Abstract
Diet provides macronutrients (carbohydrates, proteins, and fats), micronutrients (vitamins and minerals), and phytochemicals (non-nutrient bioactive compounds). Emerging evidence suggests that above dietary components can directly impact the composition and metabolic activity of the mammalian gut microbiota and in turn, affect both physical and mental health. There is a growing recognition that rise in chronic disease burden in Western countries may due to progressive loss of beneficial bacteria and microbial diversity. This perspective explores the possibility of using Indian thali, an ancient approach to diet that provides both fiber and different phytochemicals by incorporating a variety of plant foods in different colors. This variety helps to restore diversity in the gut bacteria and may potentially prevent or reverse chronic disease, such as colon cancer or type 2 diabetes.
Keywords: Plant-based diet, phytochemicals, anthocyanins, gut bacterial diversity, inflammation, colibactin, colon cancer, Ayurveda
Introduction
The vast nutritional benefits of a diet containing a wide variety of plants have long been known. However, benefits distinct from simple nutrition, such as phytochemicals have recently become clear. Diets rich in a plethora of phytochemicals can promote a healthy and diverse gut microbiota, reduce intestinal and systemic inflammation, and decrease the risk of colorectal cancer and type 2 diabetes mellitus.
Some of these benefits can be observed around the world. Many parts of India have historically low colon cancer incidence rates [1]. The Indian subcontinent has been continuously settled for millennia. Ancient cities in the Indus valley have been dated to the third and fourth millennia BC and some sites are even older. Archaeological evidence of grain cultivation, including several varieties of barley and wheat, has been found in excavations dated to the sixth millennium BC. Wheat is still a staple crop in northern India, and many other grains, including barley, were commonly cultivated and eaten until the 1950s, when wheat and white rice became dominant [2]. Although the country has many diverse cultures, some customs remain common and conventional throughout the nation. One such tradition is the form of main meals where a large round platter, the thali, holds rice or bread and several smaller bowls, or katori, which hold a separate condiment or curry to be eaten with the rice or bread at the diner’s preference [3,4]. Typical dishes include, but are not limited to, dal (legumes; prebiotics), yogurt (probiotics), and assorted spices and vegetables (provides fiber and different classes of phytochemicals) [3].
The development of agriculture early in its history has allowed India to develop rich traditions around food. These traditions have been deeply influenced by Ayurveda, the ancient Indian system of medicine. In Ayurvedic practice, food is a source of nourishment and medicine, used to both prevent and treat illness. Maintaining a proper balance of Ayurvedic elements through diet is considered an effective way to live a healthy lifestyle.
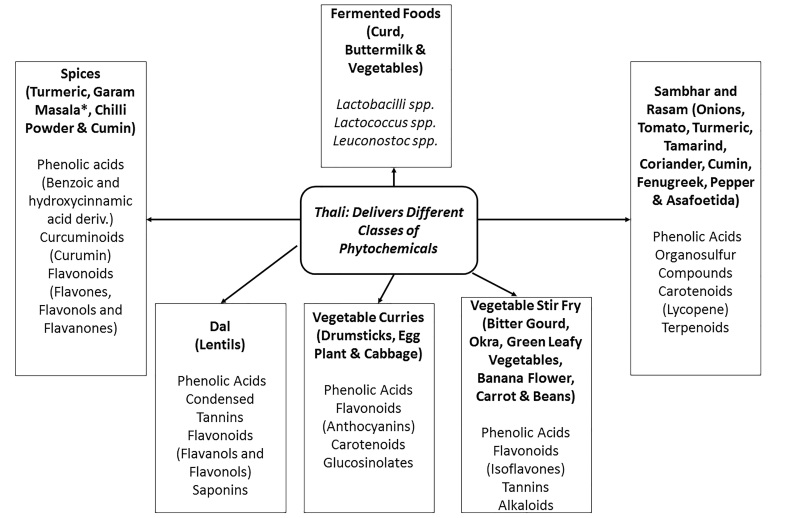
According to the Ayurvedic principles, each meal should contain a balance of the six major flavors (sweet, salty, sour, bitter, pungent, and astringent) [5]. This calls for the many small portions of a thali meal which also easily incorporate variety [6]. A variety of flavors in a meal often indicates the presence of many classes of bioactive compounds (Figure 1). Although these substances may not be macronutrients, vitamins, or minerals, they still impact human health. Polyphenols are perhaps the largest class of bioactive compounds, containing subclasses such as flavonoids, isoflavones, stilbenes, lignans, and tannins. As a flavonoid subgroup, anthocyanins are included in this class.
Figure 1.
The thali diet promotes gut bacterial diversity by delivering probiotics, prebiotics, and different classes of phytochemicals from fermented foods, dal and vegetables and spices, respectively. Indeed, sambar, a component of the thali diet suppressed chemically-induced colon carcinogenesis in vivo [67]. *Black and white peppercorns, cloves, cinnamon, mace (part of nutmeg), black and green cardamom pods, bay leaf, cumin, and coriander.
Anthocyanins are of interest in the food industry as nontoxic and water-soluble pigments, as most are colored red, purple, or blue, and many display antioxidant and anti-inflammatory activity [6]. A class of phytochemicals called polyphenols is also found in virtually all plant foods, though their quantity may be reduced by preparation methods. Rich sources of anthocyanins include deeply colored fruits and vegetables, such as blueberries, eggplants, and certain carrot and potato cultivars [6-8]. Given that many phytochemicals exert anti-inflammatory activity via promoting gut bacterial diversity, there is a growing interest in a food-based approach to countering the growing epidemic of inflammation-promoted chronic diseases such as colon cancer.
Promoting Gut Bacterial Diversity Using Thali Diet Approach
We have learned that no discussion of diet is complete without consideration of the intestinal microbiota. Trillions of bacteria, distributed throughout the gastrointestinal tract from mouth to anus, facilitate digestion and intestinal homeostasis [9]. Structural factors greatly impact the overall makeup of each community. For example, low pH prohibits many pathogenic bacteria from colonizing the stomach and the upper small intestine. The depths of the large intestine, on the other hand, is an ideal habitat for many anaerobes. The gut microbiota is a dynamic community, composed of living organisms that can alter in response to diet, disease, and other environmental pressures [10].
Changes in the intestinal microbiota were first correlated with illness in 1681 when Anton van Leeuwenhoek recorded that the microbial composition of his diarrhea differed from normal fecal samples [11]. Since then, the intestinal microbiome has been closely studied to show how it can be implicated in a variety of conditions ranging from obesity to colon cancer. A great deal of investigation into microbiota has been accomplished in the last decade. Many of these observed changes result in an overall loss of bacterial diversity in the microbiota, indicating that species diversity is associated with health [11-13]. However, the opposite may be true for cause-consequence relations, but not enough research has been brought to light.
High-throughput technologies have driven advances in identifying the trillions of microbes and the metabolic functions that live in the colon. This led to a critical insight that gut plays as dynamic of a role in metabolism as the liver. The proximity of these microbes to the intestinal mucosa and gut lymphoid tissue explains the critical role they play in health and disease. Indeed, dysbiosis plays a significant role in the development of inflammatory bowel disease, obesity, and colon cancer. Emerging evidence suggests that diet can directly influence the content and composition of gut microbiota. Thus, understanding the complex interactions between diet, gut microbiota, and the host are crucial in prevention and treatment of chronic diseases that plague our society [14]. Studies in murine models have shown rapid changes in the gut bacteria of mice being switched quickly from a standard diet to a high-calorie diet back to a standard diet [14]. In humans, surveys show that diets high in fiber correlate with higher microbial diversity and reduced populations of Enterobacteriaceae, including Escherichia and Shigella species [15]. Marked differences are also seen during consumption of animal- vs. plant-based diets [10].
While nutrients in the diet will affect intestinal microbes, other substances present in food may also have an effect. For example, most anthocyanins are not absorbed into the bloodstream in the small intestine, and so they stay in the gastrointestinal tract until they reach the colon [16]. There, they can affect the colonic microbiota in multiple ways. Firstly, anthocyanins have antioxidant activity that can reduce inflammation-induced oxidative stress on the gut bacteria [17]. Secondly, anthocyanins are a potential carbon source, which bacteria can metabolize, resulting in increased growth of certain microbes [7]. Lastly, bacterial metabolism of anthocyanins produces a variety of metabolite byproducts, some of which have antimicrobial effects on enteric pathogen species including Escherichia coli [16].
Thali Diet Approach to Countering Inflammation-Promoted Chronic Diseases
Chronic intestinal inflammation is a hallmark of certain bowel disorders, such as ulcerative colitis and Crohn’s disease, which are two major forms of inflammatory bowel diseases (IBD), and IBD is also considered a risk factor for colorectal cancer. In the latter, inflammation is generally low-grade but persists over a long period of time. Diet composition can promote or suppress chronic inflammation. Low-fiber high-calorie diets, which are typical in Western countries, may directly promote inflammation, or as already discussed, indirectly promote this through dysbiosis. Indeed, some dietary patterns associated with chronic inflammation are also linked to the reduction of total microbial diversity and imbalances in intestinal microbial groups [10,18]. Furthermore, some bacteria, including E. coli, can flourish during low-grade inflammation, where thinning of the intestinal mucus layer occurs and allows for more direct interaction between the host’s cells and the intestinal bacteria. This condition can cause a feedback loop in which contact between bacteria and epithelial cells leads to dysregulation of mucosal immune response. This contact can lead to a bacterial biofilm, formed when bacteria attach themselves to the surfaces of the aqueous environment in the gut and begin to secrete substances that allow them to affix onto the epithelium. The interaction between bacteria and epithelial cells elevates inflammation, leading to increased thinning of the mucus and direct host-bacteria interaction. The thali approach, however, combats this cycle in two different ways: by suppressing bacterial growth with anti-microbial phytochemicals (for example, curcumin), and by reducing the opportunity for inflammation to occur.
One molecular pathway involved in such a cycle involves interleukin 6 (IL6). This cytokine is normally expressed during acute inflammatory responses, and among other effects, upregulates the transcription factor STAT3. In the nucleus, STAT3 promotes cell proliferation and differentiation as well as upregulating anti-apoptosis genes. When IL6 is chronically elevated, it can lead to an apoptosis-resistant, constantly expanding T-cell population in the intestinal mucosa. These cells can further contribute to chronic inflammation [19].
Just as a certain diet may promote chronic inflammation, a change in diet can help to restore health. Various bioactive compounds, including anthocyanins, have demonstrated antioxidant activity, reducing local amounts of reactive oxygen species. Low levels of reactive oxygen species can lower the expression of some inflammatory genes, including IL6, and relieve the stresses on both the intestinal microbiota and epithelial cells caused by chronic inflammation [20,21]. In a study of pigs, we found that supplementing (10 percent w/w) a high-calorie diet (HCD) with purple potatoes that contains anthocyanins led to a six-fold reduction in levels of interleukin-6 (IL6) compared to high-fat diet control [22].
Colorectal cancer (CRC) killed nearly 774,000 people worldwide in 2015, and nearly an estimated 50,630 deaths in 2018 in America making it the third leading cause of cancer-related deaths in the United States in women and second in men [23,24]. Virtually all cases of CRC are considered to result from an interplay of exogenous and endogenous factors with respect to the variable contribution from each factor [25,26]. Some non-modifiable risk factors include old age and family history of CRC. Other risk factors, however, are associated with lifestyle or behaviors and thus can be changed.
These modifiable risk factors include smoking, obesity, low physical activity, deficiency of dietary fiber, deficiency of vitamin D, deficiency of folate, high intake of red and processed meat, and alcohol consumption [26-28].
Some of these risk factors, however, are closely related. For example, inadequate fiber intake and excessive fat intake are dietary risk factors which tend to lead to a lack of exercise which ultimately may contribute to obesity, particularly in combination. In the US, 40 percent of adults are obese, and so the risk factors discussed are common mainly due to the modern Western lifestyle. Therefore, it is no surprise that nearly half of the CRC cases arise in the developed nations.
The Western diet in its current form contains more risk factors than the calorie and fat content. Foods that contain heterocyclic amines (HCA), polycyclic aromatic hydrocarbons (PAH), and emulsifiers can also contribute to carcinogenesis. HCA and PAH are produced in meats when they are fried or grilled over an open flame. These substances have been proved to damage the DNA of colonocytes and potentially promote risk of colon cancer [29]. Emulsifiers are used in foods like ice cream to ensure an even distribution of fat molecules. Recent evidence suggests, however, that emulsifiers promote intestinal inflammation, creating an environment that favors colon carcinogenesis in mice [30]. Some of these risk factors, however, are closely related. For example, inadequate fiber intake and excessive fat intake are dietary risk factors. These tend to lead to a lack of exercise, which ultimately contributes to obesity. In the US, 40 percent of adults are obese, and so the risk factors discussed are common mainly due to the modern Western lifestyle. Therefore, it is no surprise that nearly half of CRC cases arise in developed nations. However, colon cancer has a long development period (10 to 30 years). This gives ample time for lifestyle changes to take place, including diet-based intervention.
Chronic inflammation, a condition that is promoted by dietary risk factors also contributes to the development of cancer, even in humans. Patients with inflammatory bowel disease have a significantly increased risk of developing CRC, while long-term aspirin treatment is associated with a significantly decreased risk of CRC [31]. The mechanisms by which chronic inflammation promotes tumor development often involve the immune system. For example, the IL6/STAT pathway discussed earlier is also implicated in cancer formation. Overexpression of IL6 leads to excess STAT3 transcription, causing unwanted cell proliferation not only in T cells but also in the intestinal epithelium [20,30,31]. Another inflammatory cytokine of note is TNF α (tumor necrosis factor α) [32-34].
While the intestinal bacteria can promote inflammation, they may also affect the likelihood of CRC more directly. Once the intestinal mucus layer is thinned, and direct bacterial-epithelial cell interactions occur, certain bacterial strains promote tumor development. E. coli strains bearing the pks island are of particular interest. This genetic locus codes for the secondary metabolite colibactin, along with the enzymes necessary for its production [35]. Colibactin has been shown to crosslink with DNA, producing double-stranded breaks [36]. Furthermore, pks+ E. coli strains have been shown to be prevalent in CRC patients. In one study, nearly two-thirds of CRC patients had pks+ E. coli strains in their intestinal bacteria. In the same study, pks+ E. coli also existed in about 20 percent of healthy individuals [32]. Colibactin, however, is a reactive and short-lived protein, requiring close contact with epithelial cells to cause DNA damage [32]. A healthy mucosal barrier (one not thinned by chronic inflammation) keeps colibactin at a distance and reduces the chance of affecting the intestinal epithelium.
Evidence for the pathogenic relationship between diets, Fusobacterium nucleatum, and CRC has been emerging [37,38]. The F. nucleatum levels have been shown to be higher in CRC than in adjacent normal mucosa [39]. Utilizing the molecular pathological epidemiology paradigm and methods [25,40], a recent study has shown the association of fiber-rich diets (so-called prudent dietary pattern) with decreased risk of F. nucleatum-detectable CRC, but not that of F. nucleatum-undetectable CRC [41]. Experimental evidence supports a carcinogenic role of F. nucleatum [42-45],as well as its role in modifying therapeutic outcomes [46]. The amount of F. nucleatum in CRC tissue has been associated with proximal tumor location, CpG island methylator phenotype (CIMP), microsatellite instability (MSI) [47,48], low-level CD3+ T cell infiltrate [49], high-level macrophage infiltration [50], and unfavorable patient survival [47,48]. The amount of F. nucleatum in average increased in CRC from rectum to cecum [51], supporting the colorectal continuum model [52,53]. Future studies should examine the role of diets, microbiota, and CRC in detailed tumor locations.
Dietary prevention of CRC, then, has two intertwined aims: to reduce inflammation and to promote a healthy intestinal microbiota. As already discussed, preclinical evidence implies that dietary bioactive compounds, particularly anthocyanins, can reduce symptoms of low-grade chronic inflammation as well as oxidative stress. It can also aid in balancing the intestinal microbiota by promoting the growth of beneficial bacteria and by reducing the populations of pro-inflammatory bacteria [8,20]. Clinical trials have had mixed results, but anthocyanins and some polyphenols have shown to counteract against CRC actively. More research, however, is necessary for conclusive results [54].
How, then, are individuals to consume enough bioactive compounds to have an effect on health? Some answers may be found in the food consumption practices of cultures with historically low CRC incidence. Parts of India, for example, have had some of the lowest CRC incidence rates in the world [1]; however, this status has been changing.
In recent decades, increasing urbanization and similar factors have led to progressively Westernized diet patterns and lifestyle [55]. CRC incidence rates are similarly rising, lending weight to the hypothesis that the traditional Indian diet may help prevent CRC [56]. Furthermore, Indian immigrants to Western countries have a much higher incidence of CRC [57] compared to Indians in India. Typical components of traditional Indian meals include a broad variety of flavors, as promoted in Ayurvedic medicine, and a variety of other foods. Both are facilitated by using a thali platter to serve the meal [3,4]. The traditional American main meal includes an entree (the main source of protein, usually containing meat or fish), one or more carbohydrates (potatoes, rice, pasta, bread, etc.), and one or more vegetables [5]. This basic structure can potentially be adapted with inspiration from thali meals by reducing the size of the main dish and serving more vegetables, legumes, pulses, herbs, and spices to accompany it.
A unique component to thali is the combination of many tastes and colors. The inclusion of multiple colors in a meal is desirable, because certain bioactive compounds, particularly anthocyanins are also pigments. Blue, purple, and red-purple colors in plant foods indicate high anthocyanin content. Purple-pigmented potatoes can be prepared in the same way as traditional white potatoes, but the anthocyanin content is significantly higher in the pigmented varieties [6]. Purple sweet potatoes also contain more anthocyanins than the more common orange varieties and can be easily substituted for them [58]. Other vegetables with red or purple cultivars include carrots, cauliflower, and cabbage [17]. Different colors can indicate the presence of other bioactive compounds, such as orange (carotenes), yellow (lutein and zeaxanthin), and red/pink (lycopene). Thus, healthy bioactive compound consumption may be increased by selecting colorful vegetables. Another way to increase consumption of bioactive compounds is to increase their presence in available foods. The content of bioactive compounds in plant foods is highly influenced by genetics [59-61]. The agricultural industry could greatly impact health by adopting food plant cultivars that produce bioactive compounds in larger amounts than is currently common. New cultivars may need to be developed that retain desirable characteristics such as large size, pest resistance, reduced spoilage, etc., but also have high bioactive content at the time of consumption. Bioactive compounds, with some exceptions, tend to deteriorate during storage [62-64]. Even when compounds have not deteriorated, storage may reduce the anti-inflammatory/antioxidant activity of bioactive compounds to affect health [65]. A second systemic change that would promote increased bioactive compound consumption involves reworking how fruits and vegetables are currently stored and processed, as well as reducing the average storage time and adapting processing to optimize the amount of bioactive compounds. Presently, “nutritional adequacy” does not consider many of the bioactive compounds discussed in this paper. Further clinical studies are needed to support and elucidate the role of bioactive compounds in the prevention and treatment of disease.
Conclusion
Recent research provides preclinical evidence that phytochemicals, especially anthocyanins, promote gut microbial health, reduce inflammation, and lower the risk of colorectal cancer. Clinical evidence is sparse but indicates that anthocyanins and other bioactive compounds do have an effect on colon cancer [54]. Both are consistent with low cancer rates in India, where both traditional diet and Ayurvedic medicine promote consumption of many classes of phytochemicals. Long-term, diet-based randomized clinical trials are both difficult to conduct and prohibitively expensive. However, given the strong evidence from basic studies, observational data, and randomized clinical studies with short-term surrogate outcomes, steps should still be taken to improve the consumption of bioactive compounds, particularly in countries which contain a large proportion of CRC patients [55]. Eating a wide variety of plant foods has no ill effects, and is indeed a commonly recommended part of a healthy lifestyle [55]. Increasing bioactive intake among Westerners will require modifications in both individual eating habits and food system practices [66].
Acknowledgments
We thank Justin Martin for the edits on earlier versions of the manuscript. This research was supported by USDA-NIFA NRI Integrated grant 2009-55200-05197 (to JKPV) and NIH grant R35 CA197735; Nodal Award from Dana-Farber Harvard Cancer Center (to SO).
Glossary
- CIMP
CpG island methylator phenotype
- CRC
Colorectal cancer
- HCA
heterocyclic amines
- HCD
high-calorie diet
- IBD
inflammatory bowel disease
- IL6
interleukin 6
- MSI
microsatellite instability
- PAH
polycyclic aromatic hydrocarbons
- TNF α
tumor necrosis factor α
Author Contributions
Introduction (KS and JKPV); Promoting gut bacterial diversity using Thali diet approach (KS, RK, and JKPV); Thali Diet Approach to Countering Inflammation-Promoted Chronic Diseases (JKPV, SO, AS, and RK); Conclusion (KS and JKPV), Abstract (JKPV), Figure (AS and JKPV).
References
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. Dis Colon Rectum. 2010;53(7):1099. [Google Scholar]
- Hermann K. A history of India. 6th ed. New York (New York): Routledge; 2016. [Google Scholar]
- Sen CT. Food culture in India. Greenwood Press; 2004. 197 pp. [Google Scholar]
- Sahni J. Classic Indian Cooking. 1st ed. New York: William Morrow and Company; 1980. [Google Scholar]
- Chopra A, Doiphode VV. Ayurvedic medicine: core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002. January;86(1):75–89. [DOI] [PubMed] [Google Scholar]
- Burgos G, Amoros W, Muñoa L, Sosa P, Cayhualla E, Sanchez C, et al. Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. J Food Compos Anal. 2013;30(1):6–12. [Google Scholar]
- Lacombe A, Wu VC, White J, Tadepalli S, Andre EE. The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiol. 2012. May;30(1):124–31. [DOI] [PubMed] [Google Scholar]
- Nile SH, Park SW. Edible berries: bioactive components and their effect on human health. Nutrition. 2014. February;30(2):134–44. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J Nutr. 2011;141(5):769–76. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos WM, De Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012;70 SUPPL. 1:45–56. [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008. October;105(43):16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzola F, Bernstein C, Ho GT. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes [Commentary] Inflamm Bowel Dis Monit. 2011;11(4):166. [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci Transl Med. 2009. Nov;1(6):6ra14 LP-6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010. Aug;107(33):14691 LP-14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, et al. How to feed the Mammalian gut microbiota: bacterial and metabolic modulation by dietary fibers. Front Microbiol. 2017;8(SEP):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Deng Z, Zhu H, Hu C, Liu R, Young JC, et al. Highly pigmented vegetables: anthocyanin compositions and their role in antioxidant activities. Food Res Int. 2012;46(1):250–9. [Google Scholar]
- Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice alters host barrier function favouring AIEC colonisation. Gut. 2014. Jan;63(1):116 LP-124. [DOI] [PubMed] [Google Scholar]
- Atreya R, Neurath MF. Involvement of IL6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005. June;28(3):187–95. [DOI] [PubMed] [Google Scholar]
- Esposito D, Chen A, Grace MH, Komarnytsky S, Lila MA. Inhibitory Effects of Wild Blueberry Anthocyanins and Other Flavonoids on Biomarkers of Acute and Chronic Inflammation in Vitro. J Agric Food Chem. 2014;62(29):7022–8. [DOI] [PubMed] [Google Scholar]
- Wu T, Yin J, Zhang G, Long H, Zheng X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol Nutr Food Res. 2016. March;60(3):687–94. [DOI] [PubMed] [Google Scholar]
- Sido A, Radhakrishnan S, Kim SW, Eriksson E, Shen F, Li Q, et al. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J Nutr Biochem. 2017;43:11–7. [DOI] [PubMed] [Google Scholar]
- WHO Cancer. WHO; 2018. Available from: http://www.who.int/news-room/fact-sheets/detail/cancer
- American Cancer Society Key Statistics for Colorectal Cancer. 2018 Available from: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html .
- Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011. Mar;60(3):397 LP-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20(20):6055–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PD, Buring JE, Manson JE, Giovannucci EL, Moorthy M V, Zhang S, et al. Circulating Vitamin D Levels and Risk of Colorectal Cancer in Women. Cancer Prev Res. 2015. Aug;8(8):675 LP-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R, Marley HN. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105–14. [PMC free article] [PubMed] [Google Scholar]
- Helmus DS, Thompson CL, Zelenskiy S, Tucker TC, Li L. Red Meat-derived heterocyclic amines increase risk of colon cancer: A population-based case-control study. Nutr Cancer. 2013;65(8):1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennois E, Merlin D, Gewirtz AT, Chassaing B. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77(1):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugerie L, Svrcek M, Seksik P, Bouvier A, Simon T, Allez M, et al. Risk of Colorectal High-Grade Dysplasia and Cancer in a Prospective Observational Cohort of Patients With Inflammatory Bowel Disease. Gastroenterology. 2013. July;145(1):166–175.e8. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science (80-). 2012. Oct;338(6103):120 LP-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Wu Y, Okobi Q, et al. Proinflammatory Cytokines IL-6 and TNF-αIncreased Telomerase Activity through NF-κB/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Mediators Inflamm. 2017;5958429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science (80-). 2013. Jan;339(6117):286 LP-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014. Dec;63(12):1932 LP-1942. [DOI] [PubMed] [Google Scholar]
- Vizcaino MI, Crawford JM. The colibactin warhead crosslinks DNA. Nat Chem. 2015;7(5):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22(2):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115(3):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016;27(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- RS M, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017. July;3(7):921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci. 2014. Dec;111(51):18321 LP-18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe. 2013;14(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang R, Bhattacharya R, Boulbes DR, Fan F, Xia L, et al. Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev Res. 2017. Jul;10(7):398 LP-409. [DOI] [PubMed] [Google Scholar]
- Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor−κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152(4):851–866.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017. July;170(3):548–563.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016. Dec;65(12):1973 LP-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, et al. Fusobacterium in Colonic Flora and Molecular Features of Colorectal Carcinoma. Cancer Res. 2014. Mar;74(5):1311 LP-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and t cells in colorectal carcinoma. JAMA Oncol. 2015. August;1(5):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017. September;471(3):329–36. [DOI] [PubMed] [Google Scholar]
- Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol. 2016;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012. Jun;61(6):847 LP-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012. Jun;61(6):794 LP-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Sánchez MA, González-Sarrías A, Romo-Vaquero M, García-Villalba R, Selma MV, Tomás-Barberán FA, et al. Dietary phenolics against colorectal cancer-From promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Mol Nutr Food Res. 2015;59(7):1274–91. [DOI] [PubMed] [Google Scholar]
- Rasool S, Kadla SA, Rasool V, Ganai BA. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol. 2013. October;34(5):2469–76. [DOI] [PubMed] [Google Scholar]
- Mohandas KM. Colorectal cancer in India: controversies, enigmas and primary prevention. Indian J Gastroenterol. 2011. February;30(1):3–6. [DOI] [PubMed] [Google Scholar]
- Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37(1):147–60. [DOI] [PubMed] [Google Scholar]
- Lim S, Xu J, Kim J, Chen TY, Su X, Standard J, et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr Food Res. 2013. November;57(11):1908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddivari L, Hale AL, Miller JC. Genotype, location, and year influence antioxidant activity, carotenoid content, phenolic content, and composition in specialty potatoes. J Agric Food Chem. 2007;55(20):8073–9. [DOI] [PubMed] [Google Scholar]
- Lima VL, Mélo EA, Maciel MI, Prazeres FG, Musser RS, Lima DE. Total phenolic and carotenoid contents in acerola genotypes harvested at three ripening stages. Food Chem. 2005. May;90(4):565–8. [Google Scholar]
- Khanizadeh S, Tsao R, Rekika D, Yang R, Charles MT, Vasantha Rupasinghe HP. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J Food Compos Anal. 2008;21(5):396–401. [Google Scholar]
- Klimczak I, Małecka M, Szlachta M, Gliszczyńska-Świgło A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Compos Anal. 2007;20(3-4):313–22. [Google Scholar]
- Nhung DT, Bung PN, Ha NT, Phong TK. Changes in lycopene and beta carotene contents in aril and oil of gac fruit during storage. Food Chem. 2010;121(2):326–31. [Google Scholar]
- Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, et al. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. 2009. September;53 S2:S219–219. [DOI] [PubMed] [Google Scholar]
- Madiwale GP, Reddivari L, Holm DG, Vanamala J. Storage elevates phenolic content and antioxidant activity but suppresses antiproliferative and pro-apoptotic properties of colored-flesh potatoes against human colon cancer cell lines. J Agric Food Chem. 2011;59(15):8155–66. [DOI] [PubMed] [Google Scholar]
- Vanamala J. Food systems approach to cancer prevention. Vol. 57. Crit Rev Food Sci Nutr. 2017;57(12):2573–88. [DOI] [PubMed] [Google Scholar]
- Prasad V, Reddy N, Francis A, Nayak P, Kishore A, Nandakumar K, et al. Sambar, an Indian dish prevents the development of dimethyl hydrazine-induced colon cancer: A preclinical study [Internet] Pharmacogn Mag. 2016;12(47):441 Available from: http://www.phcog.com/text.asp?2016/12/47/441/191454 [DOI] [PMC free article] [PubMed] [Google Scholar]