentially cause immune reactions and graft failure, while an aggressive decellularization process may remove essential ECM components, thus altering the mechanical properties of the tissue [14,15].
One challenge of using decellularized vessels might be limited recellularization in vivo, caused by the dense ECM of the vessel wall or chemical damage to the ECM in the decellularization process [16,17]. The limited success of current commercially available decellularized grafts has been, in part, explained with their lack of cellularity on implantation [18,19]. A viable endothelium is important to suppress thrombosis of smaller caliber vessel grafts, especially crucial for decellularized grafts with their exposed collagen luminal wall surface [20]. This makes a successful recellularization step essential, and a number of different cell sources and strategies have been employed [13,21]. The ideal cell source would be one that is readily available in sufficient amounts, can be obtained by a minimally invasive procedure, and that would willingly settle in the graft.
In 2014, Olausson et al. [22] reported two pediatric cases involving clinical transplantation of tissue engineered decellularized allogeneic veins. The veins were reported to be recellularized following exposure to 25 mL of autologous peripheral whole blood. It is known that normal human adult peripheral blood contains a small number of circulating endothelial cells (ECs), and cell cultures from blood have demonstrated endothelial outgrowth [23]. Using cells obtained from a simple blood sample for recellularization, this whole blood procedure would sidestep the time-consuming processes of harvest, isolation and expansion, and would present a promising and available approach.
The number of circulating ECs from peripheral blood has previously been estimated to four cells per mL of blood, or even less [24, 25, 26]. If the estimated 100 ECs from 25 mL blood could fully endothelialize a graft luminal surface of at least 10 cm2 in <10 days, that would indicate a great expansive potential of these cells. This would contradict published evidence, and would suggest that there may be other cells present in blood which acquire an endothelial phenotype and contribute to the formation of neointima of a vessel graft.
Since the theoretical background on the prevalence of blood ECs does not fully integrate with the positive results on blood re-endothelialization published in the Olausson et al. study [22], we decided to re-evaluate the procedure with respect to both the quantity and the quality of the re-endothelializing cells. Of note, although these are new experiments, the procedure and the laboratory performing the de-cellularization and the re-endothelialization were the same as in the re-evaluated study.
2. Methods
2.1. Harvest of Vein Segments
The Norwegian Institutional Review Boards and Ethics Committees approved all research protocols. All methods were performed in accordance with relevant guidelines and regulations.
All veins used for decellularization and recellularization were harvested at Oslo University Hospital (OUH), Norway. Informed consent had been obtained. Donors were free from infectious disease. Segments from the femoral vein (N = 9), about 5–8 cm in length, were harvested from two adult human cadavers by using vascular surgical techniques, with careful ligation of all side branches. The segments were flushed thoroughly with saline to remove all blood. Vein samples were stored in individual labelled containers filled with PBS supplemented with antibiotics (0.5% penicillin, 0.5% streptomycin, and 0.5% amphotericin B), and kept at 2–8 °C for all transportation events. Shipping time between Oslo and Gothenburg never exceeded 24 h. Samples from two cadaver veins were kept at OUH for analysis.
Native control veins were obtained from remaining saphenous vein segments after bypass operations with the informed consent of patients. Vein samples were stored in PBS supplemented with antibiotics (0.5% penicillin, 0.5% streptomycin, and 0.5% amphotericin B) at 4 °C for maximum 24 h awaiting further processing.
2.2. Decellularization
The decellularization process was carried out by NovaHep, as described in Olausson et al. [22] Minor changes were introduced in a 2015 publication by the same group [9], considered improvements to the procedure. These are mentioned specifically in the following text. The improvements were used also in the current study. The segments were washed for 72 h in distilled water, and decellularized by 9–14 cycles of sequential incubation in three different decellularization solutions (7 cycles in Olausson et al. [22]). The solutions used were 1% Triton, 1% tri-n-butyl phosphate (TnBP), and 4 mg/L DNAse.
All steps were performed at 37 °C with agitation and continuous perfusion with decellularization solution. The segments were immersed in each solution for 4 h per cycle (3 h in Olausson et al. [22]), and rinsed in distilled water between each chemical incubation. All solutions used for decellularization contained 0.5% penicillin, 0.5% streptomycin, and 0.5% amphotericin B.
After completion of the decellularization process, the segments were washed in PBS for 48 h, sterilized in EtOH and frozen at −80 °C. The complete decellularization process lasted 10 days. Samples for characterization were shipped to OUH after the sterilization step.
2.3. Recellularization
The recellularization was conducted by NovaHep, using a patented procedure developed in the lab of Sumitran-Holgersson at Sahlgrenska University Hospital [22]. Peripheral venous blood for recellularization was collected from three healthy donors aged 48, 52 and 63 in sterile heparin-coated Vacutainer tubes and transported to the laboratory within 2 h. The entire recellularization process was carried out under sterile conditions at 37 °C. Before recellularization, the veins were perfused with heparin at a concentration of 50 IU/mL in phosphate buffered saline (PBS) for 2 h. The heparin was drained off, and whole blood was immediately perfused for 48 h at 2 mL/min speed. 25 mL blood was used for recellularization of each sample.
After 48 h the blood was drained off, and the vein was rinsed with PBS containing 1% penicillin-streptomycin-amphotericin until all traces of blood were completely removed. The vein was subsequently perfused for 4 days with EC medium, and the vein segments were immersed in EC medium throughout the incubation period. Complete endothelial medium was prepared with MCDB131 (Life Technologies, Stockholm, Sweden) basal medium supplemented with 10% heat-inactivated human AB serum (Life Technologies), 1% glutamine, 1% penicillin-streptomycin-amphotericin, and EGM-2 SingleQuot kit (CC4176; Lonza). The recellularization method originally included an additional 4 day incubation step with perfusion of the graft with smooth muscle cell medium. This step was later abandoned, as Olausson et al. did not find that it significantly affected either the endothelialization or the proliferation of ECs in the graft [22]. In line with this, the vessels were not perfused with smooth muscle cell medium for the present study.
After recellularization, the segments were washed in PBS and shipped to OUH for further analysis. Upon arrival in Oslo, all segments were immediately rinsed in PBS and processed for downstream analysis.
2.4. DNA Quantification
Vein samples were freeze dried and DNA was extracted using DNeasy blood and tissue kit (Qiagen) according to the manufacturer's instructions.
2.5. Histology
Vein samples were fixed in 4% paraformaldehyde overnight. The samples were dehydrated in gradients of ethanol and xylene, and embedded in paraffin according to standard protocol. Further, blocks were cut and mounted on SuperFrost Plus Adhesion Slides. 6 μm sections were deparaffinized and stained with hematoxylin and eosin (H&E) using standard laboratory technique. Sections were imaged in an Olympus BX51 microscope.
2.6. Immunohistochemistry
Vein samples were embedded in Frozen Section Medium (Richard-Allan Scientific Neg 50, Thermo Scientific) and frozen in dry ice-cooled isopenthane. Frozen tissue blocks were stored at —80C. The samples were cut in 8 um thick sections on a CryoStar™ NX70 Cryostat (Thermo Scientific™), mounted on SuperFrost Plus Adhesion slides and immediately post-fixed for 30 s in cold 95% ethanol. Slides were stored at —20C awaiting immunostaining.
Sections were immunostained for the presence of CD31 (1:50; Abcam #ab9498), von Willebrand factor (1:500; Abcam #ab6994), VEGFR-2 (1:200; Acris Antibodies TA337222), alfa smooth muscle actin (1:500, Abcam #ab7817), CD41 (1:1000; Abcam #ab11024) and fibrinogen (1:250; Abcam #ab118488). Antibodies were diluted in blocking buffer (5% goat serum/3% BSA in PBS), and slides were incubated at 4 °C overnight. Negative controls were made omitting the primary antibody. The secondary antibodies used were goat anti-rabbit IgG conjugated to Alexa 488 and goat anti-mouse IgG conjugated to Alexa 594 (Life Technologies), used at 1:500. Each immunostaining procedure was performed in triplicate and repeated at least twice per vein sample.
The stained sections were mounted with prolong gold antifade (Invitrogen), containing DAPI for nuclear staining. Imaging was done using an upright Nikon Eclipse E600 microscope equipped with an Olympus ColorView III camera.
2.7. Scanning Electron Microscopy
Vein samples were fixed in 2.5% glutaraldehyde for 24 h and dehydrated in increasing concentrations of ethanol. Samples were mounted on aluminum stubs, coated with platinum in a vacuum sputter coater and examined in a Philips XL30 ESEM LaB6 Electron Scanning Microscope. SEM imaging was performed in quadruplicate per sample.
2.8. En Face Microscopy
Vein samples were fixed in 4% PFA for 24 h, washed in PBS and cut open longitudinally. Triplicate samples were stained with 2 μg/mL Hoechst 34,580 (Invitrogen) for 5 min, rinsed in PBS and placed on glass slides endothelial side down. The samples were viewed with a Zeiss Axio Vert 1A inverted microscope.
2.9. Data Availability
The data generated during the current study are available from the corresponding author upon reasonable request.
3. Results
3.1. Generation and Characterization of de- and Recellularized Vessel Scaffolds
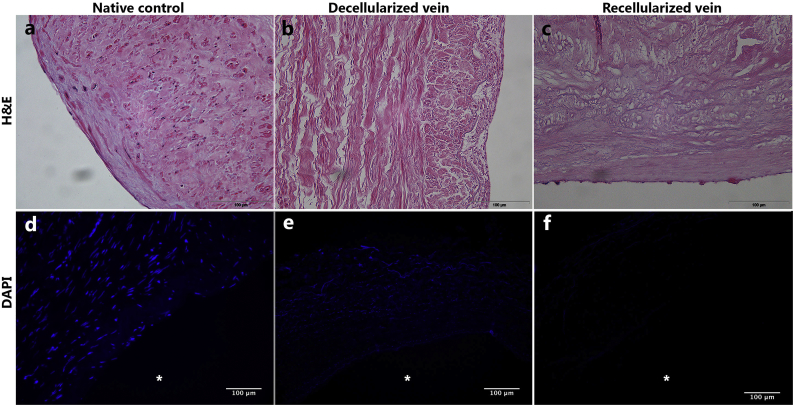
Treatment with 1% Triton and 1% tri-n-butyl phosphate through 14 decellularization cycles resulted in generation of pale and translucent veins. The vein segments did not shrink significantly, and maintained their tubular appearance. H&E staining of the control vessels showed layers of pink ECM with purple nuclei surrounded by darker pink cellular material dispersed throughout the vessel wall and along the lumen (Fig. 1a). DAPI staining showed rounded nuclei near the luminal surface, with more elongated nuclei lying parallel to the luminal surface within the outer layers. There was weak DAPI background staining between the nuclei (Fig. 1d). Staining of the cadaver veins prior to the decellularization process showed that cells were still present, and that the ECM had not been severely degraded during the five post mortem days before harvest (Fig. S1a). Decellularized veins contained multiple layers of ECM within the vessel wall, with no visible obviously intact nuclei (Fig. 1b), indicating that the cells and their nuclei had been disrupted in the decellulariazation process. DAPI staining of decellularized scaffolds showed some diffuse blue staining and perhaps scattered nuclei throughout the vessel wall, and some very rare blue condensations along the luminal surface which may represent nucleated cells (Fig. 1e). We did not observe the emergence of increased number of nuclei after the recellularization procedure (Figs. 1c; f). In particular, there was no evidence of increased numbers of nuclei along the luminal surface. Some background staining of the tunica adventitia was observed, however, there was no visible staining of the luminal surfaces. To quantify the removal of DNA from the vein wall, DNA was measured in three decellularized samples and found to be 105, 97 and 177 ng/mg tissue. For comparison, native saphenous veins contained 1110 ng/mg tissue, and cadaveric veins 723 ng/mg tissue.
Fig. 1.
Decellularization removes all nuclei throughout the vein, but no signs of successfull re-endothelialization.
(a-c) Representative images of H&E stained paraffin cross sections. a) Native control vein displays purple nuclei along the endothelial surface and throughout the tissue. b) Complete removal of nuclear components from tissue post decellularization. c) There are no visible nuclei along the luminal surface after recellularization.
(d-f) DAPI staining of cryo cross sections. d) Nuclei are evident along the endothelial surface and thoughout the tissue in the native vein. e) All intact nuclei are removed in the decellularization procedure. f) No visible nuclei along the luminal surface after the recellularization process.
3.2. Examination of the Luminal Surface
To evaluate the outcome of the recellularization process, we examined the luminal surface of the veins by en face microscopy. Hoechst staining demonstrated that native control vessels contained abundant round endothelial nuclei covering the intima, as well as the more elongated nuclei of the smooth muscle cells of the media (Fig. 2a). Decellularized vessels did not contain any nuclear structures, suggesting that intact cells were no longer present (Fig. 2b). However, there was some diffuse staining throughout the vessel wall, which may represent remaining DNA following disruption of the nuclei. The recellularized tissues also displayed some diffuse staining throughout the vessel wall, and with rare condensations of staining which may conceivably represent cell nuclei, but markedly different from the native vessels in both shape and amount (Fig. 2c).
Fig. 2.
A closer look at the luminal surface shows no evidence of recellularization.
(a-c) En face view of luminal surface. a) Native control vein displays a confluent endothelial cell layer, evident by round nuclear staining. Smooth muscle cells of the media are visible in the form of elongated nuclei. b) En face microscopy of decellularized vein shows complete removal of intact nuclei. c) En face view of recellularized vein sample reveals that a new endothelial cell layer has not emerged. Small black dots are thought to be red blood cells.
(d-i) Representative images of Scanning Electron Microscopy of luminal surface, 200× and 1000× magnifications. d and g) Native control veins display a confluent endothelial layer. e and h) Decellularized vein shows the complete removal of endothelial cells. The underlying ECM is exposed. f and i) The luminal side of recellularized vein does not contain anything resembling a confluent endothelial cell layer. The lumen appears to be covered by a fibrous meshwork.
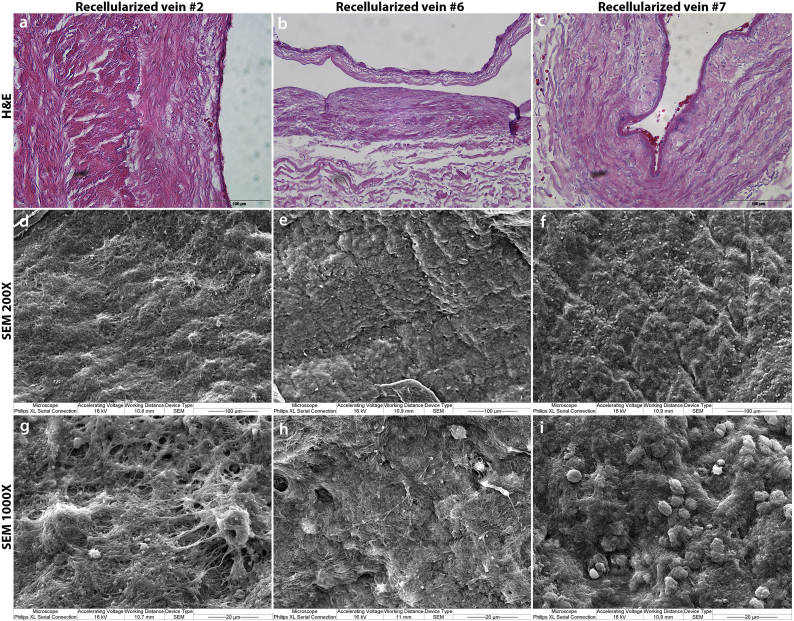
For a more comprehensive analysis of the inner structure and morphology of the veins, we conducted Scanning Electron Microscopy (SEM) studies on all samples. All control vessels displayed a densely packed surface with elongated, smooth endothelial cells lining up in the direction of the blood flow (Figs. 2d; 2 g). The decellularized samples showed a flattened surface void of cell-like structures, but with an abundance of exposed ECM fibers (Figs. 2e; 2 h). Consistent with the en face findings, our SEM images did not indicate the presence of endothelial cell layers in any of the recellularized vein samples, but showed rare structures which may represent individual cells attached to the vessel wall. (Figs. 2f; i). However, a fibrous mesh was found to cover large areas of the luminal surface.
This meshwork was also evident in H&E cross sections at very high magnification covering areas of the lumen (Fig. 3a-c). Not infrequently, red blood cells trapped in the meshwork could be seen. SEM revealed more details of the luminal surface. Instead of an endothelium established after recellularization, we found exposed ECM fibers or red blood cells, platelet-like structures and long fibers covering parts of the vessel lumens (Fig. 3c-i). The larger magnifications reveal structures very similar to platelets in different stages of activation (Fig. 3g-i).
Fig. 3.
Deposition of fibrin and platelets on the surface of recellularized veins.
(a-c) H&E stainings show the formation of darker pink layers, possibly fibrin, along the luminal side of the vessel. Red blood cells trapped within the fibrin clot are noticeable several places. No new nuclei are seen along the luminal surface following the recellularization process.
(d-f) SEM at 200× show a fibrous mesh covering the luminal surface, in addition to some round structures, especially evident in sample #7. No apparent re-endothelialization.
(g-i) SEM at 1000× reveal details of fibrous mesh. Red blood cells and platelets are trapped in a fibrin network covering the entire surface of the vein scaffold. No traces of endothelial cells can be seen.
3.3. Immunohistochemistry
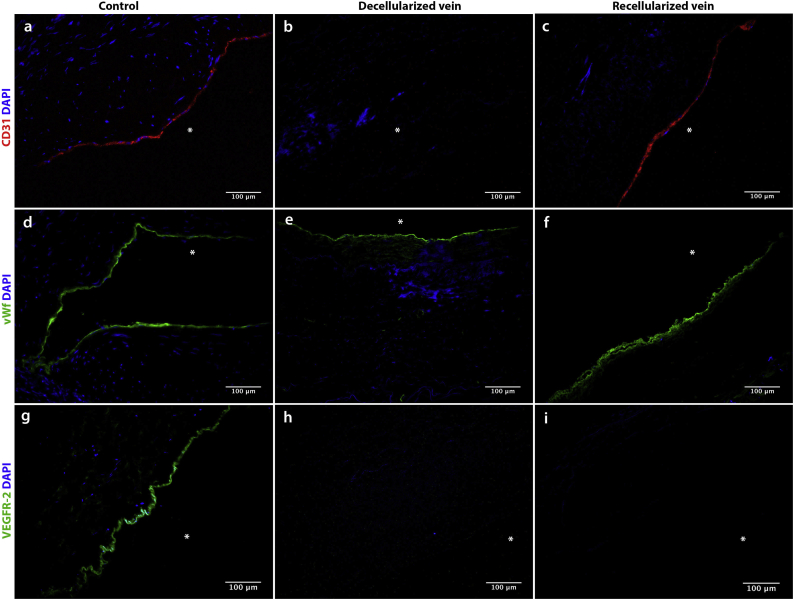
Two commonly used markers to demonstrate successful re-endothelialization are CD31 and von Willebrand factor (vWF) [22,27]. Remarkably, despite ECs not being present on the re-endothelialized surfaces, our IHC results showed that the recellularized veins stained positive for CD31 along the luminal surface (Fig. 4c). As expected, the native control veins also stained positive for CD31 (Fig. 4a), while the decellularized veins were negative for this marker (Fig. 4b). Also surprising, the staining for vWF was positive in all our preparations: the native veins, decellularized veins and the recellularized specimens (Figs. 4d-f).
Fig. 4.
CD31, von Willebrand Factor and VEGFR-2 immunostaining of native, decellularized and recellularized veins.
a-c) CD31 immunostaining of cryosections. Asterisk indicates the lumen. a) Native control vein contains a confluent layer of CD31-staining cells. b) CD31-staining cell layer has been fully removed in the decellularization process. c) A CD31-positive layer has emerged on the luminal surface after recellularization.
d-f) Von Willebrand factor immunostaining of cryosections. Asterisk indicates the lumen. d) Continuous vWF staining in the native control vein. e) Continuous vWf staining of decellularized vein. f) Continuous vWF staining of recellularized vein.
g-i) VEGFR-2 factor immunostaining of cryosections. Asterisk indicates the lumen. g) Continuous VEGFR-2 staining in the cadaver control vein. h) VEGFR-2-staining layer has been removed in the decellularization process. i) No traces of VEGFR-2-positive cells in the recellularized vein.
Anti-VEGFR-2 was used in the original study to demonstrate successful repopulation of the graft with endothelial progenitor cells. As native saphenous veins, used as control here, do not express VEGFR-2 [28], our positive control for VEGFR-2 was a sample of the cadaver vein before decellularization. Staining of both the decellularized and recellularized veins with anti-VEGFR-2 were negative, in line with our results showing no re-endothelialization of the vessels (Figs. 4g-i).
As our SEM and H&E results indicated a meshwork of fibrin and platelets forming along the luminal surface, we stained for the specific platelet marker CD41 and for fibrin. IHC results confirmed the presence of platelets and fibrin along the lumen of the recellularized vessels (Figs. 5c; f). Native and decellularized veins were all negative for these markers, demonstrating that platelets and fibrin had been deposited on the luminal surface during the recellularization process (Figs. 5a; b; d; e).
Fig. 5.
CD41 and fibrin immunostaining of native, decellularized and recellularized veins.
a-c) CD41 immunostaining of cryosections. Asterisk indicates the lumen. Native and decellularized veins stain negative (a and b), whereas c) shows continuous CD41 staining of luminal side after recellularization.
d-f) Fibrin immunostaining of cryosections. Asterisk indicates the lumen. Native and decellularized veins stain negative (d and e), whereas f) shows continuous staining for fibrin along the luminal surface following recellularization.
4. Discussion
In contrast to the proof of principle publication [22], we could not find evidence for adequate recellularization of the decellularized scaffold, and did not observe the formation of a new endothelial layer. Cross-sectional histology staining did not show nuclei along the vessel luminal surface, confirmed by en face images covering larger areas of the luminal surface. SEM images similarly made it very clear that there was no settlement of new cells along the luminal surface post recellularization. Negative VEGFR-2 staining also confirms this. However, we may have found the explanation for why some of the immunohistochemical staining procedures may have given the false impression that the scaffolds were re-endothelialized.
Interestingly, antibodies specific for both vWF and CD31 stained positively in the recellularized specimens, even though no new endothelium had been formed. Both of these markers were used to verify the presence of ECs in the Olausson et al. paper, though the vWf staining results were not shown. VWF is a collagen binding protein [29], and upon rupture of the endothelial cells in the decellularization process, we believe that the vWF released from the cells binds to the collagen component of the luminal surface of the matrix. This will give similar staining with anti-vWF antibody both in the native specimens, where the vWF is inside the endothelial cells, and in the decellularized and recellularized specimens, where the vWF is bound to the ECM making up the luminal surface of the vessel scaffold. Consequently, as long as the vWF is not removed from the tissue in the decellularization process, this marker cannot be used as evidence for recellularization.
CD31, also known as PECAM-1 (Platelet Endothelial Cell Adhesion Molecule 1), is not exclusively expressed by endothelial cells, but also on platelets and other hematopoietic cells [30]. Based on our SEM images, and the fact that the decellularized vessels were negative for CD31, we made the hypothesis that platelets from the circulating blood had attached to the luminal surface of the recellularized vessels. This was tested and confirmed by staining with antibodies specific for the platelet-specific surface marker CD41 [31]. It is also possible that soluble CD31, present in circulating blood, could have attached to structures on the luminal surface of the vessel scaffold and contributed to the staining by the anti-CD31 antibody [32]. Thus, recellularization with whole blood does not endothelialize the vessel scaffold, but leads to positive staining with anti-CD31 antibodies and a false impression that the vessel scaffold is covered with endothelial cells. When whole blood is used for recellularization, CD31 cannot be used as a definite proof for the presence of endothelial cells.
The importance of a functional endothelium in preventing thrombosis has been well documented in the literature. It is not unlikely that the naked vessel wall, combined with retained vWF, may contribute to the formation of blood clots [33]. When collagen of the vessel wall is exposed, usually caused by damage to the endothelium, platelets will bind directly to the vessel wall and eventually initiate coagulation. The interaction between platelets and collagen is strengthened by vWF, which is known to be a mediator of the initiation and progress of thrombus formation by several means, including tethering of platelets, platelet adhesion and factor VIII binding [29,33].
In addition, these samples also stained positive for fibrin. Fibrin is formed from fibrinogen, a component of normal blood plasma. Fibrin, together with platelets, are known to be an essential ingredients for the formation of blood clots [34,35]. The presence of vWF, platelets and fibrin along the luminal surface may not only be a curious side effect of the use of blood for re-endothelialization, but may predispose to thrombosis in the treated veins.
The notion of using peripheral blood to regenerate a decellularized vessel would potentially make tissue engineered blood vessels available to a large number of patients. The peripheral blood re-endothelialization method was already used22 for implantation of tissue engineered veins in extrahepatic by-pass procedures in two pediatric patients. However, two of the three grafts used in the two patients failed post implantation, at least one of them due to occlusion. Our results may offer explanations for these failures.
Successful re-endothelialization by peripheral blood derived EPCs has in fact been achieved, by Tillman et al. [36] Using a CD133+ antibody capture system to harvest ovine EPCs from 1800 mL of peripheral blood, they further expanded the cells in the laboratory for several weeks, obtaining millions of cells for recellularization. In addition to histological studies, convincing SEM images demonstrate that they get a complete re-endothelialization of the vessel graft. This shows that blood does contain cells suitable for recellularization purposes. However, our results show that whole blood preparations are not suitable for re-endothelialization. The amount of endothelial cells in whole blood is too limited, in addition to the potentially thrombogenic effects of the deposition of fibrin, platelets and vWF along the vessel wall. Thus, if peripheral blood is to be used as a source of cells for re-endothelialization, these cells need to be isolated from the blood and most likely expanded ex vivo. Alternatively, one may find autologous endothelial cells or their precursors in adipose tissue and the bone marrow [37,38]. As a complete, continuous and functional layer of ECs along the vessel wall is essential for the safe use of decellularized scaffolds in clinical applications, the work to identify the best cell source and the optimal recellularization process continues.
Funding Sources
Southern and Eastern Norway Regional Health Authority.
Author Contributions
Conception and design: MHR, JH, JOS, JEB, AR.
Analysis and interpretation: MHR, JEB, AR.
Data collection: MHR, AR.
Writing the article: MHR, JEB,
Critical revision of the article: MHR, JH, JOS, JEB, AR.
Final approval of the article: MHR, JH, JOS, JEB, AR.
Obtained funding: JEB.
Competing Financial Interests
The authors declare no competing financial interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.05.012.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoghbi W.A. Sustainable development goals and the future of cardiovascular health: a statement from the global cardiovascular disease taskforce. J. Am. Coll. Cardiol. 2014;64:1385–1387. doi: 10.1016/j.jacc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Tara S. Vessel Bioengineering. Circulation Journal. 2014:12–19. doi: 10.1253/circj.cj-13-1440. [DOI] [PubMed] [Google Scholar]
- 4.Giannoukas A.D. Pre-bypass quality assessment of the long saphenous vein wall with ultrasound and histology. Eur. J. Vasc. Endovasc. Surg. 1997;14:37–40. doi: 10.1016/s1078-5884(97)80223-7. [DOI] [PubMed] [Google Scholar]
- 5.Avci-Adali M., Perle N., Ziemer G., Wendel H.P. Current concepts and new developments for autologous in vivo endothelialisation of biomaterials for intravascular applications. Eur Cell Mater. 2011;21:157–176. doi: 10.22203/ecm.v021a13. [DOI] [PubMed] [Google Scholar]
- 6.Dahl S.L. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 2011;3:68ra69. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 7.Ravi S., Chaikof E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010;5:107–120. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehl D., Weber B., Hoerstrup S.P. Bioengineered living cardiac and venous valve replacements: current status and future prospects. Cardiovasc. Pathol. 2016;25:300–305. doi: 10.1016/j.carpath.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Kuna V.K. Successful tissue engineering of competent allogeneic venous valves. J Vasc Surg Venous Lymphat Disord. 2015;3:421–430. doi: 10.1016/j.jvsv.2014.12.002. https://doi.org/10.1016/j.jvsv.2014.12.002 e421. [DOI] [PubMed] [Google Scholar]
- 10.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quint C. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badylak S.F., Taylor D., Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pashneh-Tala S., MacNeil S., Claeyssens F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev. 2015 doi: 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane T.J., Londono R., Turner N.J., Badylak S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Roy S., Silacci P., Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1567–H1576. doi: 10.1152/ajpheart.00564.2004. [DOI] [PubMed] [Google Scholar]
- 16.Conklin B.S., Richter E.R., Kreutziger K.L., Zhong D.S., Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002;24:173–183. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 17.Gratzer P.F., Harrison R.D., Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a Decellularized matrix. Tissue Eng. 2006;12 doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 18.Chemla E.S., Morsy M. Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access. Br. J. Surg. 2009;96:34–39. doi: 10.1002/bjs.6434. [DOI] [PubMed] [Google Scholar]
- 19.Zilla P., Bezuidenhout D., Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Kaushal S. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulte J. A novel seeding and conditioning bioreactor for vascular tissue engineering. Processes. 2014;2:526–547. [Google Scholar]
- 22.Olausson M. In vivo application of tissue-engineered veins using autologous peripheral whole blood: A proof of concept study. EBioMedicine. 2014;1:72–79. doi: 10.1016/j.ebiom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y., Weisdorf D.J., Solovey A., Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinisch A. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solovey A. Circulating activated endothelial cells in sickle cell anemia. N. Engl. J. Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 26.Levy M. Circulating endothelial cells in refractory pulmonary hypertension in children: markers of treatment efficacy and clinical worsening. PLoS One. 2013;8:e65114. doi: 10.1371/journal.pone.0065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiel G.E. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12:2355–2365. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 28.Belgore F. Localisation of members of the vascular endothelial growth factor (VEGF) family and their receptors in human atherosclerotic arteries. J. Clin. Pathol. 2004;57:266–272. doi: 10.1136/jcp.2003.012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri Z.M. Structure of von Willebrand factor and its function in platelet adhesion and thrombus formation. Best Pract Res Clin Haematol. 2001;14:257–279. doi: 10.1053/beha.2001.0133. [DOI] [PubMed] [Google Scholar]
- 30.Berman M.E., Xie Y., Muller W.A. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta 2 integrin activation. J. Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- 31.Saboor M., Moinuddin M., Ilyas S. New horizons in platelets flow cytometry. Malays J Med Sci. 2013;20:62–66. [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberger A. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J. Biol. Chem. 1994;269:17183–17191. [PubMed] [Google Scholar]
- 33.Ruggeri Z.M., Mendolicchio G.L. Interaction of von Willebrand factor with platelets and the vessel wall. Hamostaseologie. 2015;35:211–224. doi: 10.5482/HAMO-14-12-0081. [DOI] [PubMed] [Google Scholar]
- 34.Becker R.C., Sexton T., Smyth S.S. Translational implications of platelets as vascular first responders. Circ. Res. 2018;122:506–522. doi: 10.1161/CIRCRESAHA.117.310939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandrashekar A. Mechanical and biochemical role of fibrin within a venous Thrombus. Eur. J. Vasc. Endovasc. Surg. 2018;55:417–424. doi: 10.1016/j.ejvs.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Tillman B.W. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J. Vasc. Surg. 2012;56:783–793. doi: 10.1016/j.jvs.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Szoke K., Beckstrom K.J., Brinchmann J.E. Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant. 2012;21:235–250. doi: 10.3727/096368911X580518. [DOI] [PubMed] [Google Scholar]
- 38.Schatteman G.C., Dunnwald M., Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1–18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data generated during the current study are available from the corresponding author upon reasonable request.