Abstract
Background
Molecular hydrogen (H2) has been widely reported to have benefiicial effects in diverse animal models and human disease through reduction of oxidative stress and inflammation. The aim of this study was to investigate whether hydrogen gas could ameliorate endotoxin-induced uveitis (EIU) in rats.
Material/Methods
Male Sprague-Dawley rats were divided into a normal group, a model group, a nitrogen-oxygen (N-O) group, and a hydrogen-oxygen (H-O) group. EIU was induced in rats of the latter 3 groups by injection of lipopolysaccharide (LPS). After that, rats in the N-O group inhaled a gas mixture of 67% N2 and 33% O2, while those in the H-O group inhaled a gas mixture of 67% H2 and 33% O2. All rats were graded according to the signs of uveitis after electroretinography (ERG) examination. Protein concentration in the aqueous humor (AqH) was measured. Furthermore, hematoxylin-eosin staining and immunostaining of anti-ionized calcium-binding adapter molecule 1 (Iba1) in the iris and ciliary body (ICB) were carried out.
Results
No statistically significant differences existed in the graded score of uveitis and the b-wave peak time in the Dark-adapted 3.0 ERG among the model, N-O, and H-O groups (P>0.05), while rats of the H-O group showed a lower concentration of AqH protein than that of the model or N-O group (P<0.05). The number of the infiltrating cells in the ICB of rats from the H-O group was not significantly different from that of the model or N-O group (P>0.05), while the activation of microglia cells in the H-O group was somewhat reduced (P<0.05).
Conclusions
Post-treatment hydrogen gas inhalation did not ameliorate the clinical signs, or reduce the infiltrating cells of EIU. However, it inhibited the elevation of protein in the AqH and reduced the microglia activation.
MeSH Keywords: Electroretinography, Lipopolysaccharides, Microglia, Uveitis
Background
Uveitis is the most common form of intraocular inflammation and is defined as a particular type of inflammation in the uvea that can involve other ocular tissues, such as vitreous and retina [1]. The incidence of uveitis was reported to be 52.4/100 000 person-years in the USA [2], and it is the cause of approximately 10% to 15% of preventable blindness in Western countries [3]. Corticosteroids and immunosuppressive drugs are the cornerstone of treating uveitis. However, they are usually associated with systemic disorders, such as increased susceptibility to microbial infection. New and effective measures against uveitis with less adverse side effects are urgently required. Despite the complicated etiology and pathological mechanism of uveitis, oxidative stress (OS) has generally been accepted to play an important role in the progress of uveitis [4,5]. Endotoxin-induced uveitis (EIU) is a widely used animal model to mimic the pathological features and investigate potential treatments for human uveitis [6]. Its pathogenesis was deemed to be closely associated with OS [7,8], and drugs that can mitigate OS have been shown to ameliorate EIU [9,10].
Molecular hydrogen (H2) has been found to possess antioxidant and anti-inflammatory properties, and has been established as a new kind of antioxidant reported to be beneficial in the prevention and treatment of several diseases [11,12]. Inflammation is a basic pathological process in many diseases. H2 has been demonstrated to alleviate inflammation in the lungs, pancreas, and liver [13,14]. The H2-induced relief of inflammation may be due to its suppression of the inflammatory mediators [15], or through its direct interaction with reactive oxygen species (ROS), which were reported to be one of the causes of inflammation [16].
Our previous study [17] found that hydrogen-rich saline (HRS) inhibited the elevation of protein in the aqueous humor (AqH), but did not obviously mitigate the clinic manifestation of EIU rats. Hydrogen gas, compared to HRS, provides a higher amount of H2 and has also been shown to be effective against multiple diseases [18,19]. Therefore, this study was conducted to investigate whether post-treatment of hydrogen gas could somewhat ameliorate EIU in rats.
Material and Methods
Animals and LPS administration
Male Sprague-Dawley (SD) rats (6 to 8 weeks old) were obtained from the Laboratory Animal Center of the Fourth Military Medical University (License number: 2014270138S, Xi’an, China), and kept under dim cyclic light (5 lux, 12 h on/off, 6 a.m.–6 p.m.), with food and water available ad libitum. All experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Care and Use of Laboratory Animals.
The rats were randomly divided into a normal group, a model group, a nitrogen-oxygen (N-O) group, and a hydrogen-oxygen (H-O) group. Lipopolysaccharide (LPS, #L6511, Sigma, USA) was stored in the dark at 4°C upon arrival and was dissolved in saline to a concentration of 2 mg/ml before use [20]. In the rats of the latter 3 groups, we induced uveitis by subcutaneous injection of 1/8 mg/kg LPS into their footpads. Twenty-four rats (6 for each group) were subjected for evaluation of the clinical manifestation in EIU under slit-lamp and electroretinography (ERG) examination, as these 2 indicators were obtained while the rats were alive. Another 36 rats were subjected for protein concentration measurement, histological examination with hematoxylin & eosin (HE) staining, and immunostaining of microglia cells. To collect these data, 12 rats were included in the other three groups except the normal group (6 rats for each group at each time point). This was aimed to minimize the number of animals according to the 3Rs principles of animal experiments.
Hydrogen gas intervention
Rats in the N-O group inhaled a gas mixture of 67% N2 and 33% O2 (the N2-O2 mixed gas, product code #10021120, Shaanxi XingHua Chemical Co. LTD., Xi’an, China) at a flow rate of 3 L/min for 1 h at 0 and 1 h after LPS injection and once a day for 3 weeks afterwards. Rats in the H-O group inhaled a gas mixture of 67% H2 and 33% O2 at a flow rate of 3 L/min at the same period. As previously reported [21], the H2-O2 mixed gas was produced using an AMS-H-01 hydrogen-oxygen nebulizer (Shanghai Asclepius Meditec Co., Ltd., China), which can extract 67% H2 and 33% O2 from water. During the period of gas inhalation, the rats were awake and freely moving in a special gas chamber consisting of a transparent closed box. The concentration of H2 in the box was monitored using thermal trace GC ultra-gas chromatography (Thermo Fisher, MA, USA), and was confirmed to be 67% throughout the study. Rats in the normal and model groups did not receive any treatment.
Clinical evaluation of EIU
Slit-lamp examination was conducted under euthanization at 12, 24, 48, 72, and 96 h after LPS injection. Pictures of the rat eyes with the slit on the middle of the cornea were acquired. Clinical scoring of uveitis was graded as previously reported [22]: iris hyperemia (0–2), flare (0–2), hypopyon (0–2), and miosis (0–2). The maximum possible score was 8.
ERG recording
ERG was carried out 1, 4, 7, 14, and 21 d after inducing EIU, as previously reported [23]. All ERG indicators were recorded and analyzed according to the ISCEV guidelines [24] by a technician blinded to the animal grouping and treatment.
Protein concentration measurement in the AqH
AqH was collected by anterior chamber puncture with an insulin syringe immediately after euthanization of rats at 1 and 4 d after LPS administration. Total protein concentration in the AqH was measured by a bicinchoninic acid (BCA) assay (#W041, Jiancheng, Nanjing, China) according to the manufacturer’s instructions.
HE staining of ICB
Eyes of rats from all groups were enucleated rapidly after an intraperitoneal injection of a lethal dose of sodium pentobarbital (#P3761,Sigma-Aldrich, USA) at 1 and 4 d after LPS administration, and were stored in 4% paraformaldehyde solution at 4°C overnight. The 4-μm sagittal sections were cut along the pupil-optic nerve axis. For each eye, 3 sections that included the optic nerve were stained with HE. Images of ICB were taken using a digital imaging system (DP71, Olympus, Japan) and analyzed by counting the infiltrating cells in the ICB at high magnification (×400).
Immunostaining
Vertical sections including the optic nerve in the paraffin-embebbed eye samples collected at 24 h after LPS administration were submerged in xylene and then rehydrated with ethanol gradient washes. Before further use, slides were washed 3 times in phosphate-buffered saline (PBS) for 5 min. The sections were then heated in a microwave oven for antigen recovery, washed in PBS, and treated with 10% normal goat serum (#AR0009, Boster, Wuhan, China) for 1 h at room temperature. The slides were subjected to immunostaining at 4°C overnight with the primary antibody against microglia cells: polyclonal rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1, #019-19741, Wako Chemicals, Japan) at 1: 500 dilution. After washing in PBS, the secondary antibody (goat anti-rabbit IgG conjugated to Alexa Fluor 594, #ZF-0516, ZSGB-BIO, Beijing, China) at a 1: 500 dilution was used. The sections were washed again in PBS and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI, #C1005, Beyotime, Nantong, China). Images of ICB in the sections were obtained using a confocal microscope (LSM510, Zeiss, Oberkochen, Germany). The activation state of microglia cells was measured by calculating the ratio of Iba1-positive cell number to DAPI-postive cell number in 3 sections for each eye and averaged.
Statistical analysis
All data were shown as mean ± standard error (S.E.) and analyzed by one-way analysis of variance (ANOVA) followed by contrast analysis (LSD-t test when equal variances were assumed and Dunnett’s T3 test when equal variances were not assumed) using SPSS software (version 16.0, Chicago, USA). Differences were considered statistically significant at P<0.05.
Results
Effects of hydrogen gas on clinical manifestation and retinal function of EIU
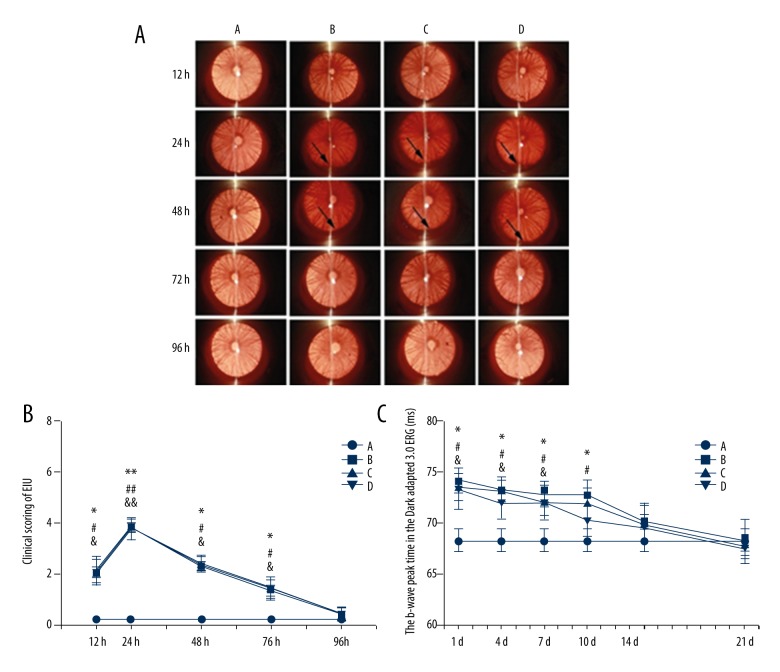
Rats in the normal group had no signs of uveitis, while rats of the other 3 groups manifested typical signs of uveitis, such as iris hyperemia and hypopyon after injection of LPS. From 12 to 74 h after LPS administration, the graded score of uveitis signs in the model, N-O, or H-O group was higher than that of the normal group (P<0.05), while there were no significant differences among the 3 groups (P>0.05). At 96 h, the uveitis of the model, N-O, and H-O groups disappeared (Figure 1A, 1B).
Figure 1.
Clinical manifestation (A) and graded scoring (B) of uveitis under slit-lamp within 96 h after LPS injection, and the b-wave peak time in the Dark-adapted 3.0 ERG within 21 d after LPS administration (C). Arrow: hypopyon in the anterior chamber. A – normal group; B – model group; C – nitrogen-oxygen (N-O) group; D – hydrogen-oxygen (H-O) group; *, #, & (**, ##, &&) P<0.05 (P<0.01): model, N-O, or H-O vs. normal group, respectively.
From 1 to 7 d after LPS injection, the b-wave peak time in the Dark Adaptation 3.0 ERG of the model, N-O or H-O group was longer than that of the normal group (P<0.05), while there were no statistical differences among the 3 groups (P>0.05). At 10 d, the b-wave peak time of the model or N-O group was statistically longer than that of the normal group (P<0.05), while the peak time of the H-O group was not significantly different from that of the normal group (P>0.05). However, no significant differences were found among the model, N-O, and H-O groups. At 14 and 21 d, no significant differences were found among the 4 groups (P>0.05) (Figure 1C).
Effects of hydrogen gas on AqH protein concentration in EIU
One day after LPS injection, protein concentrations in the AqH of the normal, model, N-O, and H-O groups were 1.65±0.09, 4.47±0.12, 4.28±0.08, and 3.34±0.46 mg/ml, respectively. The AqH protein concentration in the model, N-O or H-O group was higher than that of the normal group, with differences being statistically significant (P=0.002, the model group vs. the normal group; P=0.003, the N-O group vs. the normal group; P=0.006, the H-O group vs. the normal group). The protein concentration of the H-O group was significantly lower than that of the model or the N-O group (P=0.011, the H-O group vs. the model group; P=0.013, the H-O group vs. the N-O group). At 4 d, protein concentration returned to a normal level in the model, N-O, and H-O groups, and no significant differences were found among the 4 groups (P>0.05) (Figure 2).
Figure 2.
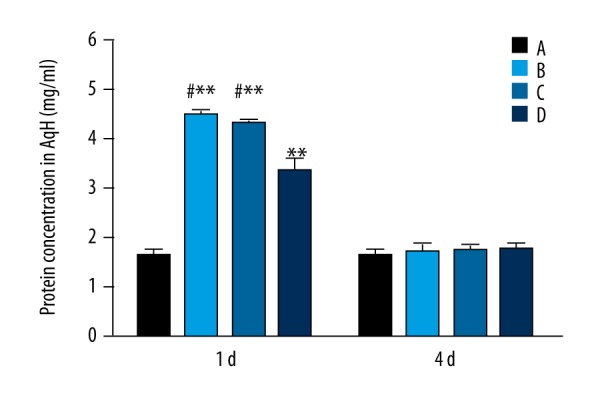
Protein concentration in the AqH 1 and 4 d after LPS injection. A – normal group; B – model group; C – nitrogen-oxygen (N-O) group; D – hydrogen-oxygen (H-O) group; ** P<0.01 vs. normal group; # P<0.05 vs. H-O group.
Effects of hydrogen gas on the cells infiltration in EIU
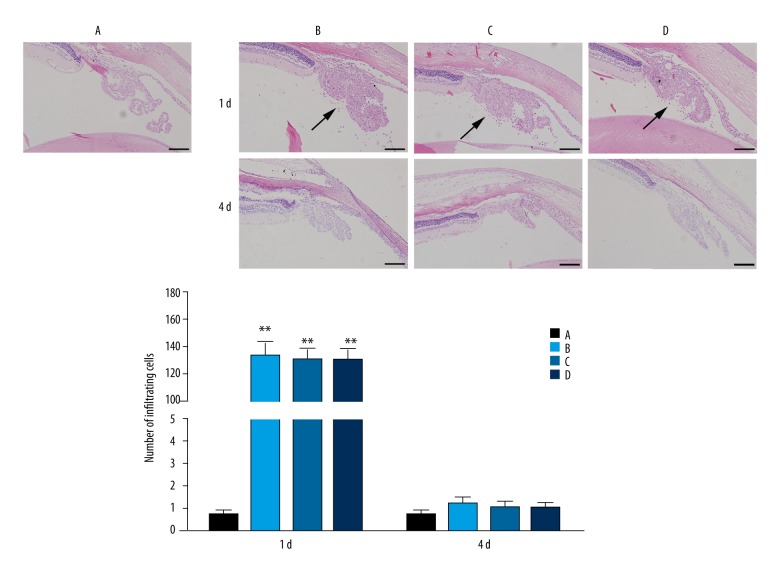
There was an obvious sign of cells infiltration in the ICB of rats from the model, N-O, and H-O groups at 1 d after LPS injection; the cell numbers were 133.02±10.5, 125.30±9.23, and 130.00±8.73, respectively. The number of infiltrating cells in the model, N-O or H-O group was significantly different from that of the normal group (0.67±0.20) (P<0.01), while there were no significant differences among the model, N-O and H-O groups (P>0.05). At 4 d, cells infiltration in the ICB disappeared in the model, N-O, and H-O groups, with no significant differences among the 4 groups (P>0.05) (Figure 3).
Figure 3.
HE staining of the ICB (×200) and number of total infiltrating cells in the ICB 1 and 4 d after LPS injection. Arrow: infiltrating cells; Scale: 100 μm. A – normal group; B – model group; C – nitrogen-oxygen (N-O) group; D – hydrogen-oxygen (H-O) group; ** P<0.01 vs. normal group.
Effects of hydrogen gas on microglia activation in EIU
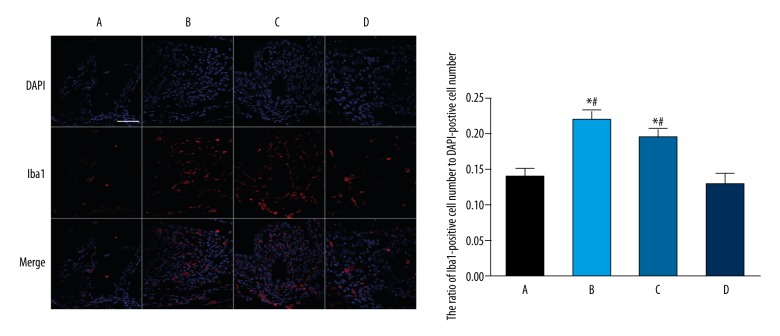
To visualize the presence of microglia cells, we used the Iba1 marker [25]. At 24 h after LPS administration, there was a substantial increase of Iba1-positive cells in the model and N-O groups, with the ratio of Iba1-positive cell number to DAPI-positive cell number significantly reduced compared to that in the normal group (P=0.013, the model group vs. the normal group; P=0.019, the N-O group vs. the normal group). The ratio of Iba1-positive cell number to DAPI-positive cell number in the H-O group was markedly lower than that of the model or the N-O group with statistically significant differences (P=0.012, the H-O group vs. the model group; P=0.018, the H-O group vs. the N-O group) (Figure 4).
Figure 4.
Representative photomicrographs of Iba1-positive microglia cells in the ICB and quantification of ratio of Iba1-positive cell number to DAPI-postive cell number 24 h after LPS injection. Scale: 50 μm; A – normal group; B – model group; C – nitrogen-oxygen (N-O) group; D – hydrogen-oxygen (H-O) group; * P<0.05 vs. normal group; # P<0.05 vs. H-O group.
Discussion
Inhalation of hydrogen gas, compared to HRS administration, provides a much higher amount of H2, keeps a higher peak concentration in the body and maintains it for a longer time [26]. Therefore, to some extent, hydrogen gas inhalation might yield a better result in disease control and prevention. It has been reported that hydrogen gas ameliorates several diseases [27]. As early as 1975, Dole et al. [28] showed that a high concentration of hydrogen gas (97.5% H2 and 2.5% O2) somewhat reduced the area of squamous cell carcinoma in albino mice. Xie et al. [29] discovered that hydrogen gas ameliorated the lung inflammation in mice with acute lung injury, which was induced by bronchial suction of LPS. By using a rat model of cardiac arrest, Hayashida et al. [30] found that hydrogen gas inhalation during cardiopulmonary resuscitation (CPR) improved heart and brain function.
In the current study, we applied a relatively high concentration of hydrogen gas (67% H2 and 33% O2) as an intervention for EIU in rats, with the control rats inhaling the N2-O2 mixed gas (67% N2 and 33% O2). The method of hydrogen gas inhalation is portable, easy to administer, and safe. The gas mixture used to prevent decompression sickness contains up to 49% of H2 [31]. Besides, the gas mixture containing up to 90% of H2 did not affect the cell viability in normally cultured RAW 264.7 macrophages [32]. Throughout our study, the H2-O2 or N2-O2 mixed gas did not influence the mental state, daily activities, diet, sleep, or body weight of rats.
EIU is an animal model of human uveitis induced by LPS, a component of the outer membranes of gram-negative bacteria [33]. A major pathological characteristic of EIU is the ocular infiltration of inflammatory cells, which manifests as hypopyon under slit-lamp and cells infiltrating in the ICB in histological examination. The majority of the infiltrating cells were polymorphonuclear leucocytes, namely neutrophils, which have been recognized as a key element in the pathogenesis of EIU [34,35]. Due to the production of proteolytic enzymes and toxic intermediates, neutrophils are essential for innate immunity in evading infection and attacking a foreign agent [36]. However, these defense processes, which occur through destroying and digesting extraneous matter, are potentially deleterious to the body tissue [37]. Thus, it is vital that neutrophils are cleared in a timely manner following the completion of their physiological function. In this study, we found that the hypopyon and the infiltration of inflammatory cells in EIU were not obviously alleviated by post-treatment hydrogen gas inhalation. In the model of EIU, the inflammatory mediator that elicited neutrophils infiltration was reported to be leukotriene B4 (LTB4) [38]. An elevated level of LTB4 in isoproterenol (ISO)-induced heart failure was shown to be reduced by hydrogen sulfide (H2S) [39], an antioxidant gasotransmitter. However, to our knowledge, whether H2 could reduce the LTB4 level has not been illustrated before. As we previously suggested [17], LTB4 may not be influenced by H2 in the form of HRS, or even in the form of a high concentration of hydrogen gas. Therefore, the signs of hypopyon and cells infiltration in EIU were not relieved by H2 intervention.
The retina is easily involved in the process of uveitis, and decreased retinal function has been reported in EIU [40]. In our study, ERG results showed that the reduced retinal function of EIU rats was not significantly improved by hydrogen gas inhalation. Studies on the animal model of light-induced retinopathy and the MNU-indcued retinal degeneration suggested an amelioration of retinal function reduction by a combination of H2-pre-treatment and H2-post-treatment [41,42]. Similarly, inclusion of hydrogen gas inhalation prior to LPS injection may be beneficial to the relief of EIU in our study.
Under normal circumstances, the protein concentration in the AqH is relatively low. Elevation of protein in the AqH suggests the breakdown of blood-AqH barrier, which is a typical sign of uveitis [43]. In our study, the AqH protein concentration in the H-O group was reduced compared to that of the model or N-O group, indicating that hydrogen gas somewhat inhibited the elevation of the AqH protein in EIU rats. Prostaglandin E2 was shown to be elevated in EIU rats and was recognized the inflammatory mediator responsible for the exudation of protein into the AqH [44]. The reduction of AqH protein by hydrogen gas might be attributed to the fact that hydrogen gas, as HRS [17], reduces the level of PGE2 in EIU.
Microglia cells are the main form of adaptive immune response in the central nervous system. In response to insults, microglia cells undergo a number of changes [45], such as processes retraction, and cell body enlargement, leading to the amoeboid shape. Overactivation and prolonged activation of microglia cells have been closely linked to various neurological diseases [46,47] and retinal degeneration [48]. The activated microglia cells are often highly mobile. The activation and migration of these cells is also regarded as a non-specific early stress response in inflammation [49]. Uveitis, as a particular type of inflammation in the uvea, might involve the activation of microglia cells [50]. Our results showed that microglia cells were actively involved in EIU rats, and the activation state of microglia cells was somewhat mitigated by hydrogen gas. Monocyte chemoattractant protein-1 (MCP-1), a chemoattractant and an inflammatory cytokine, plays a crucial role in microglia migration/infiltration and neuroinflammatory disorders [51,52]. Interleukin (IL)-6 and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) are 2 other important inflammatory factors associated with microglia activation [53,54]. The reduction of MCP-1 and IL-6 by H2 has been reported in the animal models of spontaneous hypertensive and nephrotoxicity [55,56], while H2 has no significant effect on the level of RANTES [57]. Elevation of MCP-1 and IL-6 has also been confirmed in the model of EIU [58,59]. Furthermore, suppression of microglia activation by H2 has also been demonstrated by other investigators [60,61]. Thus, the hydrogen gas-induced amelioration of microglia activation in EIU rats might due to the inhibition of MCP-1 and IL-6.
Conclusions
This study found that post-treatment hydrogen gas inhalation somewhat suppressed the elevation of AqH protein and the activation of microglia cells in EIU rats, while it did not obviously ameliorate the cells infiltrating in the ICB and reduced retinal function of EIU. The discrepant effects of molecular hydrogen on different indicators of EIU might be due to its selective suppression on some inflammatory mediators. Further investigations are needed to clarify the above hypothesis. Besides, hydrogen gas treatment prior to LPS injection might be beneficial, and would be worth investigating in the future.
Footnotes
Source of support: This work was supported by grants from the Foundation of Open Sharing Platform of Science and Technology of Shaanxi Province, R.P. China (2015FWPT-02), and a grant from the National Natural Science Foundation of China (81500724)
Conflicts of interest
None.
References
- 1.Seve P, Cacoub P, Bodaghi B, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017;16:1254–64. doi: 10.1016/j.autrev.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Prete M, Dammacco R, Fatone MC, Racanelli V. Autoimmune uveitis: Clinical, pathogenetic, and therapeutic features. Clin Exp Med. 2016;16:125–36. doi: 10.1007/s10238-015-0345-6. [DOI] [PubMed] [Google Scholar]
- 3.Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123:655–62. doi: 10.1016/j.ophtha.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Turk A, Aykut M, Akyol N, et al. Serum anti-carbonic anhydrase antibodies and oxidant-antioxidant balance in patients with acute anterior uveitis. Ocul Immunol Inflamm. 2014;22:127–32. doi: 10.3109/09273948.2013.830753. [DOI] [PubMed] [Google Scholar]
- 5.Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000;19:41–68. doi: 10.1016/s1350-9462(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 6.Tsubura A, Lai YC, Miki H, et al. Review: Animal models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. In Vivo. 2011;25:11–22. [PubMed] [Google Scholar]
- 7.Kubota S, Kurihara T, Mochimaru H, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–19. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 8.Qin YJ, Chu KO, Yip YW, et al. Green tea extract treatment alleviates ocular inflammation in a rat model of endotoxin-induced uveitis. PLoS One. 2014;9:e103995. doi: 10.1371/journal.pone.0103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncu T, Oguz E, Sezen H, et al. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq Bras Oftalmol. 2016;79:357–62. doi: 10.5935/0004-2749.20160102. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz A, Yildirim O, Tamer L, et al. Effects of caffeic acid phenethyl ester on endotoxin-induced uveitis in rats. Curr Eye Res. 2005;30:755–62. doi: 10.1080/02713680590967962. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara M, Sobue S, Ito M, et al. Beneficial biological effects and the underlying mechanisms of molecular hydrogen – comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–79. doi: 10.1055/s-0034-1395509. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Yang H, Fan Y, et al. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediators Inflamm. 2016;2016:1320365. doi: 10.1155/2016/1320365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Q, Chen C, Deng WH, et al. Hydrogen-rich saline attenuates acute hepatic injury in acute necrotizing pancreatitis by inhibiting inflammation and apoptosis, involving JNK and p38 mitogen-activated protein kinase-dependent reactive oxygen species. Pancreas. 2016;45:1424–31. doi: 10.1097/MPA.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yu P, Yang Y, et al. Hydrogen-rich saline resuscitation alleviates inflammation induced by severe burn with delayed resuscitation. Burns. 2015;41:379–85. doi: 10.1016/j.burns.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Guo SX, Fang Q, You CG, et al. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J Transl Med. 2015;13:183. doi: 10.1186/s12967-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan WM, Zhang L, Chen T, et al. Effects of hydrogen-rich saline on endotoxin-induced uveitis. Med Gas Res. 2017;7:9–18. doi: 10.4103/2045-9912.202905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato R, Nomura A, Sakamoto A, et al. Hydrogen gas attenuates embryonic gene expression and prevents left ventricular remodeling induced by intermittent hypoxia in cardiomyopathic hamsters. Am J Physiol Heart Circ Physiol. 2014;307:H1626–33. doi: 10.1152/ajpheart.00228.2014. [DOI] [PubMed] [Google Scholar]
- 19.Wu CY, Hsu WL, Tsai MH, et al. Hydrogen gas protects IP3Rs by reducing disulfide bridges in human keratinocytes under oxidative stress. Sci Rep. 2017;7:3606. doi: 10.1038/s41598-017-03513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keles S, Halici Z, Atmaca HT, et al. The ocular endothelin system: A novel target for the treatment of endotoxin-induced uveitis with bosentan. Invest Ophthalmol Vis Sci. 2014;55:3517–24. doi: 10.1167/iovs.14-14193. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Wu J, Chen Z, et al. Postconditioning with inhaled hydrogen promotes survival of retinal ganglion cells in a rat model of retinal ischemia/reperfusion injury. Brain Res. 2016;1632:82–90. doi: 10.1016/j.brainres.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Lajavardi L, Bochot A, Camelo S, et al. Downregulation of endotoxin-induced uveitis by intravitreal injection of vasoactive intestinal Peptide encapsulated in liposomes. Invest Ophthalmol Vis Sci. 2007;48:3230–38. doi: 10.1167/iovs.06-1305. [DOI] [PubMed] [Google Scholar]
- 23.Yan W, Yao L, Liu W, et al. A kind of rd1 mouse in C57BL/6J mice from crossing with a mutated Kunming mouse. Gene. 2017;607:9–15. doi: 10.1016/j.gene.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 25.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, Kurokawa R, Fujino M, et al. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep. 2014;4:5485. doi: 10.1038/srep05485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. 1975;190:152–54. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 29.Xie K, Yu Y, Huang Y, et al. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37:548–55. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 30.Hayashida K, Sano M, Kamimura N, et al. Hydrogen inhalation during normaloxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation. 2014;130:2173–80. doi: 10.1161/CIRCULATIONAHA.114.011848. [DOI] [PubMed] [Google Scholar]
- 31.Abraini JH, Gardette-Chauffour MC, Martinez E, et al. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J Appl Physiol (1985) 1994;76:1113–18. doi: 10.1152/jappl.1994.76.3.1113. [DOI] [PubMed] [Google Scholar]
- 32.Chen HG, Xie KL, Han HZ, et al. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg. 2013;11:1060–66. doi: 10.1016/j.ijsu.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Hsu YR, Chang SW, Yang CH, et al. Expression profile of cationic amino acid transporters in rats with endotoxin-induced uveitis. Mediators Inflamm. 2016;2016:6586857. doi: 10.1155/2016/6586857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang ZX, Qiu S, Lou BS, et al. Roscovitine ameliorates endotoxin-induced uveitis through neutrophil apoptosis. Mol Med Rep. 2016;14:1083–90. doi: 10.3892/mmr.2016.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mashimo H, Ohguro N, Nomura S, et al. Neutrophil chemotaxis and local expression of interleukin-10 in the tolerance of endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:5450–57. doi: 10.1167/iovs.08-1878. [DOI] [PubMed] [Google Scholar]
- 36.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witko-Sarsat V. Neutrophils in the innate immunity conundrum of cystic fibrosis: A CFTR-related matter? J Innate Immun. 2013;5:195–96. doi: 10.1159/000350215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbort CP, Okumura A, Mochizuki M. Immunopharmacological analysis of endotoxin-induced uveitis in the rat. Exp Eye Res. 1989;48:693–705. doi: 10.1016/0014-4835(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu YH, Lu M, Xie ZZ, et al. Hydrogen sulfide prevents heart failure development via inhibition of renin release from mast cells in isoproterenol-treated rats. Antioxid Redox Signal. 2014;20:759–69. doi: 10.1089/ars.2012.4888. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M, Ozawa Y, Kurihara T, et al. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–39. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 41.Chen T, Tao Y, Yan W, et al. Protective effects of hydrogen-rich saline against N-methyl-N-nitrosourea-induced photoreceptor degeneration. Exp Eye Res. 2016;148:65–73. doi: 10.1016/j.exer.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Qi LS, Yao L, Liu W, et al. Sirtuin type 1 mediates the retinal protective effect of hydrogen-rich saline against light-induced damage in rats. Invest Ophthalmol Vis Sci. 2015;56:8268–79. doi: 10.1167/iovs.15-17034. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Zhao B, Jiang R, et al. Cytokine expression profile in aqueous humor and sera of patients with acute anterior uveitis. Curr Mol Med. 2015;15:543–49. doi: 10.2174/1566524015666150731100012. [DOI] [PubMed] [Google Scholar]
- 44.Herbort CP, Okumura A, Mochizuki M. Endotoxin-induced uveitis in the rat. A study of the role of inflammation mediators. Graefes Arch Clin Exp Ophthalmol. 1988;226:553–58. doi: 10.1007/BF02169204. [DOI] [PubMed] [Google Scholar]
- 45.Walker FR, Beynon SB, Jones KA, et al. Dynamic structural remodelling of microglia in health and disease: A review of the models, the signals and the mechanisms. Brain Behav Immun. 2014;37:1–14. doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Calcia MA, Bonsall DR, Bloomfield PS, et al. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233:1637–50. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savitz SI, Cox CJ. Concise review: Cell therapies for stroke and traumatic brain injury: Targeting microglia. Stem Cells. 2016;34:537–42. doi: 10.1002/stem.2253. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Zeng Y, Chen X, et al. Neural stem cells transplanted to the subretinal space of rd1 mice delay retinal degeneration by suppressing microglia activation. Cytotherapy. 2016;18:771–84. doi: 10.1016/j.jcyt.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 50.Couturier A, Bousquet E, Zhao M, et al. Anti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammation. Mol Vis. 2014;20:908–20. [PMC free article] [PubMed] [Google Scholar]
- 51.Jin D, Yang JP, Hu JH, et al. MCP-1 stimulates spinal microglia via PI3K/Akt pathway in bone cancer pain. Brain Res. 2015;1599:158–67. doi: 10.1016/j.brainres.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 52.Deng YY, Lu J, Ling EA, Kaur C. Monocyte chemoattractant protein-1 (MCP-1) produced via NF-kappaB signaling pathway mediates migration of amoeboid microglia in the periventricular white matter in hypoxic neonatal rats. Glia. 2009;57:604–21. doi: 10.1002/glia.20790. [DOI] [PubMed] [Google Scholar]
- 53.Kitai R, Zhao ML, Zhang N, et al. Role of MIP-1beta and RANTES in HIV-1 infection of microglia: Inhibition of infection and induction by IFNbeta. J Neuroimmunol. 2000;110:230–39. doi: 10.1016/s0165-5728(00)00315-5. [DOI] [PubMed] [Google Scholar]
- 54.Stone S, La Flamme AC. Type II activation of macrophages and microglia by immune complexes enhances Th17 biasing in an IL-6-independent manner. PLoS One. 2016;11:e164454. doi: 10.1371/journal.pone.0164454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Z, Chen W, Wang L, et al. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol. 2015;8:2680–89. [PMC free article] [PubMed] [Google Scholar]
- 56.Li FY, Zhu SX, Wang ZP, et al. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol. 2013;61:248–54. doi: 10.1016/j.fct.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Zhao L, Wang YB, Qin SR, et al. Protective effect of hydrogen-rich saline on ischemia/reperfusion injury in rat skin flap. J Zhejiang Univ Sci B. 2013;14:382–91. doi: 10.1631/jzus.B1200317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida T, Honjo M, Yamagishi R, Aihara M. The anti-inflammatory effect of ripasudil (K-115), a Rho Kinase (ROCK) inhibitor, on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2017;58:5584–93. doi: 10.1167/iovs.17-22679. [DOI] [PubMed] [Google Scholar]
- 59.de Vos AF, Klaren VN, Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994;35:3873–83. [PubMed] [Google Scholar]
- 60.Imai K, Kotani T, Tsuda H, et al. Neuroprotective potential of molecular hydrogen against perinatal brain injury via suppression of activated microglia. Free Radic Biol Med. 2016;91:154–63. doi: 10.1016/j.freeradbiomed.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Ge Y, Wu F, Sun X, et al. Intrathecal infusion of hydrogen-rich normalal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PLoS One. 2014;9:e97436. doi: 10.1371/journal.pone.0097436. [DOI] [PMC free article] [PubMed] [Google Scholar]