Abstract
Left ventricular mass index (LVMI) provides a metric for cardiovascular disease risk. We aimed to assess the association of adiponectin-related genetic variants resulting from GWAS in East Asians (loci in/near CDH13, ADIPOQ, WDR11FGF, CMIP and PEPD) with LVMI, and to examine whether sleep duration modified these genetic associations in youth. The 559 subjects aged 15–28 years were recruited from the Beijing Child and Adolescent Metabolic Syndrome study. Among the six loci, CDH13 rs4783244 was significantly correlated with adiponectin levels (p = 8.07 × 10−7). The adiponectin-rising allele in rs4783244 locus was significantly associated with decreased LVMI (p = 6.99 × 10−4) after adjusting for classical cardiovascular risk factors, and further for adiponectin levels, while no significant association was found between the other loci and LVMI. Moreover, we observed a significant interaction effect between rs4783244 and sleep duration (p = .005) for LVMI; the genetic association was more evident in long sleep duration while lost in short sleep duration. Similar interaction was found in the subgroup analysis using longitudinal data (p = .025 for interaction). In this young Chinese population, CDH13 rs4783244 represents a key locus for cardiac structure, and confers stronger cardio-protection in longer sleep duration when contrasted with short sleep duration.
Keywords: Adiponectin-associated locus, CDH13, Adiponectin, Left ventricular mass index, Sleep time
Abbreviations: LVM, Left ventricular mass; LVM index, Left ventricular mass index; LVH, Left ventricular hypertrophy; GWAS, Genome wide association study; MetS, Metabolic syndrome; T2D, Type 2 diabetes; CVD, Cardiovascular diseases; BMI, Body mass index; WC, Waist circumference; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FBG, Fasting blood glucose; LDL-C, Low density lipoprotein cholesterol; HDL-C, High density lipoprotein cholesterol; TG, Triglyceride; HOMA-IR, Homeostasis model assessment of insulin resistance; IVSDT, Interventricular septal diastolic thickness; LVEDD, Left ventricular end-diastolic diameter; LVPWT, Left ventricular posterior wall thickness
Highlights
-
•
CDH13 rs4783244 represents a key locus for cardiac structure.
-
•
The effect of CDH13 rs4783244 on reduced left ventricular mass index is significantly modified by sleep duration.
-
•
Short sleep may contribute to the loss of CDH13 locus-mediated cardio-protective effects.
We analyzed the associations of five adiponectin-associated genetic variants with left-ventricular mass index (LVMI), a cardiovascular risk factor, in a population of 559 youth. We found a significant protective association of CDH13 rs4783244 with decreased LVMI, independent of adiponectin and other conventional risk factors. Sleep duration has previously been described as a risk factor for increased LVMI in this population, therefore, we assessed modification of this association in CDH13 by sleep duration, and found that short sleep attenuated the cardio-protective effect of this SNP. Our study provides important insights into pathologic mechanisms and prevention strategies for early risk of cardiovascular disease.
1. Introduction
Left ventricular hypertrophy (LVH) is initially a compensatory response to chronic stress by cardiomyocytes, but individuals with LVH are at increased risk for cardiovascular diseases (CVD), even at young ages [2, 18]. LVM, reflecting left ventricular remodeling, is related to body size, sex, and age; as such, LVM index is calculated to minimize these effects and serves as an important marker for myocardial remodeling. LVH, reflected by increased left ventricular mass (LVM) and LVM indexes are influenced by both genetic determinants and environmental factors, including lifestyle [15]. In a recent genome wide association study (GWAS), investigators reported that the cadherin 13 (CDH13) locus was associated with LVM in adults [3]. This gene has also been shown to be associated with adiponectin levels through its coding for T-cadherin, a receptor for high-molecular-weight species of adiponectin and widely expressed in vascular tissues and myocardium [7, 8]. It is known that adiponectin, a major adipokine, exhibits a board spectrum of biological effects, including anti-diabetic, anti-oxidant, and anti-atherosclerotic actions [4, 10], and low adiponectin levels have been reported to be a risk marker of cardiac remolding [4, 33, 36]. Studies that have documented the associations between CDH13 genetic variations and other cardiometabolic profiles affected by adiponectin levels have provided evidence for crosstalk between this locus, T-cadherin and adiponectin in influencing cardiac remolding [5, 11, 14, 34, 38]; however, these metabolic links remain controversial and the underlying mechanisms warrant further studies. In addition to CDH13, a number of adiponectin-associated loci, including in/near ADIPOQ, CMIP, PEPD, and WDR11FGFR etc. have been identified by GWAS [5, 7, 30, 42]. Although epidemiologic studies have reported that ADIPOQ variants are associated with metabolic syndrome (MetS), type 2 diabetes (T2D) and cardiovascular diseases (CVD) [9], the correlations between above-noted adiponectin-associated loci and cardiac remolding have not yet been fully evaluated.
In addition to genetic factors, sleep duration, a modifiable environmental factor, has been recently shown to be associated with LVM in a multiethnic elderly cohort [39]. Our previous findings from the cohort study of the ‘Beijing Child and Adolescent Metabolic Syndrome’ (BCAMS) have also demonstrated that short sleep duration is associated with increased LVM and LVM index in youth with risk for MetS [12], but this association is independent of traditional cardio-metabolic risk factors; although we also found short sleep duration was associated with cardio-metabolic risk factors in younger children at baseline from the same cohort [24]. We thus hypothesize that sleep modifications play a role in cardiac remodeling via genetic predisposition to LVH. Moreover, further study of the interactions between sleep and adiponectin-associated loci would improve the understanding of the underlying pathologic mechanisms and lead to prevention strategies to optimize cardiac remodeling.
Therefore, in our current study, we firstly aimed to determine the association of several GWAS–identified adiponectin-associated loci with parameters of cardiac structure as measured by echocardiography, a well-documented and reliable method [12, 23]. Secondly, we examined the interaction between genetic predisposition to cardiac remodeling and habitual sleep duration in this young population with risk for MetS.
2. Methods
2.1. Participants
The design of the BCAMS has been described in detail elsewhere [26, 40]. In brief, BCAMS study began in 2004, as a prospective cohort study of identifying cardiovascular risk factors from childhood to adulthood. The baseline population-based survey was conducted in a representative sample (n = 19,593, 50% boys) of school children in Beijing aged 6–18 years. In total, approximately 4500 participants were identified as being at a high risk of cardiovascular disease (CVD) due to having one of the following abnormalities: overweight/obesity as defined by body mass index (BMI between 85th percentiles in specific age and sex), high blood pressure (≥ 90th percentiles in specific age and sex), elevated lipids (total cholesterol ≥5.2 mmol/L, triglyceride ≥1.7 mmol/L), and/or fasting blood glucose (≥ 5.6 mmol/L) based on finger capillary blood tests. We conducted follow up studies in 2014. Participants were recruited consecutively through various modalities (phone, text, and/or email) and underwent medical examination at a center in the Beijing Chaoyang Hospital. Signed informed consent was obtained from all participants and/or their parents or guardians. The protocol for the follow-up examination was approved by the Ethics Committee at the Beijing Chaoyang Hospital, and conformed to standards indicated by the Declaration of Helsinki. A total of 559 individuals had complete follow-up data and thus were included into this analysis. The BCAMS study has been registered at www.clinicaltrials.gov (NCT03421444).
2.2. Clinical and Biochemical Measurements
All participants underwent a physical examination that involved measurements of height, weight, waist circumference (WC) and blood pressure in a sitting position after 15 min of rest. Standing height (to 0.1 cm) and weight (to 0.1 kg) were measured using a wall-mounted stadiometer. WC was measured by plastic tape as midway between the lowest rib and the top of the iliac crest. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times during 10 min with a standard sphygmomanometer after 5 min of rest. BMI was calculated as weight divided by height squared. In addition, questionnaires were used to obtain information on lifestyle factors and health history [25]. Physical activity was assessed as weekly minutes of moderate-to-vigorous physical activity. Participants were asked to recall all their food intake in the previous week, including whole grains, meat, fruits, vegetables, dairy and snacks, and the average daily total caloric intake was calculated. Health history information included hypertension, diabetes, dyslipidemia, and kidney, heart and thyroid diseases plus medication. Cigarette smoking was defined as current, former, or never.
Blood samples were collected via an antecubital vein after a 10 h fasting. The fasting samples were also aliquoted and frozen for future analysis of adipokines. Blood glucose, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL—C), and triglycerides (TG) were measured on an autoanalyzer (Hitachi 7060C automatic biochemistry analysis system). Insulin and adiponectin were measured by monoclonal antibody-based sandwich enzyme-linked immunosorbent assay, which was developed in the Key Laboratory of Endocrinology, Peking Union Medical College Hospital [[27], [28], [29]]. The intra-assay coefficient of variation (CVs) for insulin and adiponectin were < 4.1% and < 5.4%, respectively. The inter-assay CVs were < 7.0% and < 8.5%, respectively. Insulin resistance was assessed by the homeostasis model assessment (HOMA-IR), calculated as fasting insulin (μIU/mL) × fasting blood glucose (mmol/L)/22.5 [32]. MetS was determined using the 2009 harmonized definition [1].
2.3. Genomic DNA Extraction and Genotyping
Genomic DNA was extracted from blood samples collected from each participant using the QIAamp DNA blood midikits (Qiagen). The six most strongly adiponectin-associated SNPs were selected from previously reported GWAS of adiponectin in East Asians [5, 7, 30, 42], ADIPOQ rs10937273, rs6773957, CDH13 rs4783244, WDR11FGF rs3943077, CMIP rs2925979, and PEPD rs889140, and were genotype on the Sequenom Mass Array iPLEX genotyping platform in BioMiao Biological Technology Co, Ltd. [13, 25]. Repeated control samples were present in each genotyping plate, with the concordance rate being 100%. All these SNPs had genotyping efficiency >0.95 and were in Hardy-Weinberg equilibrium with p value >.008 (0.05/6).
2.4. Echocardiography
Ultrasound images were acquired by a non-invasive transthoracic echocardiogram using a LOGIQ P5 B-mode ultrasonogram equipped (LOGIQ P5, GE Ultrasound, Korea) with a 2.5–3.5 MHz probe. All images were obtained in the left decubitus position of participants to acquire parasternal long and short axis and apical four chamber views. The following measurements were measured by a sonographer who was blinded to group: interventricular septal diastolic thickness (IVSDT), left ventricular end-diastolic diameter (LVEDD), and left ventricular posterior wall thickness (LVPWT). The following variables were calculated: LVM = 0.8 × {1.04 × [(LVEDD + LVPWT + IVSDT)3− (LVEDD)3]} + 0.6. LVM index (a measure of hypertrophy) was calculated by dividing LVM by height in meters raised to 2.7 (LVM/height2.7) to minimize the effects of age, sex [6, 20].
2.5. Sleep Duration
Sleep duration was determined for each participant by a self-reported questionnaire (including bed time and wake up time). Sleep duration was asked by time bar reaching half-hourly from 5 to 13 h per day. In the study, sleep time was analyzed for continuous variable or classification variable as following: short sleepers (≤ 7 h/day), normal sleepers (> 7 to ≤9 h/day), and long sleepers (> 9 h/day) [12]. In addition, in subgroup retrospective analyses, subjects were reclassified into four groups according to the median of sleep durations at baseline and follow-up: 8.5 and 8 h/day, respectively, to define long term status of sleep duration from childhood to adulthood. The information of sleep duration at baseline study was described previously in detail elsewhere [24].
2.6. Data Analysis
Non-normal distribution values such as insulin, adiponectin, and HOMA-IR were natural log-transformed for analysis. Comparison between males and females was achieved using the two-sample t-test for continuous variables, while categorical variables were explored using the chi-square test. The adjustment for confounding factors was performed using the analysis of covariance in the general linear model (GLM). The association of individual SNP with echocardiographic parameters was estimated using linear regression model. A score of 0, 1, or 2 was assigned to genotypes of associated SNPs according to the number of adiponectin increasing alleles in additive model. Then, multivariable linear regression models were constructed to test SNP × sleep duration interaction. In these models, the independent variables included sleep duration, SNP, SNP × sleep duration term as well as other major commonly recognized risk factors for LVM index. A P-value <.05 (two sided) was considered to be statistically significant, except for the genetic association, which was significant when P-value was less than the Bonferroni-corrected threshold of α = 0.008 (where 0.008 = 0.05/6). Analyses were performed using the Statistical Package for Social Sciences (SPSS 19.0 for Windows, SPSS Inc., USA) and R version 3.3.3 (http://cran.r-project.org/). Linkage disequilibrium (LD) was determined using the 1000G Phase-3 population data in Haploview.
3. Results
Table 1 summarized the anthropometry data, biomarker levels and echocardiographic parameters of the study participants stratified by gender. Of the 559 participants, 294 (52.6%) were males. Compared to female, male had higher BMI, WC, SBP, DBP, TG, fasting blood glucose, IVSD, LVESD, LVPWT, LVM and LVM index, lower HDL-C and adiponectin levels (all p ≤ .001).
Table 1.
Characteristics of participants.
| Characteristics | Total | Male | Female | p value |
|---|---|---|---|---|
| n | 559 | 294 | 265 | / |
| Age (year) | 20.2 ± 2.9 | 20.0 ± 3.0 | 20.4 ± 2.8 | 0.11 |
| sleep hours (h/day) | 8.20 ± 1.25 | 8.17 ± 1.17 | 8.24 ± 1.34 | 0.501 |
| Smoke (n (%)) | 109 (19.5%) | 75 (25.5%) | 34 (12.8%) | < 0.001 |
| Moderate to high activity (h/week) | 2.2 ± 1.9 | 3.2 ± 3.8 | 1.7 ± 2.0 | < 0.001 |
| Total caloric intake(kcal/day) | 1548.7 ± 562.5 | 1668.1 ± 598.9 | 1422.2 ± 491.7 | < 0.001 |
| BMI (kg/m2) | 25.7 ± 5.7 | 27.0 ± 5.8 | 24.3 ± 5.3 | < 0.001 |
| WC (cm) | 85.2 ± 14.6 | 90.5 ± 14.6 | 79.3 ± 12.1 | < 0.001 |
| SBP (mmHg) | 115 ± 14 | 121 ± 14 | 108 ± 11 | < 0.001 |
| DBP (mmHg) | 73 ± 10 | 76 ± 10 | 70 ± 10 | < 0.001 |
| TG (mmol/L)* | 0.91 (0.67–1.31) | 0.96 (0.68–1.52) | 0.84 (0.66–1.20) | 0.001 |
| Total cholesterol (mmol/L) | 4.35 ± 0.92 | 4.29 ± 0.86 | 4.41 ± 0.99 | 0.131 |
| LDL-C (mmol/L) | 2.53 ± 0.79 | 2.56 ± 0.72 | 2.50 ± 0.86 | 0.371 |
| HDL-C (mmol/L) | 1.44 ± 0.32 | 1.34 ± 0.28 | 1.54 ± 0.34 | < 0.001 |
| FBG (mmol/L) | 4.92 ± 0.69 | 5.00 ± 0.86 | 4.82 ± 0.41 | 0.001 |
| 2 h-glucose (mmol/L) | 6.06 ± 1.84 | 6.17 ± 2.07 | 5.95 ± 1.54 | 0.179 |
| Fasting insulin (mIU/L)* | 6.9 (4.1–11.5) | 7.2 (4.3–12.0) | 6.5 (3.9–11.2) | 0.236 |
| 2 h-insulin (mIU/L)* | 37.37 (23.2–59.0) | 36.9 (20.7–60.7) | 38.3 (24.2–57.6) | 0.468 |
| HOMA-IR* | 1.48 (0.86–2.54) | 1.58 (0.91–2.70) | 1.39 (0.80–2.33) | 0.146 |
| Adiponectin (ug/mL)* | 7.09 (4.97–10.24) | 6.47 (4.11–9.18) | 7.72 (5.84–11.01) | < 0.001 |
| IVSDT (cm) | 0.89 ± 0.12 | 0.93 ± 0.12 | 0.85 ± 0.10 | < 0.001 |
| LVEDD (cm) | 4.42 ± 0.49 | 4.65 ± 0.45 | 4.17 ± 0.40 | < 0.001 |
| LVPWT (cm) | 0.89 ± 0.11 | 0.94 ± 0.10 | 0.84 ± 0.10 | < 0.001 |
| LVM (g) | 133.1 ± 39.1 | 153.4 ± 36.9 | 111.7 ± 28.4 | < 0.001 |
| LVM index (g/m2.7) | 31.51 ± 8.00 | 33.23 ± 8.32 | 29.71 ± 7.23 | < 0.001 |
Abbreviations: WC waist circumstance, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TG triglycerides, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, FBG fasting blood glucose, IVSDT inter ventricular septal diastolic thickness, LVEDD left ventricular end-diastolic diameter, LVPWT left ventricular posterior wall thickness, LVM left ventricular mass.
All values were reported as mean ± SD or median (interquartile range) or number of subjects (%). P values are for the sex differences. *Variables were ln-transformed before analysis.
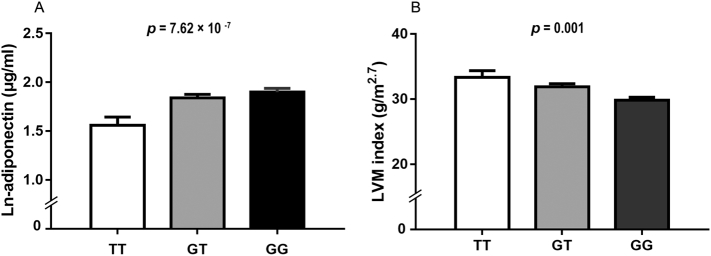
The linear association between echocardiographic parameters and the six SNPs from previously reported adiponectin-associated loci are shown in Table 2. At a Bonferroni-corrected threshold of p < .008, there was a significant association in CDH13 rs4783244 with LVM (β = −6.774 g per additional G allele, p = .004) and LVM index (β = −1.781 g/m2.7 per G allele, p = .001) after adjustment for age, sex and BMI. In addition, we also replicated a strong relationship between CDH13 rs4783244 and ln-adiponectin levels (β = 0.238 μg/ml per additional G allele, p = 8.07× 10−7, Table 2, Fig. 1A), while no significant association between other SNPs and adiponectin levels (all P > .05). Since LVM index is an important marker for LV hypertrophy, therefore, for subsequent analyses, we focused on the top hit CDH13 rs4783244 and LVM index.
Table 2.
Associations of the six SNPs with the cardiac-traits and adiponectin levels.
| Gene | SNP | Position | Allele | MAF | IVSDT (cm) | LVEDD (cm) | LVPWT (cm) |
LVM (g) |
LVM index (g/m2.7) | Adiponectin (ug/ml)# | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CDH13 | rs4783244 | intron | T/G* | 0.325 | β | −0.020 | −0.076 | −0.008 | −6.774 | −1.781 | 0.238 |
| p | 0.028 | 0.023 | 0.314 | 0.004 | 0.001 | 8.07× 10−7 | |||||
| ADIPOQ | rs10937273 | 5′ near gene | A*/G | 0.388 | β | −0.010 | −0.052 | −0.006 | −3.761 | −0.889 | 0.031 |
| p | 0.287 | 0.111 | 0.442 | 0.107 | 0.103 | 0.515 | |||||
| ADIPOQ | rs6773957 | 3’ UTR | G/A* | 0.426 | β | −0.014 | 0.008 | −0.018 | −1.840 | −0.577 | 0.012 |
| p | 0.098 | 0.805 | 0.018 | 0.416 | 0.275 | 0.800 | |||||
| WDR11FGF | rs3943077 | Between the 2 genes | G/A* | 0.370 | β | 0.011 | 0.008 | −0.004 | 1.156 | −0.286 | 0.066 |
| p | 0.215 | 0.814 | 0.616 | 0.616 | 0.595 | 0.163 | |||||
| CMIP | rs2925979 | intron | T/C* | 0.406 | β | −0.010 | −0.008 | 0.006 | −0.719 | −0.029 | 0.040 |
| p | 0.257 | 0.802 | 0.466 | 0.758 | 0.958 | 0.410 | |||||
| PEPD | rs889140 | intron | A*/G | 0.491 | β | 0.008 | 0.022 | 0.004 | 2.045 | 0.769 | 0.027 |
| p | 0.364 | 0.495 | 0.562 | 0.371 | 0.150 | 0.576 |
Abbreviation: MAF, minor allele frequency; IVSDT inter ventricular septal diastolic thickness, LVEDD left ventricular end-diastolic diameter, LVPWT left ventricular posterior wall thickness, LVM left ventricular mass.
Minor allele/major allele. *Allele with higher adiponectin levels.
#Variables were ln-transformed before analysis.
β means linear regression coefficients adjusted for sex, age and BMI.
Values in bold were significant at a Bonferroni-corrected threshold of p ≤ .008.
Fig. 1.
Comparisons of ln-adiponectin levels (A) and LVM index (B) among different genotypes in CDH13 rs4783244. LVM index means left ventricular mass index. Data are shown as means ± SE.
P value was adjusted for age, sex, SBP, TG, HDL-C, FBG, HOMA-IR, BMI, smoking, total caloric intake and physical activity in A.
P value was adjusted for the aforementioned covariates and ln-adiponectin levels in B.
To further assess whether the effect of CDH13 on LVM index is dependent on adiponectin levels, we carried out multivariable liner regression analyses with adjustment for ln-adiponectin plus other major confounding factors. As shown in Table 3, CDH13 rs4783244 was associated with LVM index after adjusting for age, sex, SBP, TG, HDL—C, fasting blood glucose, HOMA-IR and BMI (Model 1), and even further adjusted for smoking, total caloric intake and physical activity (Model 2) (all p < .001). Notably, in Model 3 when ln-adiponectin levels was entered as cofounding, the association of rs4783244 with LVM index was still unaltered (β = −2.119 g/m2.7 per additional G allele, p = 2.94 × 10−4, Table 3, Fig. 1B). Meanwhile, similar trends were evident between this variant with LVM, albeit slightly weaker than the association with LVM index.
Table 3.
Effects of CDH13 rs4783244 on LVM and LVM index.
| Model | Factors adjusted for | LVM (g) |
p | LVM index (g/m2.7) |
|
|---|---|---|---|---|---|
| β (SE) | β (SE) | p | |||
| 1 | age, sex, SBP, TG, HDL—C, FBG, HOMA-IR and BMI | −7.445 (2.366) | 0.002 | −2.001 (0.551) | 3.32 × 10−4 |
| 2 | Model 1 additionally adjusted for smoking, total caloric intake and physical activity | −7.137 (2.344) | 0.003 | −1.916 (0.551) | 5.85× 10−4 |
| 3 | Model 2 additionally adjusted for ln-adiponectin. | −7.324 (2.463) | 0.003 | −2.119 (0.578) | 2.94× 10−4 |
| 4 | Model 3 additionally adjusted for sleep duration | −6.601 (2.449) | 0.007 | −1.978 (0.577) | 6.99× 10−4 |
Abbreviation: LVM left ventricular mass, BMI body mass index, SBP systolic blood pressure, TG triglycerides, HDL-C high-density lipoprotein cholesterol, FBG fasting blood glucose, HOMA-IR homeostatic model assessment of insulin resistance.
β represents changes in outcomes for the increasing number of G allele of the SNP rs4783244. Boldfacetype indicates nominally significant values (P < 0.008).
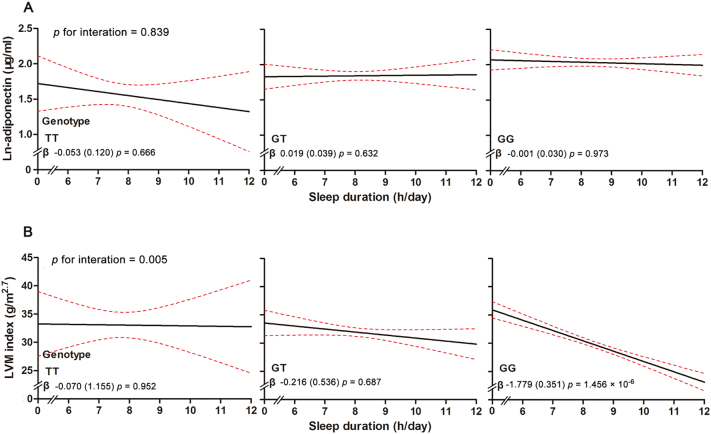
Next, as we found that short sleep duration had a possible direct effect on cardiac remodeling in our previous study [12], we investigated whether sleep duration has the effect via modifying the association of CDH13 with these traits. In the multivariable liner regression analyses for LVM index, where the SNP in CDH13 and continuous sleep duration were independent variables, these two factors were significantly associated with LVM index when controlling for other potential confounders (Table 3 Model 4). Moreover, when we tested for interaction between sleep duration and CDH13 in influencing LVM index, a significant interaction was evident between CDH13 rs4783244 and sleep duration after adjusting for the aforementioned covariates (p = .005, Fig. 2B). It was obvious that the variation of LVM index in subjects with GG genotype was sensitive to the protection of long sleep duration, while individuals with the T allele showed no significant response to sleep duration. Further, in the stratification of categorical sleep duration (Table 4), the adiponectin–raising allele G at the rs4783244 variant was significantly associated with reduced LVM index in longer sleep duration groups after adjusted for age, sex, SBP, TG, HDL—C, fasting blood glucose, HOMA-IR, BMI, smoking, total caloric intake and physical activity (β = −1.528 g/m2.7 per additional G allele, p = .042 for 7–9 h/day group; β = −4.209 g/m2.7, p = 6.58 × 10−4 for >9 h/day group), compared to a non-significant associations (p > .05) in participants with sleep duration ≤7 h/day. Moreover, these associations of CDH13 and LVM index remained significant after further adjusted for adiponectin levels. In contrast, neither significant associations between sleep duration and adiponectin levels nor obvious interaction between sleep duration and CDH13 genotype on adiponectin levels were observed after adjusting for the aforementioned covariates (Fig. 2A, all P > .6).
Fig. 2.
Results of CDH13 × sleep duration interaction on ln-adiponectin and LVM index. LVM index means left ventricular mass index. X-axis represents the average sleep duration (h/day) and Y- axis represents LVM index (g/m2.7) or ln-adiponectin levels (ug/ml). Three groups of genotypes for rs4783244 are represented. Red dotted line represents the 95% confidence intervals and black line represents the regression line. The interaction p value is listed for ln-adiponectin and LVM index. P value for interaction in the liner regression model was adjusted for age, sex, CDH13 genotype, sleep duration, SBP, TG, HDL—C, FBG, HOMA-IR, BMI, smoking, total caloric intake and physical activity in A. P value was adjusted for the aforementioned covariates and ln-adiponectin levels in B.
Table 4.
Linear regression analysis of CDH13 rs4783244 genotypes on LVM index stratified by sleep duration.
| Sleep duration (h/day) | ≤ 7 h/day |
7–9 h/day |
> 9 h/day |
p | ||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | ||
| Model 1 | 1.037 (1.357) | 0.448 | −1.561 (0.730) | 0.034 | −4.056 (1.029) | 2.47 × 10−4 |
| Model 2 | 0.856 (1.433) | 0.553 | - 1.528 (0.746) | 0.042 | −4.209 (1.144) | 6.58 × 10−4 |
| Model 3 | 1.065 (1.547) | 0.495 | −1.667 (0.728) | 0.023 | −5.766 (1.006) | 6.00 × 10−7 |
Abbreviation: LVM left ventricular mass, BMI body mass index, SBP systolic blood pressure, TG triglycerides, HDL-C high-density lipoprotein cholesterol, FBG fasting blood glucose, HOMA-IR homeostatic model assessment of insulin resistance.
Model 1: adjusted for age, sex, SBP, TG, HDL-C, FBG, HOMA-IR and BMI.
Model 2: Model 1 additionally adjusted for smoking, total caloric intake and physical activity.
Model 3: Model 2 additionally adjusted for ln-adiponectin.
β represents changes in outcomes for the increasing number of G allele of the SNP rs4783244.
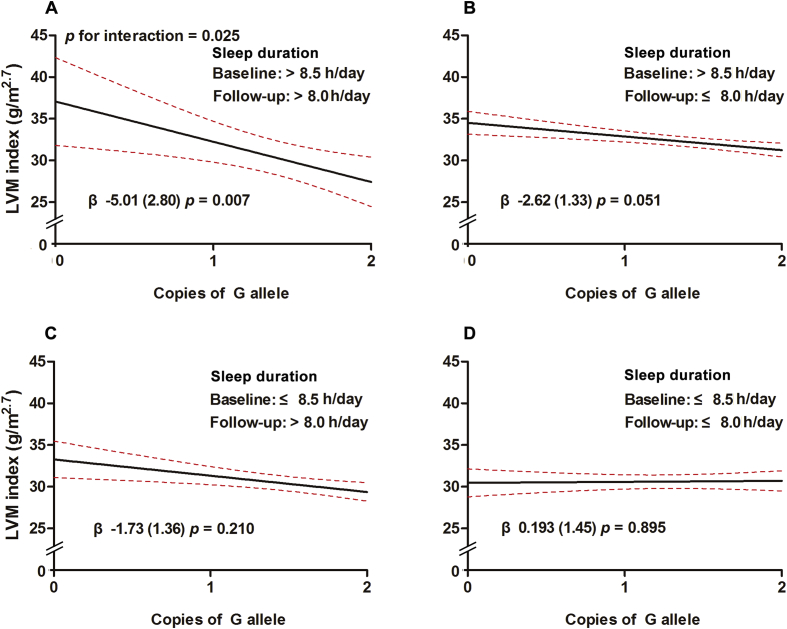
Lastly, to provide longitudinal evidence, we further examined whether long-term sleep status would modify the association of CDH13 locus with LVM index in BCAMS cohort, based on the information of habitual sleep duration collected both at baseline and 10-year follow-up. As listed in Table 5 and Fig. 3, subjects were reclassified into four groups according to the median of sleep durations at baseline and follow-up, i.e. 8.5 h/day and 8 h/day, respectively; we found that the strongest associations between CDH13 locus LVM index exhibited in the long-term status of long sleep group (β = −5.01 g/m2.7 per additional G allele, p = .007), while in the long-term short sleep group, the protection effect at this locus became obviously nonsignificant (β = 0.193 g/m2.7 per additional G allele, p = .895).
Table 5.
Associations between CDH13 rs4783244 and LVM index (g/m2.7) in different sleep status from baseline to 10-year follow-up.
| Sleep duration at baseline (mean age 12 ys) |
Sleep duration at follow-up (mean age 21 ys) |
n | β (SE) | p |
|---|---|---|---|---|
| Long (> 8.5 h/d) | Long (> 8.0 h/d) | 67 | −5.01 (2.80) | 0.007 |
| Long (> 8.5 h/d) | Short (≤ 8.0 h/d) | 104 | −2.62 (1.33) | 0.051 |
| Short (≤ 8.5 h/d) | Long (> 8.0 h/d) | 77 | −1.73 (1.36) | 0.210 |
| Short (≤ 8.5 h/d) | Short (≤ 8.0 h/d) | 89 | 0.193 (1.45) | 0.895 |
Abbreviation: LVM index, left ventricular mass index.
Four groups according to the baseline and follow-up sleep duration are represented. Long and short sleep durations were classified by the median of sleep durations at baseline and follow-up, i.e. 8.5 h/day and 8 h/day, respectively.
P value in the liner regression model was adjusted for baseline age, follow-up time, sex, CDH13 genotype, SBP, TG, HDL-C, FBG, HOMA-IR, BMI, smoking, total energy intake and physical activity at follow-up. Boldfacetype indicates nominally significant values (P < 0.008).
Fig. 3.
Results of CDH13 rs4783244 × long-term sleep status interaction on LVM index at 10-year follow-up. LVM index means left ventricular mass index. X-axis represents the copies of the G allele in CDH13 rs4783244 and Y- axis represents LVM index (g/m2.7). The median of sleep durations at baseline and follow-up were 8.5 and 8 h, respectively. Subjects were classified into four groups according to the median of sleep durations at baseline and follow-up, respectively. A, sleep duration >8.5 h/day at baseline (long) and > 8.0 h/day at follow-up (long); B, > 8.5 h/day at baseline (long) and ≤ 8.0 h/day at follow-up (short), C, ≤ 8.5 h/day at baseline (short) and > 8.0 h/day at follow-up (long), and D, sleep duration ≤8.5 h/day at baseline (short) and ≤ 8.0 h/day at follow-up (short). Red dotted line represents the 95% confidence intervals and black line represents the regression line. The interaction p value is listed for LVM index. P value for interaction in the liner regression model was adjusted for baseline age, follow-up time, sex, CDH13 genotype, SBP, TG, HDL-C, FBG, HOMA-IR, BMI, smoking, total energy intake and physical activity at follow-up.
4. Discussion
Left ventricular remodeling, as reflected by increased LVM index, contributes to heart failure and cardiovascular death, while adiponectin has displayed a broad spectrum of cardiometabolic effects. To our knowledge, this is the first study to explore the association of common genetic variants resulting from GWAS of adiponectin levels (CDH13 rs4783244, ADIPOQ rs10937273 and rs6773957, WDR11FGF rs3943077, CMIP rs889140, and PEPD rs889140) with cardiac structure. In addition to replication of the established relationship between CDH13 rs4783244 and adiponectin levels, we found this locus is independently associated with LVM index in youths at risk of developing MetS, suggesting that it might be a key susceptibility locus contributing to cardiac remodeling at young ages. Moreover, we found that this genetic association is significantly modified by habitual sleep duration in the cross-sectional and longitudinal analysis, suggesting a modifiable behavior i.e. longer sleep duration may strengthen cardiac protection conferred by the CDH13 locus, while short sleep duration may contribute to loss of cardio-protective effects at this locus.
Adiponectin-associated genes have recently received increasing attention for the vital cardiometabolic effects of adiponectin [36]. Various GWAS studies conducting meta-analysis have reported numerous candidate genetic loci for adiponectin levels [5, 7, 30, 42], including ADIPOQ, CDH13, WDR11FGF, CMIP and PEPD genes. In addition, the CDH13 rs4783244 was found to be the strongest associated variant with adiponectin in East Asian populations [5, 14]. In this study, we replicated the association between CDH13 rs4783244 and adiponectin levels; consistent with previous studies [5, 14], we found that circulating adiponectin levels increase across the genotypes of rs4783244 from minor TT to major GG genotypes. We did not replicate the relationship between the selected SNP in /near ADIPOQ, WDR11FGF, CMIP and PEPD with adiponectin levels but the direction of effect for adiponectin was consistent with previous studies [5, 7, 30, 42]. This is likely due to differences in samples or insufficient statistical power of the current study, however, we did replicate the associations of all the above-mentioned loci, except for CMIP, with adiponectin levels by achieving study-wide significance in our large cohort at baseline (n = 3514, age 6–18 years, data not shown).
In addition to the association with adiponectin, it is worthy to note that CDH13 rs4783244 was associated with cardiac structure in our study. LVH is an independent risk factor for cardiovascular events such as heart failure and cardiovascular mortality. Given that adiponectin may also have cardio-protective effects by reducing fibrosis and apoptosis, and preventing myocyte hypertrophy, and has been reported to be associated with LVM and LVM index in addition to cardiometabolic diseases [4, 33], it is likely that adiponectin-related genes are genetic candidates for cardiac remolding phenotypes. Yet the data are still sparse. A previous study of 62 adults with uncomplicated obesity reported that subjects with the GG genotype of the SNP in the adiponectin gene (+276 G > T) showed significant higher LVM/body surface area and LVM index than those carrying T alleles [17]. Most recently, a GWAS of LVM index firstly revealed that one SNP rs9646331 in CDH13 showed suggestive association with four measured LVM traits [3], but this study did not analyze the adiponectin levels, and thus do not know whether these associations were mediated by adiponectin levels. In our study, we first found CDH13 rs4783244, although not in LD with rs9646331 (r2CBH and JPT = 0.014 with rs9646331), is also associated with increased LVM index after adjusting for other major potential confounders, suggesting that CDH13 rs4783244 is a key susceptibility locus for LVM index.
The mechanisms that underlie the association between CDH13 rs4783422 and cardiac remodeling remain unclear. The CDH13 gene encodes T-cadherin, a novel receptor for hexameric high-molecular-weight (HMW) adiponectin and is widely expressed in cardiovascular tissues [16, 37]. The binding of adiponectin to T-cadherin displays important functions in metabolism homeostasis [16]. In addition, associations between CDH13 polymorphisms and cardiometabolic profiles were reported in epidemiological studies although they remain controversial. For instance, CDH13 rs3865188 (LD with rs4783244, r2 CEU > 0.8) is associated with lower adiponectin levels and increased metabolic risks in a French population study [34], while some previous studies reported an association of T allele in CDH13 rs4783244 with lower adiponectin levels but with better metabolic profiles [5, 14]. Nonetheless, those findings lead to the speculation that the association of CDH13 variant with cardiac structure is mediated by its influence on either adiponectin level or metabolic profile. However, in our study, the association of CDH13 rs4783244 with LVM index was unexpectedly independent of both adiponectin levels and other conventional cardiovascular risk factors, suggesting that these associations are not merely mediated by adiponectin levels and conventional cardiometabolic risk factors.
How might this variation in the CDH13 gene directly influence cardiac structure? Clearly, this study does not allow us to address this directly, but it is interesting to note that a recent study from Korean population showed that a CDH13 promoter SNP rs12444338, which is in strong LD with rs4783244 (r2 CHB and JPT > 0.9), has a strong effect on gene expression in vitro [19]. Moreover, some other studies found that the expression of T-cadherin was abundant in the myocardium where it provided protection from pathological cardiac remodeling induced by stress [8], and decreased T-cadherin level was in association with the severities of CVD [22]. Given the close linkage to nearby functional variation, we speculated that this intron variant rs4783244 at CDH13 may also influence T-cadherin expressions, thereby playing a potential protective role against cardiac remodeling. Thus, further studies are needed to explore whether this variation in cardiac tissue affects CDH13 expressions, local adiponectin sensitivity, cardiac structure, and ultimately CVD risk [38].
Another intriguing finding in our study was the interaction effect between CDH13 and sleep duration for LVM index. Sleep duration is known to be associated with T2D, MetS and hypertension, which are risk factors of cardiac remodeling [21, 31, 41]. Our previous findings suggested that short sleep duration was directly associated with increased LVM and LVM index independent of adiponectin level and other cardiometabolic risk factor, though the mechanisms were unclear [12]. Therefore, we explore the mechanism implication whether CDH13 was the link between sleep duration and LVH. To our knowledge, no study has assessed these associations before. Interestingly, a strong significant interaction effect between the CDH13 polymorphisms and sleep duration on LVM index was observed, that is, carrying an additional adiponectin-raising G allele can decrease LVM index by up to 5.8 g/m2.7 in subjects with sleep duration >9 h/day compared with 1.1 g/m2.7 increase in subjects with sleep duration ≤7 h/day (Table 3), which suggests that the cardio-protective effects at the CDH13 locus is most evident in subjects with long sleep duration, but these effects were lost in the status of short sleep duration. In addition, the protective associations of longer sleep duration with LVM index were obvious in individuals with GG genotype but not in carriers of T alleles.
Notably, given this important finding in a cross-sectional setting, to provide longitudinal evidence, we further retrospectively analyzed the information of habitual sleep duration collected both at baseline and 10-year follow-up in this cohort, and examined the modification of long-term sleep status on the effect of CDH13 locus with LVM index. As expected, we found that the long-term status of longer sleep duration from childhood to adulthood can strengthen the cardiac protection conferred by this locus, while the long-term status of short sleep duration has the converse effect.
Additionally, neither significant associations between sleep duration and adiponectin levels nor obvious interaction between sleep duration and CDH13 genotype on adiponectin levels were found, further supporting the notion that the effects of CDH13 and sleep duration on LVM index are not mediated by adiponectin levels. Given that sleep deprivation has been reported to alter epigenetic processes [35], further work with the epigenetic modification of sleep on the expressions of CDH13 [38], are likely to provide the fruitful approach to understanding the mechanism and pathways whereby this variant influences the risk of LVH. However, our study gives a new sight to delineate specific thresholds of sleep duration for preventing CVD risks at young age with risk of MetS, especially in those with sensitive genetic backgrounds.
Strengths of this study are that we studied six very relevant GWAS implicated adiponectin-related loci, and the novel finding that CDH13 rs4783244 was associated with LVM index and modified by long-term sleep duration. Despite the strengths, there are also some key limitations to our study. First, the sample in the present study was drawn from youths at risk of cardiovascular diseases; future replication study is needed to evaluate the generalizability of our findings to older adults and other ethnical populations. Secondly, given that rs4783244 at the CDH13 locus, which encodes a receptor for HMW adiponectin, was reported to be more strongly associated with HMW adiponectin than total adiponectin [14], we measured total adiponectin level but not HMW adiponectin. However, HMW adiponectin constitutes the most abundant isoform of total adiponectin [4]. Thirdly, we collected the sleep duration by questionnaire, and were unable to validate self-reporting by actigraphy or other physiological monitoring. Fourthly, we did not collect sleep duration on weekdays and weekends, or total sleep and night sleep duration, separately, so could lead to a degree of bias for the evaluation of sleep time. Lastly, we found a relationship between CDH13 rs4783244 and LVM index, and the modification of long-term sleep duration even verified by a retrospective longitudinal analysis, but causality is difficult to infer. Our ongoing follow-up observation and/or experimental studies are required to establish these mechanistic links and promote understanding of the physiologic significance of these observations.
In conclusion, our study shows that individuals with at least one protective G allele of CDH13 rs4783244 had decreased LVM index when compared to carriers of T alleles. Specifically, we observed highly significant attenuation of the cardio-protective effects associated with G allele in rs4783244 in youths who had short sleep duration. We also found that this allele was strongly correlated with adiponectin levels, while this effect of CDH13 on adiponectin levels did not appear to translate into effects on LVM index. Although our observation will require further replication, it provides important insights into the potential mechanisms and the prevention strategies of cardiac remodeling. Future work is warranted to focus on experimental designs to determine mechanistic pathway.
Acknowledgments
Acknowledgements
We thank Prof. Jie Mi and all the BCAMS study members, and all participants for their continuing participation in this research effort.
The funders had no role in study design; data collection, analysis or interpretation; or writing of the report.
Parts of this study have been accepted as a poster presentation at the Annual World Congress on Insulin Resistance, December 1-3, 2016 in Los Angeles, CA. The entire paper is not being submitted to any other journal.
Contribution Statement
G.L. and D.F. contributed to data analysis and wrote the study. Y.H.W., J.L.F., L.W.H. and L.J.L. contributed to the data collection and performed the immunoassay and genotype analysis. M.Y.L. and S.F.G. contributed to interpretation of the data, and revised the manuscript. S.G. and M.L. were responsible for the study design, protocol development, and interpretation of data and revised the manuscript. All authors have approved the final version of the article.
Funding
This work was supported by grants from key program of Beijing Municipal Science &Technology Commission (#D111100000611001; #D111100000611002), National Key Research program of China (#2016YFC1304801), Beijing Natural Science Foundation (#7172169), Development Program of Beijing Chaoyang Hospital (#JXPY201606), Graduate innovation fund of Peking Union Medical College (#2017–1002–1-15), Beijing Science & Technology Star Program (#2004A027), Novo Nordisk Union Diabetes Research Talent Fund (#2011A002) and National Key Program of Clinical Science (#WBYZ 2011–873).
Conflict of Interest
The authors have no competing interests to declare.
Contributor Information
Ming Li, Email: liming@pumch.cn.
Shan Gao, Email: gaoshanmw@163.com.
References
- 1.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong A.C., Jacobs D.R., Jr., Gidding S.S., Colangelo L.A., Gjesdal O., Lewis C.E. Framingham score and LV mass predict events in young adults: CARDIA study. Int. J. Cardiol. 2014;172(2):350–355. doi: 10.1016/j.ijcard.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barve R.A., Gu C.C., Yang W., Chu J., Davila-Roman V.G., De Las Fuentes L. Genetic association of left ventricular mass assessed by M-mode and two-dimensional echocardiography. J. Hypertens. 2016;34(1):88–96. doi: 10.1097/HJH.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiara T.D., Argano C., Scaglione A., Corrao S., Pinto A., Scaglione R. Circulating adiponectin: a cardiometabolic marker associated with global cardiovascular risk. Acta Cardiol. 2015;70(1):33–40. doi: 10.1080/ac.70.1.3064591. [DOI] [PubMed] [Google Scholar]
- 5.Chung C.M., Lin T.H., Chen J.W., Leu H.B., Yang H.C., Ho H.Y. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts Cardiometabolic outcomes. Diabetes. 2011;60(9):2417–2423. doi: 10.2337/db10-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels S.R., Kimball T.R., Morrison J.A., Khoury P., Meyer R.A. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am. J. Cardiol. 1995;76(10):699–701. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 7.Dastani Z., Hivert M.F., Timpson N., Perry J.R., Yuan X., Scott R.A. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8(3) doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denzel M.S., Scimia M.C., Zumstein P.M., Walsh K., Ruiz-Lozano P., Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Invest. 2010;120(12):4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enns J.E., Taylor C.G., Zahradka P. Variations in Adipokine genes AdipoQ, Lep, and LepR are associated with risk for obesity-related metabolic disease: the modulatory role of gene-nutrient interactions. J. Obes. 2011;2011 doi: 10.1155/2011/168659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasshauer M., Bluher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Fava C., Danese E., Montagnana M., Sjogren M., Almgren P., Guidi G.C. A variant upstream of the CDH13 adiponectin receptor gene and metabolic syndrome in swedes. Am. J. Cardiol. 2011;108(10):1432–1437. doi: 10.1016/j.amjcard.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 12.Feng D., Zhang J., Fu J., Wu H., Wang Y., Li L. Association between sleep duration and cardiac structure in youths at risk for metabolic syndrome. Sci. Rep. 2016;6 doi: 10.1038/srep39017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriel S., Ziaugra L., Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009 doi: 10.1002/0471142905.hg0212s60. Chapter 2: Unit 2.12. [DOI] [PubMed] [Google Scholar]
- 14.Gao H., Kim Y.M., Chen P., Igase M., Kawamoto R., Kim M.K. Genetic variation in CDH13 is associated with lower plasma adiponectin levels but greater adiponectin sensitivity in east Asian populations. Diabetes. 2013;62(12):4277–4283. doi: 10.2337/db13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gjesdal O., Bluemke D.A., Lima J.A. Cardiac remodeling at the population level--risk factors, screening, and outcomes. Nat. Rev. Cardiol. 2011;8(12):673–685. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 16.Hug C., Wang J., Ahmad N.S., Bogan J.S., Tsao T.S., Lodish H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. U. S. A. 2004;101(28):10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobellis G., Petrone A., Leonetti F., Buzzetti R. Left ventricular mass and +276 G/G single nucleotide polymorphism of the adiponectin gene in uncomplicated obesity. Obesity (Silver Spring) 2006;14(3):368–372. doi: 10.1038/oby.2006.48. [DOI] [PubMed] [Google Scholar]
- 18.Iwashima Y., Horio T., Kamide K., Rakugi H., Ogihara T., Kawano Y. Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension. 2006;47(2):195–202. doi: 10.1161/01.HYP.0000200033.14574.14. [DOI] [PubMed] [Google Scholar]
- 19.Jee S.H., Sull J.W., Lee J.E., Shin C., Park J., Kimm H. Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 2010;87(4):545–552. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury P.R., Mitsnefes M., Daniels S.R., Kimball T.R. Age-specific reference intervals for indexed left ventricular mass in children. J. Am. Soc. Echocardiogr. 2009;22(6):709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.Y., Yadav D., Ahn S.V., Koh S.B., Park J.T., Yoon J. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16(12):1511–1515. doi: 10.1016/j.sleep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Kostopoulos C.G., Spiroglou S.G., Varakis J.N., Apostolakis E., Papadaki H.H. Adiponectin/T-cadherin and apelin/APJ expression in human arteries and periadventitial fat: implication of local adipokine signaling in atherosclerosis? Cardiovasc. Pathol. 2014;23(3):131–138. doi: 10.1016/j.carpath.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Li L., Fu J., Yu X.T., Li G., Xu L., Yin J. Sleep duration and Cardiometabolic risk among Chinese school-aged children: do Adipokines play a mediating role? Sleep. 2017;40(5) doi: 10.1093/sleep/zsx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Yin J., Cheng H., Wang Y., Gao S., Li M. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: data from the BCAMS study. J. Clin. Endocrinol. Metab. 2016;101(4):1816–1825. doi: 10.1210/jc.2015-3760. [DOI] [PubMed] [Google Scholar]
- 26.Li M., Fisette A., Zhao X.Y., Deng J.Y., Mi J., Cianflone K. Serum resistin correlates with central obesity but weakly with insulin resistance in Chinese children and adolescents. Int. J. Obes. 2009;33(4):424–439. doi: 10.1038/ijo.2009.44. [DOI] [PubMed] [Google Scholar]
- 27.Li M., Wu C., Song A. Development and preliminary application of enzyme-linked immunosorbent assay for human net insulin in serum. Chin. J. Endocrinol. Metab. 1997;13:214–217. [Google Scholar]
- 28.Li M., Yin J.H., Zhang K., Wu C.Y. A highly sensitive enzyme-linked immunosorbent assay for measurement of leptin secretion in human adipocytes. Zhonghua Yi Xue Za Zhi. 2008;88(46):3293–3297. [PubMed] [Google Scholar]
- 29.Li Q., Lu Y., Sun L., Yan J., Yan X., Fang L. Plasma adiponectin levels in relation to prognosis in patients with angiographic coronary artery disease. Metabolism. 2012;61(12):1803–1808. doi: 10.1016/j.metabol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Ling H., Waterworth D.M., Stirnadel H.A., Pollin T.I., Barter P.J., Kesaniemi Y.A. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS study. Obesity (Silver Spring) 2009;17(4):737–744. doi: 10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lou P., Zhang P., Zhang L., Chen P., Chang G., Zhang N. Effects of sleep duration and sleep quality on prevalence of type 2 diabetes mellitus: a 5-year follow-up study in China. Diabetes Res. Clin. Pract. 2015;109(1):178–184. doi: 10.1016/j.diabres.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Mcmanus D.D., Lyass A., Ingelsson E., Massaro J.M., Meigs J.B., Aragam J. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham offspring study. Obesity (Silver Spring) 2012;20(9):1882–1886. doi: 10.1038/oby.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas A., Aubert R., Bellili-Munoz N., Balkau B., Bonnet F., Tichet J. T-cadherin gene variants are associated with type 2 diabetes and the fatty liver index in the French population. Diabete Metab. 2017;43(1):33–39. doi: 10.1016/j.diabet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson E.K., Bostrom A.E., Mwinyi J., Schioth H.B. Epigenomics of Total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS. 2016;20(6):334–342. doi: 10.1089/omi.2016.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker-Duffen J.L., Walsh K. Cardiometabolic effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28(1):81–91. doi: 10.1016/j.beem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philippova M., Joshi M.B., Kyriakakis E., Pfaff D., Erne P., Resink T.J. A guide and guard: the many faces of T-cadherin. Cell. Signal. 2009;21(7):1035–1044. doi: 10.1016/j.cellsig.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 38.Putku M., Kals M., Inno R., Kasela S., Org E., Kožich V. CDH13 promoter SNPs with pleiotropic effect on cardiometabolic parameters represent methylation QTLs. Hum. Genet. 2014;134(3):291–303. doi: 10.1007/s00439-014-1521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos A.R., Jin Z., Rundek T., Russo C., Homma S., Elkind M.S. Relation between long sleep and left ventricular mass (from a multiethnic elderly cohort) Am. J. Cardiol. 2013;112(4):599–603. doi: 10.1016/j.amjcard.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Yin J., Xu L., Cheng H., Zhao X., Xiang H. Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health. 2013;13:249. doi: 10.1186/1471-2458-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T., Lu J., Wang W., Mu Y., Zhao J., Liu C. Sleep duration and snoring associate with hypertension and glycaemic control in patients with diabetes. Diabet. Med. 2015;32(8):1001–1007. doi: 10.1111/dme.12809. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Gao H., Li H., Tabara Y., Nakatochi M., Chiu Y.F. A meta-analysis of genome-wide association studies for adiponectin levels in east Asians identifies a novel locus near WDR11-FGFR2. Hum. Mol. Genet. 2014;23(4):1108–1119. doi: 10.1093/hmg/ddt488. [DOI] [PMC free article] [PubMed] [Google Scholar]