Abstract
Background
The aim of this study was to characterize adenovirus-associated acute respiratory infection (ARI) and observe correlations between inflammatory markers and severity of human adenovirus type 7 (HAdV-7) infection, and to evaluate the potential of inflammatory markers to predict progression from upper-respiratory infection (URI) to adenovirus pneumonia (AdP).
Material/Methods
A total of 81 patients with adenovirus-associated ARI and confirmed HAdV-7 infection were enrolled. Cases were classified according to severity, as AdP and URI. Demographic and clinical data were collected retrospectively. Clinical features and serum inflammatory markers were evaluated and compared according to the severity of adenoviral infection.
Results
We observed high-grade fever and strong inflammatory response in patients with HAdV-7–associated ARI. Procalcitonin (PCT), interleukin 6 (IL-6), and C-reactive protein concentrations were higher in patients with AdP than in those with URI. The mean erythrocyte sedimentation rate (ESR) was significantly higher in patients with AdP (p=0.008). Reduced serum prealbumin levels were observed in patients with HAdV-7 infection. In the analysis of URI to AdP prediction ability, areas under the curve (AUCs) for all inflammatory markers were <0.9. We found that 35.9% of pneumonia had ≥2 lobars of lung infiltrate and bilateral lung infiltrate, and 20% of patients with SP had pleural effusion and atelectasis.
Conclusions
IL-6 and ESR were associated with the severity of HAdV-7 respiratory infection. No inflammatory marker in our study predicted URI-to-AdP progression accurately. Lung infiltration and consolidation are common in HRCT in AdP. Multiple- or single-lobar/segment consolidation was most common in SP. SP progressed very quickly after onset.
MeSH Keywords: Adenovirus Infections, Human; Respiratory System; Systemic Inflammatory Response Syndrome
Background
Adenovirus is associated with a broad spectrum of clinical diseases in humans, including acute respiratory and gastrointestinal infections. More than 65 types of human adenovirus (HAdV) have been characterized and classified into 7 species (A–G) [1]. Among them, human adenovirus-B7 (HAdV-7) is a documented cause of severe infections such as pneumonia [2–4]. HAdV-7 can cause severe respiratory symptoms and complicated pneumonia. It can easily turn into a severe or critical illness and can even cause serious consequences. Once adenovirus pneumonia is developing, especially when severe pneumonia develops rapidly, it can deteriorate in a short time, leading to acute respiratory distress syndrome. If it is not managed properly, patients die quickly.
Adenovirus-associated acute respiratory infection (ARI) is characterized by prolonged high-graded fever and strong inflammatory response [5–7]. Inflammatory responses are mediated by numerous cytokines. Elevated levels of some cytokines, such as tumor necrosis factor alpha (TNF-α), have been reported to be associated with severe or fatal adenoviral infections [8]. Thus, there is a need for better understanding of the inflammatory cytokine levels in patients with HAdV-7-associated acute respiratory infection. However, such data are scarce. In addition, no previous publication has described inflammatory markers of ARI in adults. The objectives of the present study were two-fold. First, we aimed to characterize ARI with serum inflammatory marker concentrations in patients with HAdV-7 infection. Second, we assessed the performance of inflammatory markers in predicting the progression of upper-respiratory infection (URI) to adenovirus pneumonia (AdP).
Material and Methods
Subjects
We conducted a retrospective cohort study using data collected from an HAdV-7 outbreak that occurred in a military training camp in China. ARI cases were detected between 20 January and 21 February 2015. Only laboratory-confirmed HAdV-7 infection cases were recruited in this study. Polymerase chain reaction (PCR) was then performed with type-specific primers targeting the hexon coat protein. The PCR products were sequenced and the NCBI database was searched with the Basic Local Alignment Search Tool (BLAST) to identify adenovirus type. The primers used in this study were taken from the existing literature and have been described elsewhere [9,10].
Patients with HIV infection, neutropenia, or a diagnosis of pneumonia or other infectious disease in the last 30 days, and those receiving immunosuppressive chemotherapy, were excluded.
Disease diagnoses
According to guidelines for the diagnosis and treatment of adenovirus infection [11], cases were classified into 2 groups based on their chest radiographic results: AdP and URI. We further divided cases in the AdP group into common pneumonia (CP) and severe pneumonia (SP) subgroups according to their clinical features and computed tomography (CT) findings.
Upper-respiratory infection (URI)
Diagnosis of HAdV-7 URI was accepted when conditions 1 and 5, below, were observed, together with condition 2, 3, or 4:
HAdV-7-specific nucleic acid of throat swab specimens using real-time quantitative PCR (RT-PCR) detection is positive;
Acute onset of fever (temperature more than 37.5°C);
Cough and expectoration, sore throat, fatigue, nausea, loss of appetite;
Pharyngeal hyperemia, tonsil enlargement, surface visible patchy gray white secretion, bilateral cervical lymph nodes;
Chest X-ray or CT examination did not reveal lung lesions.
Common pneumonia (CP)
CP was diagnosed when conditions 1 and 4 were observed, together with condition 2 or 3 shown below:
Positive RT-PCR detection of HAdV-7–specific nucleic acid in throat swab specimen;
Continuous fever (>38.5°C);
Cough with an irritated pharynx, rapid breathing, and tightness in the chest;
Chest X-ray or CT examination revealed lung lesions.
Severe pneumonia (SP)
SP was diagnosed when the AdP diagnostic criteria were met, as well as any of the following conditions being met:
Sustained high fever (>39°C) for more than 5 days, accompanied by a frequent and severe irritating cough;
Heart rate >100 beats/min and (or) respiratory rate >30 breaths/min;
Rapidly progressing lung shadow with multiple- or single-lobar/segment consolidation;
PaO2 <70 mmHg and (or) SpO2 <90% that does not improve with oxygen supplementation.
Data collection
Demographic and clinical data, including age, sex, clinical symptoms, body temperature and maximum temperature (Tmax), vital signs, and physical examination findings, were collected for all cases. Laboratory findings, including the results of routine blood tests (white blood cell, neutrophil, and lymphocyte counts) and data on co-infection pathogens, were recorded. Throat swabs, sputum, and blood samples were collected at the clinic and submitted to the Clinical Microbiology Laboratory of the People’s Liberation Army General Hospital for detection of co-infection pathogens. Sputum cultures were considered valid only when microscopic analysis showed >25 neutrophils and <10 epithelial cells per field of view under low-power (100×) microscopy. Blood cultures were performed, and serum samples were analyzed to detect Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila antibody titers. Throat swabs and sputum specimens were subjected to RT-PCR for detection of viruses, including rhinovirus, influenza A and B viruses, and respiratory syncytial viruses A and B.
Data on ESR and serum concentrations of PCT, IL-6, CRP, and PA were also collected. The concentrations of these cytokines were detected within 2 days after the disease onset. Normal ranges were considered as follows: PCT, <0.5 ng/ml; IL-6, <7.0 pg/ml; CRP, <8 mg/l; PA, 0.2–0.4 g/l; and ESR, <15 mm/h.
Statistical analysis
Study subjects and selected serum inflammatory marker concentrations were characterized by means ± standard deviations for continuous variables, or medians and interquartile ranges when normal distribution could not be assumed. To test if selected serum inflammatory marker concentrations varied across different ARI diagnoses, univariate comparisons between groups were performed using the t test for normally distributed data and the nonparametric Mann-Whitney U test for non-normally distributed data. To assess if serum inflammatory marker concentrations were correlated with disease severity, Pearson correlation tests were performed to analyze correlations between serum inflammatory marker concentrations and Tmax. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the performance inflammatory markers to predict progression of URI to AdP. A statistically-derived value based on the Youden index that maximized the sum of sensitivity and specificity was set as the optimal cutoff values. All analyses were performed with SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). P values ≤0.05 were considered to be statistically significant.
Results
Clinical and laboratory characteristics of the study sample
The study sample comprised 81 HAdV-7–associated acute respiratory infection cases with 39 subjects diagnosed with AdP and the other 42 with URI. Among the 39 subjects with AdP, 29 patients were diagnosed with CP and 10 with SP. All patients were adult males with a median age of 18.96 (range, 17–24) years. Mean ages of patients with URI and AdP were 18.86±1.46 and 19.08±1.50 years, respectively (Table 1). All patients were previously healthy, except for 1 patient with URI who reported a history of albuminuria and hyperuricemia. No patient was immunocompromised.
Table 1.
Clinical and laboratory characteristics of patients with adenovirus type 7 infection.
| Characteristic | All cases (n=81) | AdP (n=39) | URI (n=42) |
|---|---|---|---|
| Age (years) | 18.96±1.47 | 19.08±1.49 | 18.86±1.46 |
| Fever | 81/81 (100) | 39/39 (100) | 42/42 (100) |
| Tmax (°C) | 39.40±0.59 | 39.51±0.69 | 39.31±0.47 |
| WBC, median (IQR) (109/l) | 7.65 (2.1–19.3) | 7.73 (2.8–19.3) | 7.58 (2.1–15.5) |
| N, median (IQR) (109/l) | 5.47 (0.65–15.22) | 5.47 (0.65–15.22) | 5.47 (0.65–13.84) |
| L, median (IQR) (109/l) | 1.42 (0.61–6.41) | 1.52 (0.75–6.41) | 1.33 (0.61–3.04) |
AdP – adenovirus pneumonia; URI – upper respiratory infection; Tmax – maximum body temperature; WBC – white blood cell; IQR – interquartile range; N – neutrophil; L – lymphocyte.
High-grade fever (39.4±0.59°C) was observed in all patients, with 69.5% having temperatures exceeding 39.0°C. The highest temperature recorded was 41°C but temperatures did not differ significantly between groups. Most patients presented flu-like symptoms such as cough (96.3%), expectoration (70.4%), and sore throat (79.0%). Physical examination most commonly revealed throat congestion (93.8%) and anti-aduncus (75.5%). Complications observed in patients with AdP were hypoproteinemia (17.9%), hyponatremia (15.4%), and respiratory failure and decreased heart rate (7.7%). Pleural effusion (5.1%) and hemorrhagic cystitis (5.1%) were also observed. No patient had a laboratory-confirmed bacterial infection. Complete blood counts showed increased band-form neutrophils in 26 individuals, with no significant difference between groups.
The characteristics of chest radiography results in patients
All 81 patients were examined with high-resolution computed tomography (HRCT) scanning (Figures 1, 2, Table 2). The HRCT scans in all 42 URI patients were normal. In 35.9% (14/39) of pneumonia (including CP and SP) patients, we observed more than 2 lobars of lung infiltration and bilateral lung infiltration. Multiple- or single-lobar/segment consolidation was most common, followed by ground-glass opacities among pneumonia (including CP and SP) patients. Single-lobar patchy infiltration was observed in 58.6% (17/29) of the patients with CP. Pleural effusion and atelectasis were observed in 20% (2/10) of SP patients (Table 2).
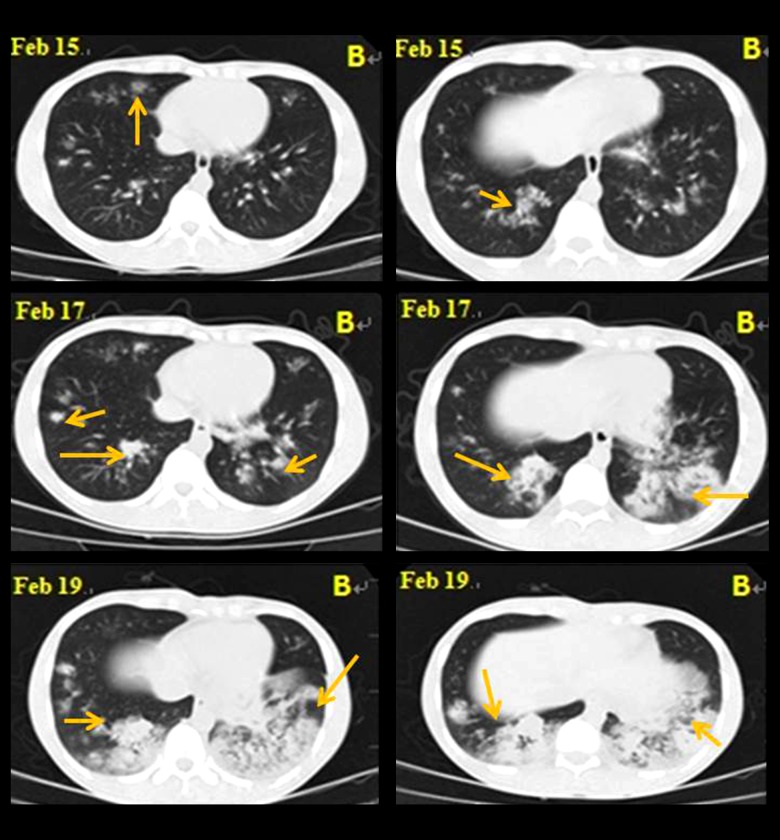
Figure 1.
HRCT in a 22-year-old patient with SP, showing fuzzy boundaries in bilateral lung scatter with flakey/patchy infiltration. Lung lesions progressed very quickly. The double-lung patchy shadow progressed into consolidation within 4 days.
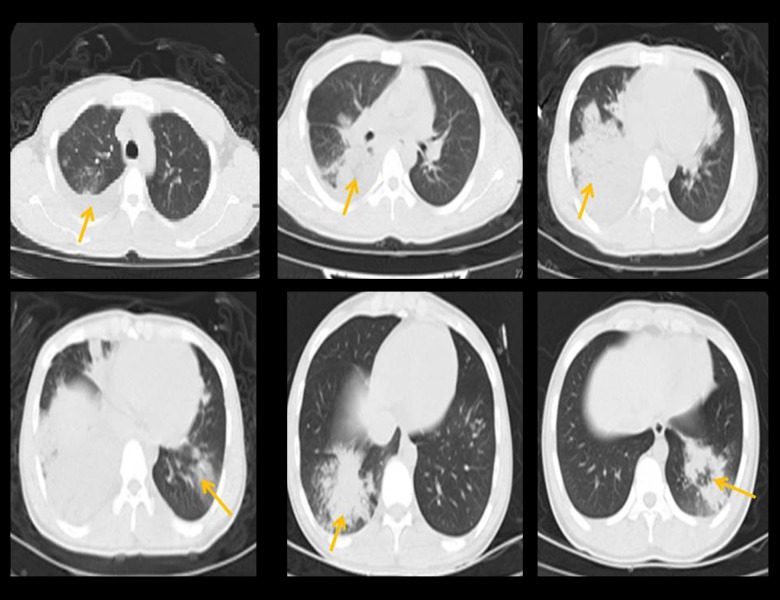
Figure 2.
Multiple- or single-segment consolidation was observed in HRCT and it was followed by ground-glass opacities. Ground-glass-like changes, bronchial signs, and interstitial and nodular changes are visible. Pleural effusion and atelectasis also can be observed.
Table 2.
The characteristics of chest radiography who developed to adenovirus pneumonia.
| Characteristics | All pneumonia cases (n=39) | SP (n=10) | CP (n=29) |
|---|---|---|---|
| No. of lobar involved ≥2 | 14 (35.9) | 6 (60.0) | 8 (27.6) |
| Bilateral infiltrate | 14 (35.9) | 6 (60.0) | 8 (27.6) |
| Pleural effusion | 2 (5.1) | 2 (20.0) | 0 (0.0) |
| Atelectasis | 2 (5.1) | 2 (20.0) | 0 (0.0) |
| Consolidation | |||
| Mutiple lobar or segment | 14 (35.9) | 6 (60.0) | 8 (27.6) |
| Single lobar or segment | 26 (66.7) | 4 (60.0) | 22 (75.9) |
| Consolidation and muti-focal patchy/ground glass opacities | 39 (100.0) | 10 (100.0) | 29 (100.0) |
| Patchy infiltration (single lobar) | 21 (53.8) | 4 (40.0) | 17 (58.6) |
| Diffuse ground-glass opacities | 2 (5.1) | 1 (10.0) | 1 (3.4) |
AdSP – severe pneumonia; AdP – pneumonia. Categorical variables are expressed as no. of patients with the presence of the characteristics.
Serum inflammatory markers
Serum cytokine concentrations are presented in Table 2. Most PCT concentrations were within the normal range and did not significantly differ among the 3 groups. Elevated IL-6 concentrations were observed in all 3 subgroups, with the SP group reporting significantly higher levels than in the other 2 groups (p<0.05). There was no significant difference between the CP and URI groups in IL-6 concentration. The mean ESR of patients with URI was within the normal range, but that of patients with AdP was higher than normal (p=0.008). Mean ESR was higher in patients with SP than in those with CP (34.20±28.70 vs. 17.74±13.17 mm/h; Table 3). Higher than normal serum CRP concentrations were found in all patients with HAdV-7 respiratory tract infection, but no significant differences among groups were observed. PA concentrations were lower in the URI and AdP groups. Among patients with normal CRP levels, the mean PA concentration was 0.19±0.07 g/l, while among patients with elevated CRP levels, the mean PA concentration was lower (0.16±0.04 g/l; p>0.05). Reduced PA level was negatively correlated with increased CRP level in patients with AdP (r=−0.536, p=0.003). The most common co-infection pathogens were Mycoplasma and Chlamydia (14.81%). Epstein-Barr virus (4.94%) and influenza B (2.47%) were also identified concomitantly with HAdV-7 infection. Among cytokines, serum PCT concentrations were higher in patients with than in those without co-infection, but no significant difference for any inflammatory marker was found among groups (Table 4).
Table 3.
Serum inflammatory marker concentrations.
| Group | PCT (ng/ml) | IL-6 (pg/ml) | CRP (mg/l) | ESR (mm/h) | PA (g/l) |
|---|---|---|---|---|---|
| AdP (n=39) | 0.33±0.78 | 40.91±28.51 | 45.53±31.10 | 22.19±19.65 | 0.16±0.05 |
| CP (n=29) | 0.17±0.14 | 36.15±23.30 | 45.85±33.20 | 17.74±13.17 | 0.17±0.04 |
| SP (n=10) | 0.78±1.50 | 54.69±38.22&,# | 44.50±24.75 | 34.20±28.70 | 0.12±0.04 |
| URI (n=42) | 0.13±0.11 | 30.78±20.14 | 48.74±36.50 | 12.79±7.75* | 0.18±0.03 |
| ALL (n=81) | 0.23±0.56 | 35.72±24.95 | 47.21±33.87 | 17.43±15.50 | 0.17±0.04 |
p<0.01, AdP vs. URI;
p<0.01, SP vs. URI;
p<0.05, SP vs. CP.
PCT – procalcitonin; IL-6 – interleukin 6; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; PA – prealbumin; AdP – adenovirus pneumonia; CP – common pneumonia; SP – severe pneumonia; URI – upper respiratory infection.
Table 4.
Serum inflammatory marker concentrations in patients with and without co-infection.
| Marker | Without co-infection (n=63) | With co-infection (n=18) |
|---|---|---|
| PCT (ng/ml) | 0.17±0.16 | 0.44±1.14 |
| IL-6 (pg/ml) | 37.05±24.40 | 31.13±26.97 |
| ESR (mm/h) | 18.41±16.45 | 14.06±11.44 |
| CRP (mg/l) | 48.98±33.33 | 47.85±34.78 |
| PA (g/l) | 0.17±0.04 | 0.17±0.04 |
PCT – procalcitonin; IL-6 – interleukin 6; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; PA – prealbumin.
Correlations between serum inflammatory marker concentrations and Tmax
Tmax was positively correlated with serum PCT concentration (r=0.558, p=0.001) and negatively with PA concentration (r=−0.306, p=0.005;). No significant correlation was observed with IL-6, CRP, or ESR concentration.
Predicting performance of inflammatory markers
The ROC curve for assessing the predicting performance of using inflammatory markers to predict progression from URI to ADP is illustrated in Figure 3. The best performance was seen in PCT, which had an area under the curve (AUC) of 0.656 [95% confidence interval (CI), 0.531–0.782]. The optimal cutoff point for defining the progression was 0.35 with a sensitivity of 81.1% and specificity of 51.4%. IL-6 concentration had a similar predicting performance, with an AUC of 0.640 (95% CI, 0.513–0.767). The optimal cut off point was 32.23, with 78.4% for sensitivity and 45.9% for specificity. Regarding ESR, the AUC was 0.638 (95% CI, 0.509–0.767). The optimal cutoff value for was 16.5 mm/h, with a sensitivity of 45.9% and a specificity of 83.8%. The AUCs for CRP concentration and PA concentration were close to 0.5, indicating no difference in their distribution between URI and AdP groups.
Figure 3.
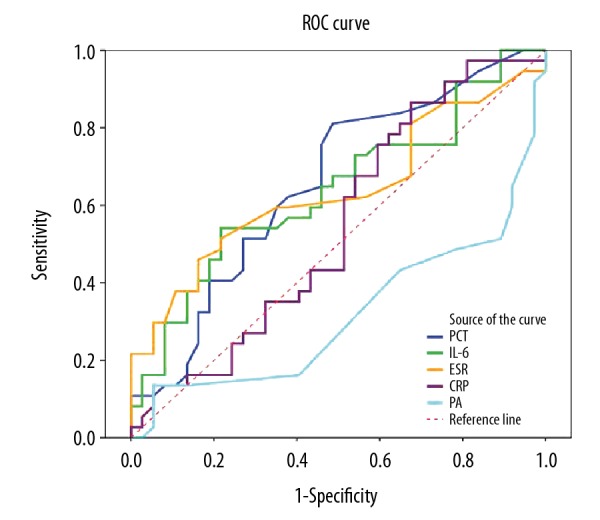
Receiver operating characteristic (ROC) curves showing the diagnostic performance of inflammatory markers and the erythrocyte sedimentation rate (ESR) in distinguishing upper-respiratory infection from adenovirus pneumonia. PCT – procalcitonin; IL-6 – interleukin 6; CRP – C-reactive protein; PA – prealbumin.
Discussion
Strong inflammatory responses were observed in patients with HAdV-7-associated acute respiratory infection in this study. Unlike other viral diseases, adenovirus infection typically results in elevated ESR and CRP levels [8]. In our study, mean ESR was not elevated in patients with URI. Tae et al. [12] found that ESR was normal in all of their patients with H1N1 influenza virus infection during the 2009 pandemic. Jung-Woo et al. [13] found that ESR tended to increase with age in children and adults affected by the pandemic. In the present study, the ESR was significantly elevated in patients with AdP and was higher in patients with SP than in those with CP. ESR may thus be associated with the severity of ARI. However, it was not correlated with Tmax.
The mean IL-6 level was higher than normal, and this concentration tended to be higher in patients with AdP than in those with URI. The IL-6 concentration was significantly elevated in patients with SP compared with those with CP and URI. Elevated IL-6 concentrations have been associated with severe or fatal adenoviral infections [7]. Mistchenko et al. [14] reported that serum values of IL-6, IL-8, and TNF-α were elevated in patients with adenoviral respiratory infection, and that these values were associated with severity of the illness. These findings suggest that IL-6 plays an important role in the pathogenesis of adenoviral respiratory infection in the acute phase.
In our study, HAdV-7 infection resulted in marked elevation of CRP concentrations. This finding is consistent with previous reports [15–17]. Kawaski et al. [18] found a strong positive correlation between CRP and IL-6 levels in patients with adenovirus infection, but we did not observe this correlation in our study. Inflammatory events leading to increased CRP concentration in adenoviral infections are incompletely understood. An obvious explanation would be bacterial co-infection. Korppi et al. [19] found evidence for bacterial co-infection in 9 of 20 children with adenovirus infection, but it was not associated with an elevated CRP level. Ruuskanen et al. [20] found that adenovirus infection was complicated less frequently by bacterial otitis media than by respiratory syncytial virus and influenza. We observed no bacterial co-infection in the present study, whereas 18 patients were co-infected with other pathogens (Mycoplasma or Chlamydia, as well as Epstein-Barr B virus and influenza B). No significant difference in inflammatory markers was found between patients with and without co-infection. Our data refute the hypothesis that bacterial co-infection is responsible for elevated CRP levels in patients with adenovirus infection.
PA is a well-known negative acute-phase protein that is downregulated during inflammation [21]. Reduced serum PA levels were observed in both groups in our study, and PA concentration was correlated negatively with Tmax. Serum PA insufficiency is widely thought to be related to a greater recurrence risk after inflammatory processes [22]. Some authors have recommended the monitoring of PA and CRP levels to determine the severity of the inflammatory response [23]. PA level is negatively correlated with the CRP level. Hrnciarikova et al. [24] also demonstrated subnormal initial mean PA values and a highly significant negative correlation between reduced PA and increased CRP levels in hospitalized geriatric patients. In our study we also found this negative correlation in patients with AdP. In the inflammatory context of HAdV-7, PA may act as a negative protein.
The main chest radiography features of adenovirus pneumonia are multiple- or single-lobar/segment consolidation. The solid form can be nodular and patchy, with high density and clear boundary. Wen et al. [25] reported the chest radiography characteristics of HAdV-7 pneumonia are ground-glass opacities or segmental opacities. Some patients may have pleural thickening and pleurisy. In the present study, 75.9% of patients had single lung involvement. Tubo et al. [26] reported that the characteristic of chest radiography in the early stage of adenovirus type 55 infection was unilateral (72.73%), consistent with our findings, in which 60% of patients with SP had more than 2 lobars infiltration or bilateral lung infiltration. Chuang [27] also found multiple lobar involvement in patients with SP and adenovirus, some of which were fused into lobar consolidations. SP can progress very quickly after onset. Adenovirus infection can occur along the airway, and when the lesion involves the bronchioles, it develops rapidly. SP is often associated with bronchiolitis obliterans, bronchiectasis, and pulmonary fibrosis. Some SP appears with severe pleural effusion, mostly unilateral, and 2 cases in our study were observed pleural effusion.
Conclusions
Our study findings confirm that HAdV-7 infection is associated with changes in inflammatory markers. Specifically, we found that IL-6 concentration and ESR are associated with severity of HAdV-7 respiratory infection. This infection can also result in marked elevation of CRP concentrations. PA may act as a negative modulator protein in the inflammatory context of HAdV-7. Future studies will need to address the role of inflammatory markers in the pathogenesis of adenovirus infection to provide a basis for treatment and prevention. Lung infiltration and consolidation are common in HRCT. Multiple-or single-lobar/segment consolidation is most common in SP. SP progresses very quickly after onset.
Footnotes
Source of support: National Science and Technology Major Project of China (grant number 2015ZX09J15105-004)
Conflicts of interest
None.
References
- 1.Walsh MP, Seto J, Jones MS, et al. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol. 2010;48:991–93. doi: 10.1128/JCM.01694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdman DD, Xu W, Gerber SI, et al. Molecular epidemiology of adenovirus type 7 in the United States, 1966–2000. Emerg Infect Dis. 2002;8:269–77. doi: 10.3201/eid0803.010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YC, Lu PL, Lin KH, et al. Molecular epidemiology and phylogenetic analysis of human adenovirus caused an outbreak in Taiwan during 2011. PLoS One. 2015;10:e0127377. doi: 10.1371/journal.pone.0127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu P, Ma C, Nawaz M, et al. Outbreak of acute respiratory disease caused by human adenovirus type 7 in a military training camp in Shaanxi, China. Microbiol Immunol. 2013;7:553–60. doi: 10.1111/1348-0421.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruuskanen O, Meuman O, Saekkinen H. Adenoviral diseases in children: A study of 105 hospital cases. Pediatrics. 1985;76:79–83. [PubMed] [Google Scholar]
- 6.Putto A, Meurman O, Ruuskanen O. C-reactive protein in the differentiation of adenoviral, Epstein-Barr viral and streptococcal tonsillitis in children. Eur J Pediatr. 1986;145:204–6. doi: 10.1007/BF00446066. [DOI] [PubMed] [Google Scholar]
- 7.Mistchenko AS, Diez RA, Mariani AL, et al. Cytokines in adenoviral disease in children: association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J Pediatr. 1994;124:714–20. doi: 10.1016/s0022-3476(05)81360-5. [DOI] [PubMed] [Google Scholar]
- 8.Cruse JM, Lewis RE. Atlas of immunology. chap 10. Boca Raton, FL: CRC Press; 1999. Cytokines; pp. 185–206. [Google Scholar]
- 9.Xu W, McDonough MC, Erdman DD. Species-specific identification of human adenoviruses by a multiplex PCR assay. J Clin Microbiol. 2000;38(11):4114–20. doi: 10.1128/jcm.38.11.4114-4120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Erdman DD. Type-specific identification of human adenovirus 3, 7, and 21 by a multiplex PCR assay. J Med Virol. 2001;64(4):537–42. doi: 10.1002/jmv.1083. [DOI] [PubMed] [Google Scholar]
- 11.Infectious diseases Specialized Committee. Guidelines for diagnosis and treatment of adenovirus infection. Med J Chin PLA. 2013;38(7):529–34. [Google Scholar]
- 12.Yun TJ, Kwon GJ, Oh MK, et al. Radiological and clinical characteristics of a military outbreak of pandemic H1N1 2009 influenza virus infection. Korean J Radiol. 2010;11(4):417–24. doi: 10.3348/kjr.2010.11.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhim JW, Go EJ, Lee KY, et al. Pandemic 2009 H1N1 virus infection in children and adults: A cohort study at a single hospital throughout the epidemic. Int Arch Med. 2012;5(1):13. doi: 10.1186/1755-7682-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Wang C, Huang X, et al. Differential proteome profiling of pleural effusions from lung cancer and benign inflammatory disease patients. Biochim Biophys Acta. 2012;1824:692–700. doi: 10.1016/j.bbapap.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Appenzellera C, Ammanna RA, Duppenthalera A, et al. Serum C-reactive protein in children with adenovirus infection. Swiss Med Wkly. 2002;132:345–50. doi: 10.4414/smw.2002.10040. [DOI] [PubMed] [Google Scholar]
- 16.Putto A, Meurman O, Ruuskanen O. C-reactive protein in the differentiation of adenoviral, Epstein-Barr viral and streptococcal tonsillitis in children. Eur J Pediatr. 1986;145:204–6. doi: 10.1007/BF00446066. [DOI] [PubMed] [Google Scholar]
- 17.Ruuskanen O, Putto A, Sarkkinen H, et al. C reactive protein in respiratory virus infections. J Pediatr. 1985;107:97–100. doi: 10.1016/s0022-3476(85)80624-7. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki Y, Hosoya M, Katayose M, Suzuki H. Correlation between serum interleukin 6 and C-reactive protein concentrations in patients with adenoviral respiratory infection. Pediatr Infect Dis. 2002;21:370–74. doi: 10.1097/00006454-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Korppi M, Leinonen PH, Launiala K. Mixed infection is common in children with respiratory adenovirus infection. Acta Paediatr Scand. 1991;80:413–17. doi: 10.1111/j.1651-2227.1991.tb11875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruuskanen O, Arolo M, Putto-Laurila A, et al. Acute otitis media and respiratory virus infection. Pediatr Infect Dis J. 1989;8:94–99. [PubMed] [Google Scholar]
- 21.Aliyazicioglu Y, Deger O, Karahan C, et al. Reference values of cord blood transferrin, ceruloplasmin, alpha-1 antitrypsin, prealbumin, and alpha-2 macroglobulin concentrations in healthy term newborns. Turk J Pediatr. 2007;49:52–54. [PubMed] [Google Scholar]
- 22.Bae HJ, Lee HJ, Han DS, et al. Prealbumin levels as a useful marker for predicting infectious complications after gastric surgery. J Gastrointest Surg. 2011;15:2136–44. doi: 10.1007/s11605-011-1719-z. [DOI] [PubMed] [Google Scholar]
- 23.Xie QH, Zhou Y, Xu ZY, et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol. 2011;12:30. doi: 10.1186/1471-2369-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrnciarikova D, Juraskova B, Hyspler R, et al. A changed view of serum prealbumin in the elderly: prealbumin values influenced by concomitant inflammation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):273–76. doi: 10.5507/bp.2007.046. [DOI] [PubMed] [Google Scholar]
- 25.Weng AM, Yang XD, Wang PJ, et al. [The chest high resolution computed tomography (HRCT) manifestation and dynamic changes in adult patients with adenovirus type 7 pneumonia]. Miliary Med J. 2015;17(2):154–56. [in Chinese] [Google Scholar]
- 26.Tu P, Xie XY, Nie WM, et al. [Clinical characteristics of respiratory tract infection caused by adenovirus type 55]. China Health Care & Nutrition. 2012;12:275–76. [in Chinese] [Google Scholar]
- 27.Yu C, Chiu CH, Wong KS, et al. Severe adenovirus infection in children. J Microbiol Immunol Infect. 2003;36(1):37–40. [PubMed] [Google Scholar]