Abstract
In critically ill patients, lung and gut microbiomes undergo profound changes. Lung microbiome might become enriched with gut-associated microbes as recently demonstrated in sepsis and acute respiratory distress syndrome (ARDS). It has been proposed that in these conditions, bacteria from the gut might enter the lungs via translocation, a process facilitated by increased gut and alveolo-capillary permeability. In patients requiring mechanical ventilation after severe trauma, lung microbiome enrichment with gut-associated microbes was found to correlate with the development of ARDS. The lungs in ARDS are increasingly susceptible to opportunistic infections which can further perpetuate alveolar inflammation and injury. Undoubtedly, more research on the gut-lung crosstalk in critically ill patients is needed to identify causal relationships between the altered microbiome, infections, inflammation, and acute lung injury. With further insights, this area of investigation could lead to the development of novel, microbiome-targeted, and immunomodulation strategies with the potential to improve outcomes of critically ill patients with sepsis, trauma, and ARDS.
Keywords: ARDS, sepsis, critically ill, microbiome, bacterial translocation, nosocomial infection
Introduction
Alveolar space is never sterile – microbes or their products continuously reach alveoli via oropharyngeal aspiration, gastroesophageal reflux aspiration, or inhalation [1,2]. Although differences exist among individuals, alveolar microbiome of a non-diseased lung is typically composed of non-pathogenic anaerobes (Prevotella, Veillonella, Fusobacterium) that originate from oropharyngeal flora [3-5]. Despite their relatively low numbers in the alveoli, these bacteria play a key role in maintaining lung immune homeostasis [6]. In the lungs of critically ill patients, the normal microbiome becomes rapidly disrupted. Bacterial diversity is decreased, and commensals might become displaced by potential pathogens, often originating from other ecosystems (gut, skin) [3,7]. Illustrating some of these complex events are recent studies in sepsis, trauma, and ARDS in which culture-independent methods were utilized to investigate dynamics of lung microbiome. In these conditions, pulmonary microbiome was frequently found to be enriched with gut-associated bacteria, primarily represented by Bacteroidetes and Enterobacteriaceae [8,9]. The phenomenon of “more of the gut in the lung” appears clinically important, as this microbiome shift is associated with increased markers of adverse inflammation and lung injury [8,10,11].
How do the gut microbes enter the lungs of critically ill? In patients that require invasive mechanical ventilation due to respiratory failure, the pathogenic inoculum is traditionally believed to originate from oral flora, composition of which becomes gradually altered during the ICU stay [12]. In a susceptible host, continuous micro-aspiration of potential pathogens may lead to the development of respiratory infection [13]. Therefore, ICU “ventilator bundles” include interventions aimed at reducing aspiration such as head of the bed elevation, oral care and intermittent tracheal suctioning [14]. Despite these interventions, nosocomial pneumonia remains a frequent complication of the ICU stay, significantly contributing to the mortality of critically ill patients [13]. Recent studies in sepsis, ARDS, and stroke suggest that during these conditions, certain gut microbes might increasingly translocate across the bowel wall and even enter the lung [9,15] (Figure 1). Such mechanism of bacterial entry to the lung is believed to be facilitated by increased gut and alveolar permeability [9,16,17]. In this review, we will discuss the influence of gut microbes on alveolar inflammation, infection, and acute lung injury. Although the complex links between the gut and lung microbiomes are yet incompletely understood, a mounting evidence suggests that a perturbed gut-lung axis might play a key role in the pathogenesis of pulmonary complications in critically ill patients [8,9,18].
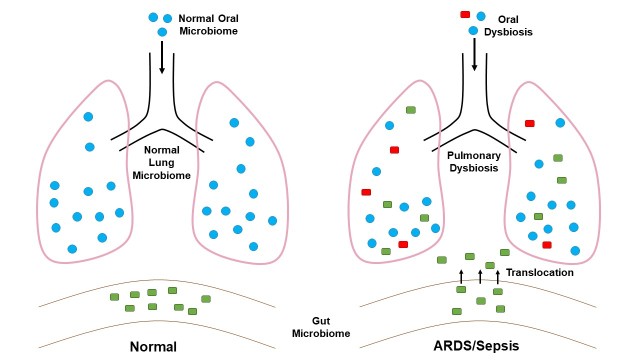
Figure 1.
Pulmonary microbiome in health and critical illness. In healthy individuals, lungs and gut harbor markedly different microbiomes. Composition of lung microbiome closely resembles the one of oropharynx. In critically ill patients with sepsis, severe trauma, or ARDS, lung microbiome might become enriched with gut-associated microbes. Potential pathogens can enter lung via aspiration and possibly translocation from the gut. The resulting state of dysbiosis promotes inflammation and acute lung injury.
How Gut and Lung Microbiomes Become Closer in Critically Ill Patients
Mucosal lining of respiratory and gastrointestinal tract is de facto continuous, allowing micro-aspiration of oropharyngeal flora to normally occur even in healthy individuals [1,19,20]. The anaerobes which in this way enter alveolar spaces are typically non-pathogenic constituents of normal oral flora [1,8,19,20]. In critically ill patients, lung microbiome undergoes substantial changes [3,9]. In patients receiving mechanical ventilation, bacterial diversity is often decreased, and a single population, often an opportunistic pathogen, might eventually become dominant [3,7,21].
Multiple factors acting in concert likely promote this microbial dysbiosis. In patients with sepsis or ARDS, the adverse inflammation alters the physiochemical (pH, oxygen tension, free radicals) and metabolic environment (presence of nutrients) of the alveoli [22-24]. The anaerobic zones that arise due to the alveolar edema or alveolar collapse (atelectasis) in the injured lungs are more permissive to the growth of potential pathogens [24]. The presence of an endotracheal tube in mechanically ventilated patients allows continuous micro-aspiration of the oropharyngeal flora while impairing natural airway clearance mechanisms [25].
Recent evidence suggests that lung microbiome might also be altered as a result of pathogenic links that exist between the gut and the lungs of critically ill patients [8,10]. Using a culture-independent method of 16S rRNA gene sequencing, Dickson and colleagues found that lung microbiome in experimental sepsis in mice as well as in patients with established ARDS becomes enriched with gut-associated bacteria, of which Bacteroides, an anaerobic gut commensal, was most commonly found in both settings [9]. Identified in lungs of mice with sepsis were also other gut commensals, such as Enterococcus faecalis and species belonging to the Enterobacteriaceae family.
Given that these gut microbes were not detected in the upper airways of intubated subjects with ARDS or mice with sepsis, authors suggested that translocation, rather than aspiration, was the primary mechanism of microbial entry to the lung. While definitive data are yet lacking, it has been proposed that the route of bacterial migration might involve gut-draining lymphatics, portal, or systemic circulation [9,26-28]. Increased gut and alveolar permeability are believed to be required for this process to take place. Gut permeability is known to be increased during sepsis and possibly during other acute conditions involving the digestive tract such as bowel obstruction, ischemia, severe pancreatitis, or enterocyte toxicity due to chemotherapy [29-33].
Recently, increased gut permeability has also been found in experimental stroke and hypothesized to be a result of autonomic dysregulation (increased sympathetic tone) that occurs in this setting [15]. In ARDS, alveolo-capillary membrane becomes increasingly permeable as a result of a direct (primarily epithelial) or an indirect (primarily endothelial) injury that can occur in the whole host of acute conditions including sepsis, pancreatitis, trauma, or pneumonia [34]. Hence, it is reasonable to posit that critically ill patients with conditions that increase both gut and alveolo-capillary permeability might be at the highest risk of gut-lung bacterial translocation.
What are the clinical implications for the ICU care? If translocation of gut bacteria to the lungs occurs in at least some critically ill patients, it would represent an additional mechanism that potentially contributes to lung dysbiosis, pneumonia or lung injury. Current pneumonia prevention measures focused at reducing aspiration in mechanically ventilated patients would be ineffective against this alternative route of bacterial entry. In fact, despite broadly used “ventilator bundles,” pneumonia continues to be a frequent complication of ICU stay [35]. Gut-associated anaerobes such as Bacteroides, even if increasingly present in the lungs during sepsis or ARDS would remain undetected by conventional culture techniques [36]. Furthermore, microbiology studies of bronchoalveolar fluid would be pursued only when infection is suspected by treating clinicians. Gut anaerobes, however, might be increasingly present in the injured lungs even when these do not appear to be infected. It is also important to note that at present, anaerobic antibiotic coverage is not uniformly administered to critically ill patients with ARDS unless a clinical suspicion for an anaerobic infection is high [37].
It therefore remains to be determined whether these potential pathogens, when present in the lungs of patients at risk for ARDS, are associated with adverse outcomes and therefore need to be identified early and targeted therapeutically [10]. Further research is needed to identify these potential causal relationships. A recent study by Panzer and colleagues suggests that a link exists between pulmonary dysbiosis in critically ill patients and the risk of subsequent development of ARDS [8]. The authors found that in mechanically ventilated patients with severe trauma, the enrichment of lung microbiome with gut microbes, particularly Enterobacteriaceae, correlated with development of ARDS. As the evidence for the adverse roles of gut-associated microbes in ARDS increases, it is likely that their timely detection and therapeutic targeting could represent a new opportunity to improve patient outcomes.
A Circle of Dysbiosis, Infection, Inflammation, and Lung Injury
Both sepsis and ARDS, regardless of their specific etiologies, are accompanied by adverse forms of inflammation [38,39]. However, targeting select immune pathways with the goal to favorably modify the inflammatory milieu in these conditions has thus far failed to improve patient outcomes in clinical trials [40,41]. As close links between inflammation and microbiome continue to be unraveled, it is being increasingly recognized that therapeutic interventions in sepsis and ARDS might need to involve a modulation of the microbiome that is itself profoundly altered in critically ill patients [42,43]. Recent studies in patients with ARDS have revealed the enrichment of lung microbiome with gut-associated microbes including Bacteroidetes and Enterobacteriaceae [8]. While these findings could simply reflect a generalized state of dysbiosis in these critically ill patients, the study by Panzer and colleagues also points to a potentially active role of these microbes in ARDS pathogenesis [8]. The authors found that in severely injured mechanically ventilated patients, an early lung dysbiosis is associated with increased markers of inflammation (IL-6, IL-8) and in these patients, subsequent development of ARDS is more likely [8]. Dickson and colleagues have suggested that translocation might be the key process by which gut bacteria immigrate into the lung during ARDS [9]. While the evidence for translocation in ARDS still remains only indirect, these new findings further expand the already exhaustive list of mechanisms by which the digestive tract might promote lung inflammation and injury in critically ill patients.
As a result of circulatory and neuroendocrine dysregulation in critically ill, gut barrier function is frequently impaired [44]. In this setting, increased amounts of luminal components, mainly from small intestine (whole bacteria, bacterial DNA, lipopolysaccharide, pancreatic enzymes, pro-inflammatory cytokines, high mobility group box 1 protein), might enter either portal circulation or draining mesenteric lymph vessels and eventually reach the lung, promoting alveolar inflammation [44].In contrast to portal blood, mesenteric lymph bypasses the liver, allowing for high concentrations of gut-derived “danger molecules” to directly enter central circulation via thoracic duct and potentially cause lung injury, primarily through toxic effects upon pulmonary microvasculature and recruitment of neutrophils [28,44]. The pathogenic role of mesenteric lymph has been demonstrated in experimental models of hemorrhagic shock when ligation of the thoracic duct and the mesenteric ducts was protective against lung injury, likely by preventing the influx of the inflammatory mediators from the gut to the lung [28,48].
Increasing evidence also points to the detrimental role of an altered gut microbiome upon the lungs of the critically ill [32,44]. Gut ecosystem of critically ill patients is profoundly disrupted and characterized by decreased bacterial diversity and decreased numbers of key anaerobic commensals (Bacteroidetes, Firmicutes). An overgrowth of a single taxon (Escherichia coli, Enterococcus, Clostridium difficile, Salmonella, Pseudomonas) occurs in a substantial proportion of patients [45,46]. These changes occur as a result of multiple factors including mesenteric hypoperfusion, interruptions in enteral nutrition, and administration of vasoactive agents, opioids, stress ulcer prophylaxis, and antibiotics during the intensive care [11]. Experimental evidence suggests that the altered gut microbiome has detrimental effects on pulmonary defense against pathogens [49-52]. Antibiotic-treated and germ-free mice manifest an increased susceptibility to lung infections with Streptococcus pneumoniae and Klebsiella pneumoniae which is linked to low pulmonary levels of interleukin (IL)-17 and granulocyte macrophage colony stimulating factor (GM-CSF) in these mice. That IL-17 and GM-CSF are required for an effective lung defense was confirmed by experiments blocking these mediators in vivo [49]. Gray and colleagues demonstrated that an intact gut microbiome promotes development of innate lymphoid cells, influx of which to the lung in the early postnatal period is required for pulmonary host defense [50]. The impairment of anti-infectious immunity in the critically ill is a major clinical problem [40,53] as patients with sepsis, trauma, or ARDS are highly susceptible to the development of nosocomial infections such as ventilator-associated pneumonia [54]. Impaired clearance of the pathogens due to the acquired immune dysfunction might perpetuate the circle of inflammation and tissue injury [10]. Importantly, it has become increasingly recognized that both immune dysfunction [40] and dysbiosis might persist for extended time periods even after physiologic “recovery” of the critically ill patient has occurred [11]. The survivors continue to be susceptible to infections with opportunistic pathogens and are more likely to develop another episode of sepsis [55,56]. Hence, restoring the immune competence [40,57,58] as well as a normal composition of the microbiome [46] might be needed to improve long-term outcomes of critically ill.
Conclusion and Future Directions
Despite decades of research into molecular mechanisms of sepsis and ARDS, current management of these conditions remains largely supportive [59,60]. In severe cases of sepsis and ARDS, mortality reaches 40 percent [61,62] and is typically a result of a refractory multiple organ failure [63]. Non-resolving inflammation and immune dysregulation have been implicated in multiple organ dysfunction syndrome [40,64].
However, immune-targeted strategies expected to favorably modify the adverse inflammation have not improved outcomes in clinical trials of sepsis or ARDS [40]. It has therefore been advocated by many that the list of potential therapeutic targets might need to be expanded beyond the components of the dysregulated immune system [10].
It is now broadly acknowledged that critical illness is associated with a profound and rapid disruption of all microbiomes [11,65,66]. For instance, an abnormal gut microbiome has long been considered to play a key role in sepsis by driving systemic inflammation and immune suppression simultaneously [32,45]. Recent evidence also suggests that an altered lung microbiome might promote inflammation and lung parenchymal injury in ARDS [8-10]. Further complicating matters is the simultaneous impairment of anti-infectious immunity which predisposes critically ill patients to infections with opportunistic pathogens, often leading to prolonged treatment with broad spectrum antibiotics [67,68]. The complex circle of dysbiosis, inflammation, infection and tissue injury that characterizes these conditions creates major challenges when designing rational therapeutic interventions [10]. The ideal interventions in sepsis and ARDS would not only target the adverse inflammation but also simultaneously restore the immune competence as well as normal composition of altered microbiomes. On the other hand, the microbes causing infection or the ones promoting adverse inflammation would be targeted by a highly specific antibiotic therapy. Small steps have already been taken in some of these directions. Clinical trials with immune checkpoint inhibitors (PD-1 antagonist), drugs that are now broadly used in cancer immunotherapy are currently ongoing in sepsis [40,57]. Reversing the “immune exhaustion” by these drugs in the right patient populations might be beneficial in reducing opportunistic infections and recurrent sepsis episodes [57,68]. First microbiome-targeted interventions consisting of either depleting [69] or adding microbiota [43] have already been tested in patients with multiple organ failure, sepsis, and C. difficile colitis. However, a large amount of additional research is needed for these interventions to become broadly utilized in clinical care.
As our knowledge on microbiome dysregulation in sepsis and ARDS increases, new questions also arise as to the value of serially monitoring the dynamics of the microbiome during ICU care, for example, in cases of non-resolving multiple organ dysfunction or severe ARDS. Of note, recent identification of gut anaerobes in lungs of patients with ARDS was made possible with the use of culture-independent techniques that are not routinely used by clinical laboratories. Because these potential pathogens are likely to remain undetected using routine culture methods [36,70], their antibiotic targeting would also remain only empiric. As the evidence for pathogenic roles of gut-associated microbes in sepsis, trauma, and ARDS further expands, clinical practice might need to adjust to the new requirements for precise and timely bacterial identification, characterization, and antibiotic susceptibility testing. The exact process of bacterial translocation to the lung during sepsis and ARDS also requires further study. Given that the current evidence is only indirect, the use of radio-imaging techniques to visualize bacterial migration to the lung might be warranted when testing novel interventions to attenuate this potentially important pathogenic process [71].
Acknowledgments
This research was supported by a grant from the National Institute of General Medical Sciences (T32 GM007592) to Dusan Hanidziar. We want to thank Ruby Feng, BA for her assistance with preparing the figure
Glossary
- ARDS
adult respiratory distress syndrome
- ICU
intensive care unit
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL-17
interleukin 17
Author Contributions
Samiran Mukherjee, MBBS conducted literature search and prepared the draft of the manuscript. Dusan Hanidziar, MD PhD provided guidance on the topic, provided mentorship during the manuscript preparation process, wrote parts of the manuscript, and edited the manuscript.
References
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. [DOI] [PubMed] [Google Scholar]
- Scales BS, Dickson RP, Huffnagle GB. A tale of two sites: how inflammation can reshape the microbiomes of the gut and lungs. J Leukoc Biol. 2016;100(5):943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BJ, Imai I, Bittinger K, Laughlin A, Fuchs BD, Bushman FD, et al. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12:150–6. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunology. 2017;6(3):e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol. 2016;196(12):4839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrami K, Sweeney DA. The microbiome of the critically ill patient. Curr Opin Crit Care. 2017:1. [DOI] [PubMed] [Google Scholar]
- Panzer AR, Lynch SV, Langelier C, Christie JD, McCauley K, Nelson M, et al. Lung Microbiota Is Related to Smoking Status and to Development of Acute Respiratory Distress Syndrome in Critically Ill Trauma Patients. Am J Respir Crit Care Med. 2018;197(5):621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP. The Lung Microbiome and ARDS: It’s Time to Broaden the Model. Am J Respir Crit Care Med. 2017;197(5):549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP. The microbiome and critical illness. Lancet Respir. 2016;4(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SD, Woeltje KF, Angenent LT. Endotracheal tube biofilm inoculation of oral flora and subsequent colonization of opportunistic pathogens. Int J Med Microbiol. 2010;300(7):503–11. [DOI] [PubMed] [Google Scholar]
- Kalanuria A, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer TP, Ewan V, Wilson P, Simpson AJ. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc. 2016;17(3):238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22(11):1277–84. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33(4):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377(6):562–72. [DOI] [PubMed] [Google Scholar]
- Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. MBio. 2017;8(1):2287–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Huffnagle GB. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015;11(7):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Hountras P, Wunderink RG. The microbiome in mechanically ventilated patients. Curr Opin Infect Dis. 2017;30(2):208–13. [DOI] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Cell Mol Physiol. 2015;309(10):1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017;10(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir S, Zerimech F, Jaillette E, Artru F, Balduyck M. Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets. 2011;11(4):413–23. [DOI] [PubMed] [Google Scholar]
- Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-Donadille A, et al. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J Oncol. 2017;2017:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Moore RJ, Wong CH. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. 2018;8(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207:103–11. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generoso S V, Viana ML, Santos RG, Arantes RME, Martins FS, Nicoli JR, et al. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur J Nutr. 2011;50(4):261–269. [DOI] [PubMed] [Google Scholar]
- König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: A prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45(2):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond). 1997;92(4):385–9. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Zeman RL. The Acute Respiratory Distress Syndrome: pathogenesis and Treatment. Annu Rev Pathol. 2011;6:147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wip C, Napolitano L. Bundles to prevent ventilator-associated pneumonia: how valuable are they? Curr Opin Infect Dis. 2009;22(2):159–66. [DOI] [PubMed] [Google Scholar]
- Brook I. Clinical review: bacteremia caused by anaerobic bacteria in children. Crit Care. 2002;6(3):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194(3):855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M, Ward PA. The Inflammatory Response in Sepsis. Trends Immunol. 2013;34(3):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NR, Galley HF. Immunomodulation in the critically ill. Br J Anaesth. 2009;103(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and “dysbiosis therapy” in critical illness. Curr Opin Crit Care. 2016;22(4):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016;20(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PR, González-Navajas JM, Jansen NJ. The digestive tract as the origin of systemic inflammation. Crit Care. 2016;20(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MC, Haak BW, Hugenholtz F, Wiersinga WJ. Gut microbiota and host defense in critical illness. Curr Opin Crit Care. 2017;23(4):257–63. [DOI] [PubMed] [Google Scholar]
- Cabrera-Perez J, Badovinac VP, Griffith TS. Enteric immunity, the gut microbiome, and sepsis: rethinking the germ theory of disease. Exp Biol Med (Maywood). 2017;242(2):127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J Immunol Res. 2015;2015:489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He GZ, Zhou KG, Zhang R, Wang YK, Chen XF. Impact of intestinal ischemia/reperfusion and lymph drainage on distant organs in rats. World J Gastroenterol. 2012;18(48):7271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8(1):1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. 2017;9(376):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Lankelma JM, Scicluna BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asehnoune K, Roquilly A, Abraham E. Innate Immune Dysfunction in Trauma Patients. Anesthesiology. 2012;117(2):411–6. [DOI] [PubMed] [Google Scholar]
- Bauer TT, Ewig S, Rodloff AC, Muller EE. Acute Respiratory Distress Syndrome and Pneumonia: A Comprehensive Review of Clinical Data. Clin Infect Dis. 2006;43(6):748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMerle KM, Royer SC, Mikkelsen ME, Prescott HC. Readmissions for Recurrent Sepsis. Crit Care Med. 2017;45(10):1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014;5(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret G, Venet F. A rapidly progressing lymphocyte exhaustion after severe sepsis. Crit Care. 2012;16(4):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Davis AM. Management of Sepsis and Septic Shock. JAMA. 2017;317(8):847. [DOI] [PubMed] [Google Scholar]
- Howell MD, Davis AM. Management of ARDS in Adults. JAMA. 2018;319(7):711. [DOI] [PubMed] [Google Scholar]
- Santos RS, Silva PL, Rocco JR, Pelosi P, Rocco PR. A mortality score for acute respiratory distress syndrome: predicting the future without a crystal ball. J Thorac Dis. 2016;8(8):1872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and Timing of Death in Patients With ARDS. Chest. 2005;128(2):525–32. [DOI] [PubMed] [Google Scholar]
- Pinsky MR. Dysregulation of the Immune Response in Severe Sepsis. Am J Med Sci. 2004;328(4):220–9. [DOI] [PubMed] [Google Scholar]
- McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, et al. Extreme Dysbiosis of the Microbiome in Critical Illness. MSphere. 2016;1(4):e00199–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MB, Firek B, Shi M, Yeh A, Brower-Sinning R, Aveson V, et al. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome. 2016;4(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway Morris A, Anderson N, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, et al. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. Br J Anaesth. 2013;111(5):778–87. [DOI] [PubMed] [Google Scholar]
- van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016;315(14):1469. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, Zandstra DF, Marshall JC, Gregori D, Gullo A. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med. 2010;38(5):1370–6. [DOI] [PubMed] [Google Scholar]
- Strobel HJ. Basic Laboratory Culture Methods for Anaerobic Bacteria. Methods Mol Biol. 2009;581:247–61. [DOI] [PubMed] [Google Scholar]
- Wang L, Llorente C, Hartmann P, Yang A, Chen P, Schnabl B, et al. Methods To Determine Intestinal Permeability and Bacterial Translocation During Liver Disease. J Immunol Methods. 2015;421:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]