Abstract
Lupus nephritis (LN) is one of the most severe complications of systemic lupus erythematosus (SLE) caused by uncontrolled activation of the complement system. Mesenchymal stem cells (MSCs) exhibit clinical efficacy for severe LN in our previous studies, but the underlying mechanisms of MSCs regulating complement activation remain largely unknown. Here we show that significantly elevated C5a and C5b-9 were found in patients with LN, which were notably correlated with proteinuria and different renal pathological indexes of LN. MSCs suppressed systemic and intrarenal activation of C5, increased the plasma levels of factor H (FH), and ameliorated renal disease in lupus mice. Importantly, MSCs transplantation up-regulated the decreased FH in patients with LN. Mechanistically, interferon-α enhanced the secretion of FH by MSCs. These data demonstrate that MSCs inhibit the activation of pathogenic C5 via up-regulation of FH, which improves our understanding of the immunomodulatory mechanisms of MSCs in the treatment of lupus nephritis.
Keywords: Lupus nephritis, C5, MSCs, FH
Graphical Abstract
Highlights
-
•
Extensively activated C5 might contribute to the progression of lupus nephritis.
-
•
MSCs ameliorated renal disease in lupus mice through inhibiting systemic and intrarenal activation of C5.
-
•
MSCs increased plasma FH in lupus mice and patients, while interferon-α enhanced the secretion of FH from MSCs.
Lupus nephritis (LN) is one of the most common manifestations of systemic lupus erythematosus (SLE). Here we show that obviously increased C5a and C5b-9 were found in patients with LN, and which were notably correlated with some clinical parameters of LN. Mesenchymal stem cells (MSCs) reduced renal disease in lupus mice through factor H (FH), one of complement regulatory proteins, suppressing C5 over-activation. Interestingly, enhanced secretion of FH from MSCs could be induced by interferon-α. The findings would expand our understanding of the complement regulatory mechanisms of MSCs in treating lupus nephritis.
1. Introduction
Lupus nephritis (LN) is one of the most common and severe complications in patients with systemic lupus erythematosus (SLE) [1]. Nearly 50–60% patients develop renal involvement in the first 10 years after disease onset [2], and approximately 25% LN patients gradually progress into end stage renal failure within 10 years due to continuous disease activity [3]. There is increasing evidence that uncontrolled activation of the complement system plays a crucial role in the pathogenesis of LN [[4], [5], [6]]. Components of the complement system, including the classical pathway, alternative pathway, and the lectin pathway, were found to be accumulated in renal tissues of LN patients [[7], [8], [9]]. Notably, deregulation and abnormality of complement regulatory proteins have also been observed in LN patients [10, 11]. Additionally, preclinical studies and clinical investigations have shown that C1q knockout mice or individuals with defective C1q genes developed SLE-like autoimmune diseases [12, 13], and that C3 deficiency aggravated proteinuria in lupus mice [14]. Obviously, further studies are needed to identify which component is a causal factor for LN. Several studies reported that application of anti-C5 monoclonal antibody improved renal outcomes in lupus mice and ameliorated clinical symptoms of LN patients refractory to conventional therapies [[15], [16], [17], [18]], which suggests that limiting the cascade on the C5 level or selectively blocking activated C5 might be an effective therapeutic strategy for the treatment of LN.
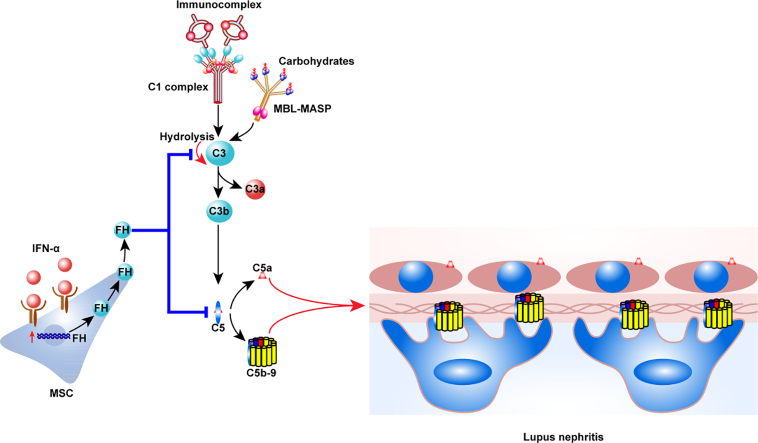
Mesenchymal stem cells (MSCs) have multiple-lineage differentiation potentials and a wide range of immunoregulatory functions [19]. We have treated >100 patients with refractory SLE, including patients with LN, using allogeneic mesenchymal stem cells transplantation (MSCT), and achieved good clinical efficacy [[20], [21], [22], [23]]. However, the exact underlying mechanisms of the MSCs-mediated therapeutic effects remain to be determined. Few data are available regarding the effects of MSCT on the complement system [24]. In particular, it has yet to be elucidated whether MSCT influences the function of the complement system in LN. LN is a type I interferon-driven autoimmune disease manifested with chronic inflammation [25]. Although inflammatory factors affect the immunomodulatory effects of MSCs [19, 26], it is still unknown whether this immunoregulatory activity could be affected by interferon-α (IFN-α) in the context of LN with extensive complement activation.
In this study, over-activated C5 was found in lupus patients and mice, and we demonstrated that MSCT attenuated glomerulonephritis in lupus mice via inhibiting the extensive activation of C5. MSCs produced Factor H (FH) upon IFN-α stimulation in vitro and, importantly, MSCT up-regulated the amount of circulating FH in vivo.
2. Materials and Methods
2.1. Patients and Healthy Controls
66 patients with SLE, and 40 age- and sex-matched healthy blood donors were recruited. All patients fulfilled the 1997 revised criteria of the American College of Rheumatology for SLE [27]. Renal involvement of SLE patients was clinically diagnosed through persistent abnormal urine tests [28]. We also calculated the disease activity score ranging from 0 to 105 for each patient [29]. Renal tissues of 40 patients with LN and 9 patients with nephrectomy were obtained from our department. The severity of LN was assessed according to the abbreviated version of the International Society of Nephrology/Renal Pathology Society classification [30]. The present study was approved by the Ethics Committee at Drum Tower Hospital. Informed consent was obtained from all of the participants prior to their enrollment in this study.
2.2. Human Umbilical Cords–Derived MSCs Isolation and Culture
Human MSCs were isolated and expanded from umbilical cords obtained from normal deliveries after receiving informed consent according to a standard protocol [22]. Unless otherwise stated, human umbilical cords–derived MSCs from passages 4–10 were used for all the following experiments.
2.3. Treatment of Mice
Female MRL/lpr mice of C57BL/6 background (B6.lpr) were obtained from the Chinese Academy of Military Medical Experimental Animal Center (Beijing, China) and were maintained at the Animal Laboratory Center of Drum Tower Hospital. After two weeks of adaptive breeding, B6.lpr mice were randomly allocated into three groups (8 per group), with one group receiving infusion of MSCs (1 × 106) via the tail vein, one group intraperitoneally injected with C5a receptor antagonist (C5aRA, Merck)1 mg/kg three times per week, and a control group intraperitoneally injected with an equal volume of 0.5%DMSO [31]. Levels of proteinuria and creatinine were periodically monitored. After 16 weeks of treatment, mice were sacrificed and perfused. All animal protocols were approved by the Animal Care and Use Committee at Drum Tower Hospital.
2.4. Kidney Histopathology
Kidneys were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 3 μm sections. Tissue sections were stained with HE, PAS, PASM and Masson's trichrome. The glomerulonephritis and perivascular cell infiltration were blindly evaluated by two renal pathologists according to a semi-quantitative criterion [32].
2.5. Immunohistochemistry
For detection of C5a (1:100, Abcam) and C5b-9 (1:50, Abcam) in renal biopsies, 3 μm slides were incubated with 0.5 mg/ml proteinase K (VETEC) for 10 min at 37 °C. For detecting C5a (1:100, Usbio), C5b-9 (1:100, Abcam), and MBL (1:50, Abcam) in mouse kidney cross-sections, 3 μm slides were immersed in citrate buffer (0.01 M, pH 6.0) and then a heat-mediated antigen retrieval procedure was performed. Slides ereincubated with the above primary antibodies overnight at 4 °C. The secondary antibody (GENETECH) was incubated for 30 min at 37 °C. Negative controls were included each time. The positive signals in the glomeruli were quantified as the mean optical density by the Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD).
2.6. Immunofluorescence
Mouse kidney tissues were OCT-embedded, snap-frozen, and cut into 3 μm sections. For direct immunofluorescence, sections were labeled with TRITC-conjugated goat anti-mouse IgG and FITC-conjugated donkey anti-mouse IgM (both 1:100 diluted, Jackson ImmunoResearch), FITC-conjugated goat anti-mouse IgA (1:100, Abcam), and FITC-conjugated rat anti-mouse C3 (1:50, Santa Cruz) for 30 min at 37 °C. For indirect immunofluorescence, sections were incubated with the anti-mouse C1q antibody (1:50, Abcam) and rabbit anti-mouse properdin (1:10, Abcam) overnight at 4 °C. After washing off the primary antibodies, sections were stained with FITC-conjugated goat anti-mouse IgG (1:800, MultiSciences Biotech) or Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:100, ThermoFisher). Negative controls were included each time. Immunofluorescence staining intensity of glomeruli was scored on a scale of 0–3 under an Axio Observer A1 inverted fluorescence microscope (Carl Zeiss) [32].
2.7. Western Blotting
Frozen kidneys were homogenized and lysed in radio-immunoprecipitation assay buffer with a halt protease inhibitor mixture (Cell Signaling Technology) and PMSF (Beyotime Biotech.) according to the manufacturer's instructions. Anti-mouse C1q (1:50, Abcam), properdin (1:500, Abcam) and GAPDH (1:1000, Cell Signaling Technology) antibodies were utilized to probe the blots according to standard procedures.
2.8. In Vitro Study
MSCs were re-suspended in low glucose DMEM/F12 (Gibco) supplemented with 0.2% BSA (Biosharp). 104–105 MSCs were seeded into 24- or 12- well plates and different concentrations of IFN-α1b (KEXING BioTech, China) were added into each well. Cell lysates and culture supernatants were harvested at 0, 12, 24, 48, and 72 h.
2.9. Real-Time Quantitative PCR
Transcripts of human FH were detected via AceQ qPCR SYBR Green Master Mix (Vazyme) according to the manufacturer's instructions. Primer sequences were as follows: FH: 5′-TCTGCATGTTGGCCTTCCTGTC-3′ (forward), 5′-CTTCCTTGTAAATCTCCACCTG-3′ (reverse); GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward), 5′-GAAGATGGTGATGGGATTTC-3′ (reverse).
2.10. Umbilical Cord Mesenchymal Stem Cells Transplantation (UC MSCT)
UC MSCs were prepared by the Stem Cell Center of Jiangsu Province (Beike Bio-Technology). All of the infused UC MSCs were derived from passages 2–5, with rigorous purification and quality control as we previously described [22]. Cells (1 × 106/kg of body weight) were administered by intravenous infusion, which was approved by the Ethics Committee at Drum Tower Hospital and registered at http://ClinicalTrials.gov (identifier: NCT00698191).
2.11. Enzyme-Linked Immunosorbent Assay (EILSA)
Quantification of human C5a, soluble C5b-9 (BD Pharmingen), and FH (Biolegend) were determined in the plasma or supernatants of MSCs. Levels of anti-dsDNA antibody (SHIBAYAGI), C3 (Abcam), C5a (R&D), soluble C5b-9 (LSBio), MBL (Abcam), and FH (Biolegend) were measured in the plasma or kidney homogenates of lupus mice.
2.12. Cofactor Assay
Cofactor activity of FH was assessed using a Factor I (FI)-mediated proteolysis of C3b as described previously [33]. In brief, C3b, FI, and a range of FH (Complement Tech.) were mixed in a final reaction volume of 20 μl. After this, the mixtures were incubated for 30 min at 37 °C. The reaction was terminated by adding SDS-PAGE loading buffer followed by boiling for 2 min. Samples were assessed by 10% SDS-PAGE with coomassie blue staining. MSCs-derived FH was purified via streptavidin magbeads. Briefly, cell-free supernatant was mixed with a biotinylated mouse anti-human Factor H/streptavidin magbeads complex and incubated overnight at 4 °C. After washing and eluting the beads, the supernatant containing FH was neutralized and saved for the following experiment. MSCs-derived FH was identified by Western blotting using a rabbit anti-FH pAb (1 μg/ml, Abcam).
2.13. Statistical Analysis
Independent sample t-tests or Mann-Whitney non-parametric tests were applied to compare variables between two groups. In some cases, paired t-tests were utilized. One-way ANOVA or Kruskal-Wallis non-parametric tests were used to account for multiple comparisons. A two-tailed p < 0.05 was considered statistically significant.
3. Results
3.1. Abnormal Activation of C5 is Related With the Progression of LN
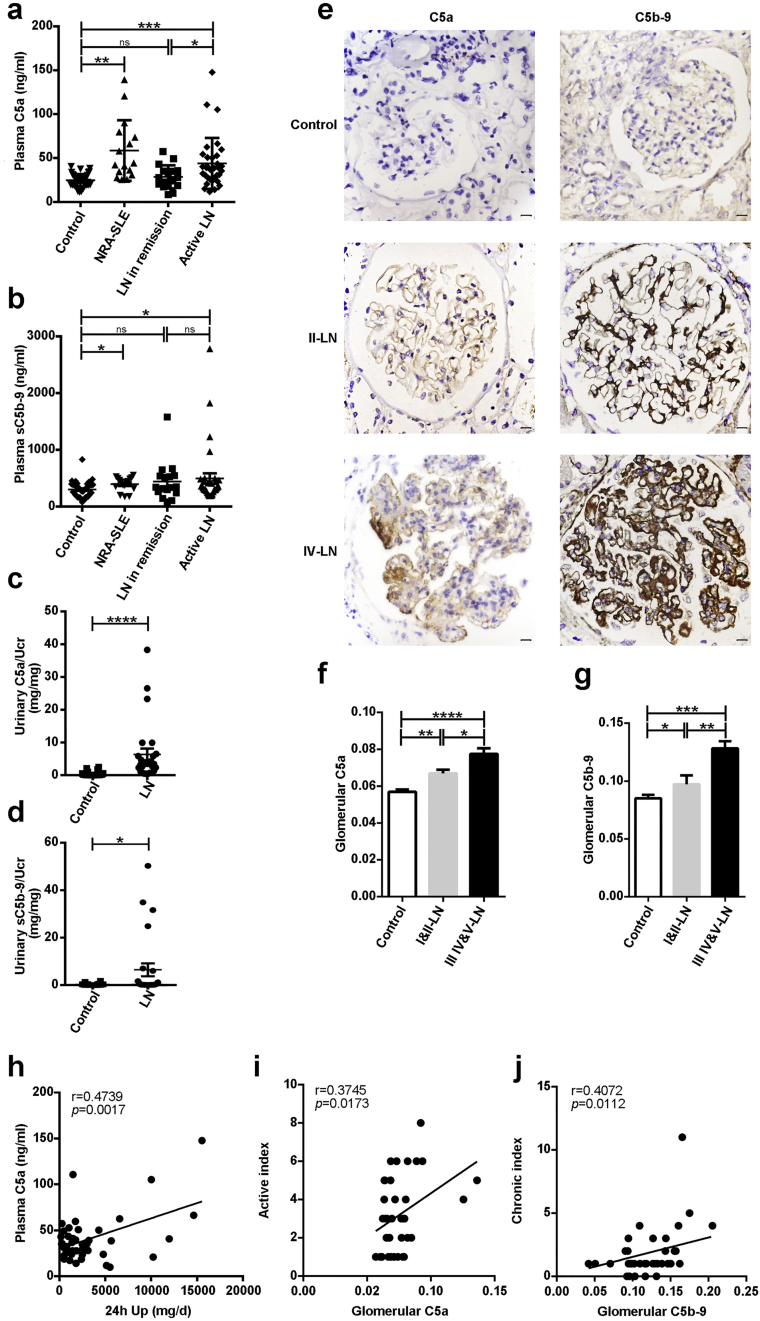
To examine whether the activation of C5 plays a role in the development of LN, we first investigated the products of activated C5 in the plasma of patients with LN. We found that plasma C5a levels were dramatically elevated in patients with active LN compared to those in remission and healthy controls (Fig. 1a). Levels of soluble C5b-9 (sC5b-9) were also significantly increased in active LN compared with healthy controls, although no significant difference was found between LN patients with active disease and those in remission (Fig. 1b). Confirming these results, urinary C5a and sC5b-9 were also significantly elevated in LN patients (Fig. 1c and d). We next examined their expression in renal specimens of LN patients, and found that both C5a and C5b-9 were deposited in the mesangium and capillary walls of affected glomeruli (Fig. 1e). Importantly, although the mean optical density of C5a and C5b-9 were significantly higher in both mild (i.e., types I and II) and severe (i.e., types III, IV, and V) LN (Fig. 1f and g) than in control samples, their expression levels were significantly lower in mild LN than those in severe LN (Fig. 1f and g).
Fig. 1.
Abnormal activation of C5 in LN. (a-b) Plasma C5a and soluble C5b-9 (sC5b-9) in patients with active lupus nephritis (LN), remission of LN, non-renal involvement active SLE (NRA-SLE), and in healthy controls. (c-d) Urinary C5a and sC5b-9 in LN patients and healthy controls. (e) Immunohistochemical staining of C5a and C5b-9 in renal specimens. Scale bar = 10 μm. (f-g) The mean optical density of C5a and C5b-9 deposition in glomeruli of patients with class I and II LN, class III, IV, and V LN, and controls. (h) Correlation between plasma C5a and 24 h proteinuria (24 h Up) in LN patients (n = 41). (i) Correlation between glomerular C5a and active index of LN (n = 40). (j) Correlation between glomerular C5b-9 and chronic index of LN (n = 38). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Values are the mean ± SEM.
Correlation analysis showed that the plasma levels of C5a were positively correlated with 24-h proteinuria in LN patients (Fig. 1h) and SLEDAI scores in SLE patients (Fig. S1). Furthermore, the expression of C5a in the glomeruli was positively correlated with the active index (Fig. 1i), whereas, the deposition of C5b-9 was positively correlated with the chronic index of LN (Fig. 1j). Taken together, these data suggest that C5 is extensively activated in LN patients and it is correlated with the progression of LN.
3.2. MSCs Inhibit Circulating C5 Activation and Ameliorate Renal Disease in Lupus Mice
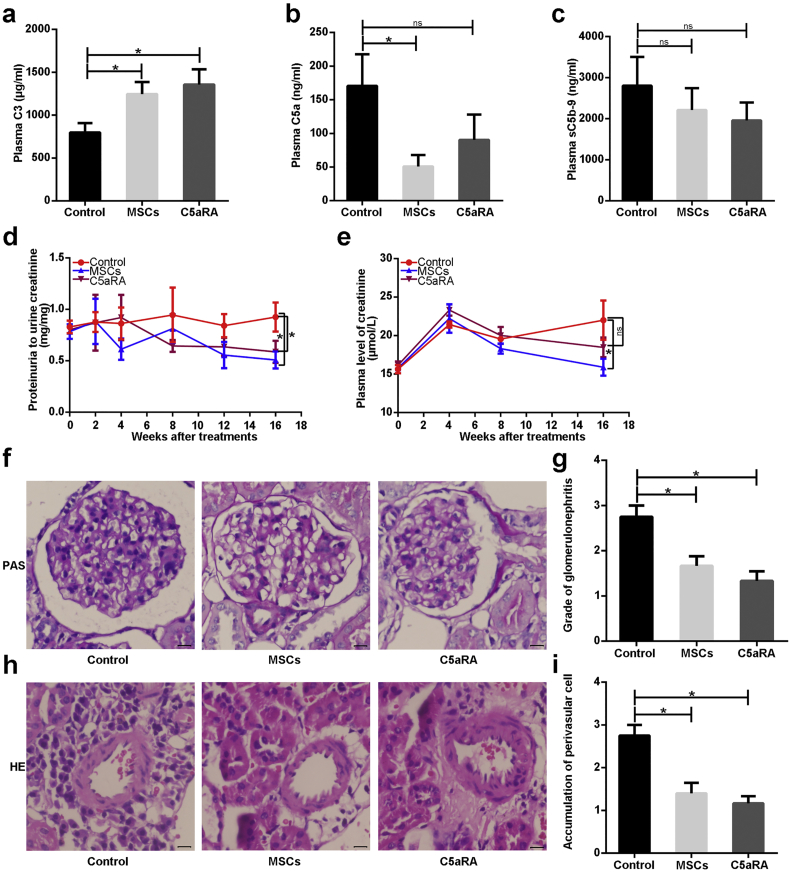
Given our finding that complement C5 is ubiquitously activated in LN, we hypothesized that MSCT, an effective clinical therapy for LN, may participate in the regulation of C5 activation, and selective blockade of C5a might ameliorate renal lesions in LN. We tested this hypothesis by treating lupus mice with MSCT or C5a receptor 1 antagonist (C5aRA). As shown in Fig. 2a, plasma levels of C3 were significantly elevated in mice treated with MSCT or C5aRA compared to untreated mice. Strikingly, mice treated with MSCT, but not C5aRA, exhibited a marked decrease in circulating C5a (Fig. 2b). No significant reduction of circulating sC5b-9 or anti-dsDNA antibody was observed in the treated mice (Fig. 2c and S2a). Intriguingly, we found that the ratios of urinary protein to urinary creatinine were significantly decreased in both treatment groups compared to untreated lupus mice (Fig. 2d). Plasma levels of creatinine were reduced in MSCT-treated mice, but not in C5aRA-treated mice (Fig. 2e), indicating that MSCT improved renal function.
Fig. 2.
MSCs inhibit circulating C5 activation and ameliorate renal disease in lupus mice. (a-c) Plasma C3, C5a, and soluble C5b-9 (sC5b-9) in B6.lpr mice at 42 weeks (n = 4–6 per group). (d-e) The ratio of proteinuria to urinary creatinine, and plasma creatinine in B6.lpr mice during the course of treatments (n = 4–8 per group). (f) PAS staining of a representative glomerulus from three groups. Scale bar = 10 μm. (g) PathologIcal scores of glomerulonephritis in B6.lpr mice (n = 4–6 per group). (h) HE staining of a representative perivascular area from three groups. Scale bar = 10 μm. (i) The severity score for perivascular inflammatory cells infiltrated in kidneys of B6.lpr mice (n = 4–6 per group). *p < 0.05; **p < 0.01; ns, not significant. Values are the mean ± SEM.
In addition to proteinuria and renal function, we also evaluated renal pathological changes in lupus mice after MSCT and C5aRA treatments. As expected, untreated lupus mice exhibited typical proliferative glomerulonephritis manifested by glomerular hypercellularity, inflammatory cells infiltration, and excess mesangial matrix (Fig. S2c). Both MSCT- and C5aRA-treated groups displayed significantly fewer proliferative lesions in the glomeruli than untreated mice (Fig. 2f and g).
Additionally, MSCT- and C5aRA-treated mice also showed significantly decreased perivascular infiltration of inflammatory cells (Fig. 2h and i). These results collectively suggest that MSCT reduced renal damage in lupus mice by inhibiting systemic C5 activation.
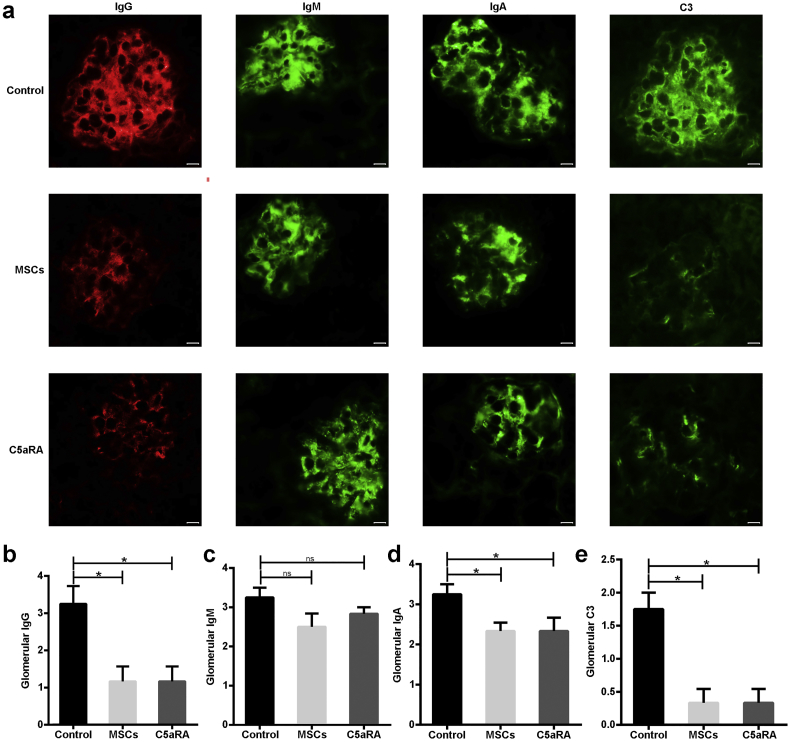
3.3. MSCs Decrease Deposits of Immune Complexes and C3 in Lupus Mice
The deposition of immune complexes and complement components in the glomeruli is the most common and characteristic feature of LN. We thus examined the deposition of IgG, IgM, IgA, and C3 in the glomeruli, and found that all of them were obviously deposited in the mesangium and capillary walls in the untreated lupus mice (Fig. 3a). However, treatment with MSCT or C5aRA significantly decreased the deposition of IgG and diminished C3 in the glomeruli of lupus mice, although the deposition of IgM did not show a significant reduction (Fig. 3b, c, and e).
Fig. 3.
MSCs decrease deposits of immune complexes and C3 in lupus mice. (a) Immunofluorescent staining of IgG, IgM, IgA, and C3 deposition in a representative kidney section from three groups (n = 4–6 per group). Scale bar = 10 μm. (b-e) The grade of fluorescence intensity of IgG, IgM, IgA, and C3 in glomeruli from three groups (n = 4–6 per group). *p < 0.05; ns, not significant. Values are the mean ± SEM.
Notably, glomerular IgA was also significantly reduced in MSCT-treated mice (Fig. 3d). Thus, MSCT limits the complement activation through decreasing glomerular immunocomplexes and C3 deposition in lupus mice.
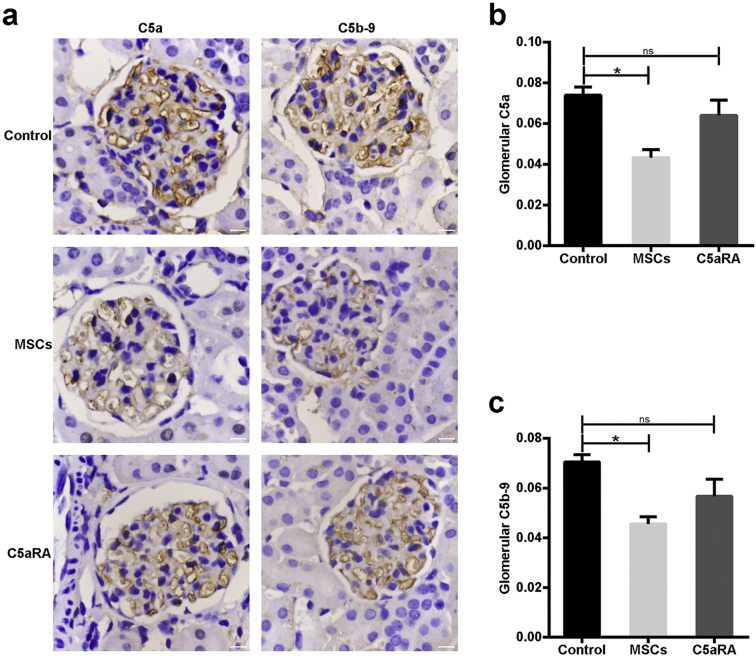
3.4. MSCs Suppress Intrarenal Activation of C5 in Lupus Mice
Apart from inducing systemic inflammatory responses, C5a and C5b-9 are also the major molecules that trigger tissue injury in affected organs [6]. For this reason, we sought to assess the expression of C5a and C5b-9 in the glomeruli of lupus mice treated with MSCs or C5aRA. Immunohistochemical analysis showed that C5a and C5b-9 were clearly detected in the mesangium and capillary walls of glomeruli in untreated lupus mice (Fig. 4a). This similar distribution pattern suggested that C5 was activated locally. Notably, mice that were treated with MSCs showed significantly decreased accumulation of C5a and C5b-9 in the glomeruli (Fig. 4a–c). In contrast, C5aRA treatment did not affect C5a and C5b-9 expression in the glomeruli (Fig. 4a–c), but it inhibited the receptor (C5aR1) binding to the ligand C5a (Fig. S3). These observations indicate that MSCT could inhibit the renal activation of C5 in lupus mice.
Fig. 4.
MSCs suppress intrarenal activation of C5 in lupus mice. (a) Immunohistochemical staining of C5a and C5b-9 in a representative kidney section from three groups (n = 4–6 per group). Scale bar = 10 μm. (b-c) The mean optical density of C5a and C5b-9 accumulation in glomeruli of B6.lpr mice (n = 4–6 per group). *p < 0.05; **p < 0.01; ns, not significant. Values are the mean ± SEM.
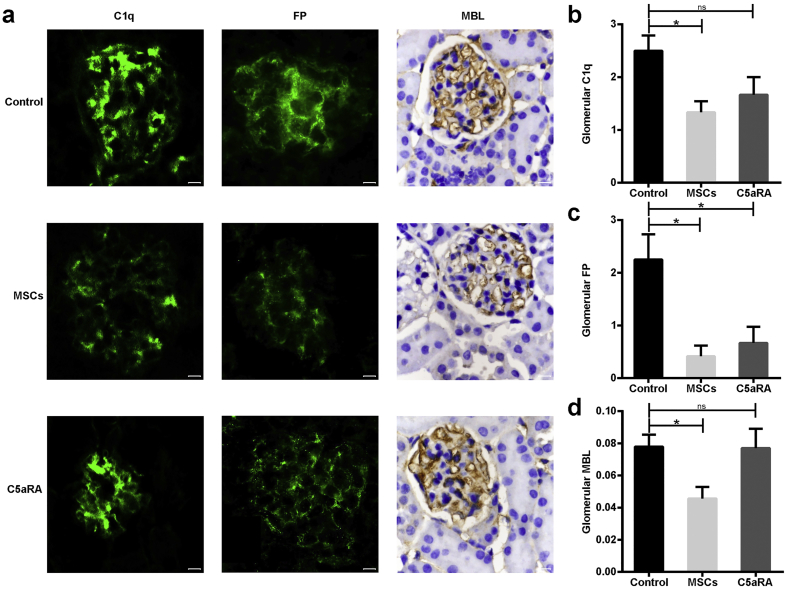
3.5. MSCs Block the Initiation of Complement Cascade in Lupus Mice
To identify which pathway is involved in the intrarenal activation of the complement system, we next examined the expression of the corresponding initiators for the classical pathway (CP), alternative pathway (AP) and lectin pathway (LP), respectively, namely, C1q, Factor P (FP, i.e., properdin), and mannose binding lectin (MBL), respectively. As presented in Fig. 5, all of these initiators were clearly detected in the mesangium and capillary walls of the glomeruli in untreated lupus mice (Fig. 5a). Importantly, we found that treatment with MSCT or C5aRA substantially decreased the deposition of FP in the glomeruli compared to untreated mice (Fig. 5a and c). However, MSCT, but not C5aRA treatment, markedly reduced glomerular C1q and MBL expression compared to untreated mice (Fig. 5a, b, and d). We also confirmed the above findings via Western blotting or ELISA (Fig. S4). Altogether, our data suggest that MSCT could block the glomerular activation of the complement cascade by interfering with the initiation of three pathways in lupus mice.
Fig. 5.
MSCs block initiation of complement cascade in lupus mice. (a) Immunofluorescent staining of C1q, factor P (FP), and immunohistochemical staining of MBL in a representative kidney section from three groups (n = 4–6 per group). Scale bar = 10 μm. (b-c) The grade of fluorescence intensity of C1q and FP in glomeruli from three groups (n = 4–6 per group). (d) The mean optical density of MBL deposition in glomeruli of B6.lpr mice (n = 4–6 per group). *p < 0.05; ns, not significant. Values are the mean ± SEM.
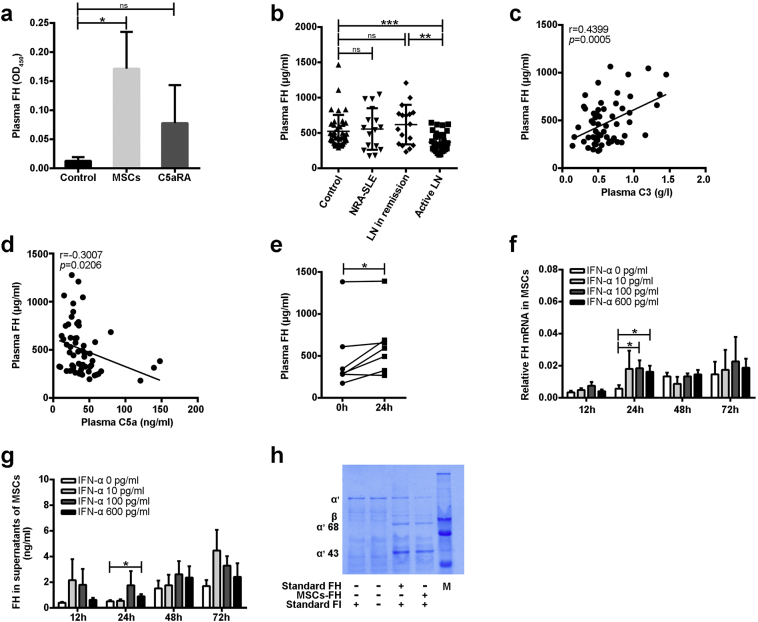
3.6. MSCs Up-Regulate FH in Lupus Mice and Patients
The aforementioned over-activation of complement C5 in LN patients, combined with the significant suppression of C5 activation by MSCT in lupus mice, encouraged us to study the mechanisms underlying this effect. To this end, we investigated whether MSCT influenced plasma levels of complement regulatory proteins. Factor H (FH) is the most abundant complement regulatory protein in the plasma [34]. We therefore first determined that FH was significantly elevated in the plasma of MSCT-treated mice, but this increase was not observed in C5aRA-treated mice (Fig. 6a). We also observed that the plasma levels of FH were significantly decreased in active LN patients compared to those in remission and healthy controls (Fig. 6b). Correlation analysis showed that plasma FH was positively correlated with circulating C3 but negatively correlated with plasma C5a in SLE patients (Fig. 6c and d). Importantly, we found that the levels of FH were also significantly up-regulated in the plasma of seven LN patients at 24 h after MSCT (Fig. 6e). These data indicate that decreased FH might be related to the pathogenesis of LN and that MSCT restores the level of circulating FH in LN patients.
Fig. 6.
MSCs up-regulate FH in lupus mice and patients. (a) Plasma FH in B6.lpr mice at 42 weeks (n = 4–6 per group). (b) Plasma FH in patients with active lupus nephritis (LN), remission of LN, non-renal involvement active SLE (NRA-SLE), and in healthy controls. (c-d) Correlation between plasma FH and C3 or C5a in SLE patients (n = 59). (e) Plasma FH in patients with LN before and after MSCT (n = 7). (f) MSCs were stimulated with IFN-α1b (0–600 pg/ml) for 12 h, 24 h, 48 h, and 72 h. Relative expression of FH mRNA was performed by qPCR, and (g) supernatants of MSCs was detected for FH. (h) C3b and FI were incubated with standard or MSCs-derived FH for 30 min at 37 °C. Samples were analyzed by SDS-PAGE with Coomassie Blue staining. Degradation of C3b was indicated by the appearance of 43 kDa and 68 kDa fragments. Data represents three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. Values are the mean ± SEM.
To study whether the elevated FH was attributable to the production by MSCs directly, we examined the transcripts of FH in MSCs. Analysis by quantitative PCR (qPCR) showed that MSCs expressed FH at a basal level (Fig. 6f). Of particular note, we found that IFN-α stimulation significantly up-regulated the expression of FH in MSCs in vitro (Fig. 6f and g). FH effectively cleaved C3b in vitro and consequently inactivated complement activation (Fig. S5). FH secreted by MSCs upon IFN-α stimulation indeed cleaved C3b as efficiently as recombinant FH in vitro (Fig. 6 h), suggesting that MSCs suppress C5 activation by secreting functional FH.
4. Discussion
Lupus nephritis is an immune complex (IC) disease [35]. Circulating immune complexes (CICs) induce systemic complement activation, while deposition of CICs and formation of ICs in situ lead to intrarenal complement activation. As one of the common terminal effector molecules in the complement cascade, C5a is a potent anaphylatoxin and which is 10–100 times more active than C3a [36]. C5b-9 is another prominent inflammatory mediator [37]. Several animal studies showed that targeted inhibition of C5 could contribute to control excessive complement activation involved in glomerular diseases including LN [18, 38]. Recently, clinical case reports demonstrated that short-term application of anti-C5 monoclonal antibody dramatically improved clinical symptoms of refractory LN [15, 16]. These data suggested that excessive activation of C5 might contribute to the pathogenesis of LN. In this study, we found that C5 is extensively activated in active LN. Interestingly, glomerular expression of C5a and C5b-9 gradually increased with the aggravation of LN, and positive correlations were found between activated C5 and histopathological lesions of LN. Taken together, these data strongly support the notion that over-activation of C5 possibly accelerates the development and progression of LN.
Based on these observations, we reasoned that the existing treatment options for LN might be related to inhibition of C5 over-activation, and blocking C5a should benefit renal lesions in lupus mice. Allogeneic MSCT has achieved clinical efficacy for refractory SLE, including LN patients [[20], [21], [22], [23]], and non-peptide C5a receptor antagonist (C5aRA) has been found to be therapeutic for the inflammatory diseases [31, 39]. In the present study, we therefore explored the possibility that MSCs are involved in regulating C5 activation. We determined here that MSCs and C5aRA indeed suppressed systemic C3 activation and C3 deposition in the glomeruli of B6.lpr mice. As C3 plays an important role in maintaining the solubility of ICs [14], decreased C3 consumption by these treatments significantly inhibit the deposition of IgG in the glomeruli of lupus mice. However, future studies are needed to determine whether immunoglobulins could be disposed directly by MSCs due to their phagocytic activity or by MSCs-educated macrophages [52]. It should be noted that control of C3 activation did not mean thoroughly abolishing C5 activation [40]. In our study, we demonstrated that only MSCT significantly inhibited the accumulation of C5a and C5b-9 in the glomeruli of lupus mice, whereas, C5aRA seemed to have no direct effect on over-activation of C5 in the glomeruli except for preventing C5a from binding to its receptor. Apart from being activated by ICs, complement activation can be triggered and enhanced by the alternative pathway (AP), and inhibition of AP activity improved renal disease in lupus mice [41]. We here found that both MSCT and C5aRA diminished the expression of Factor P, an initiator of AP, and reduced proteinuria in lupus mice. Notably, MSCT, but not C5aRA treatment, also significantly decreased glomerular C1q and MBL, and this further improved the renal outcome. Collectively, our study found that MSCT limited the three pathways of activation and effectively inhibited the common terminal pathway, i.e., cleavage of C5 and the subsequent process.
The AP is tightly controlled by complement regulation proteins to avoid excessive damage to host cells. It is worth mentioning that the AP accounts for up to 80–90% of the total complement activity [42]. Notably, the Factor H (FH) is the major inhibitor of the AP [43]. It was reported that FH deletion was associated with C3 glomerulopathy and thrombotic microangiopathy [44, 45] and accelerated the progression of LN in MRL/lpr mice [46]. However, FH supplement could alleviate renal injury in those model mice [47, 48]. We here found that plasma FH was drastically decreased in active LN, suggesting that decreased FH is involved in the progression or flare-up of LN. Significantly, MSCs increased circulating FH in lupus mice and LN patients. Mechanistically, IFN-α, a typical inflammatory cytokine in SLE, promotes the secretion of FH by MSCs. FH in turn can inhibit the formation of C5 convertase [43], and eventually inhibit the activation of C5. Moreover, FH can also compete with C1q to bind to the activators in the classical pathway, thereby inhibiting C1q mediated CP activation [49, 50]. Given that the structure of MBL is similar to that of C1q [51], the possibility that FH might exert some regulatory effect beyond the AP cannot be completely excluded.
In conclusion, we have demonstrated that over-activation of C5 may contribute to the progression of LN, and that MSCT suppresses C5 activation by secreting FH, which provides profound insights into understanding the therapeutic effects of MSCT in treating LN.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Conceptualization, H.J.M., and L.Y.S.; Methodology, H.J.M., C.L., Z.Y.Z., R.H.F., and X.G; Software, H.J.M. and C.L.; Formal Analysis, H.J.M., W.J.C., and L.Y.S.; Investigation, H.J.M., C.L., and L.Y.S.; Resources, H.J.M., B.Y.S., M.H.G, and L.Y.S.; Data Interpretation, H.J.M., W.J.C., and L.Y.S.; Writing – Original Draft, H.J.M., W.J.C., and L.Y.S.; Writing – Review & Editing, H.J.M., W.L.L., W.J.C., S.T.S., and L.Y.S.; Visualization, H.J.M. and L.Y.S.; Supervision, L.W.L., W.J.C., S.T.S., and L.Y.S. All authors critically reviewed the important intellectual content and approved the final version of the manuscript.
Acknowledgments
This work was funded by the Major International (Regional) Joint Research Project (81720108020), Jiangsu Province Major Research and Development Program (BE2015602), and Jiangsu Province 333 Talent Grant (BRA2016001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.05.034.
Appendix A. Supplementary data
Supplementary material
References
- 1.Moroni G., Raffiotta F., Ponticelli C. Remission and withdrawal of therapy in lupus nephritis. J Nephrol. 2016;29:559–565. doi: 10.1007/s40620-016-0313-6. [DOI] [PubMed] [Google Scholar]
- 2.Singh J.A., Hossain A., Kotb A., Oliveira A., Mudano A.S., Grossman J. Treatments for lupus nephritis: a systematic review and network metaanalysis. J Rheumatol. 2016;43:1801–1815. doi: 10.3899/jrheum.160041. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh S.C., Tsai C.Y., Yu C.L. Potential serum and urine biomarkers in patients with lupus nephritis and the unsolved problems. Open Access Rheumatol. 2016;8:81–91. doi: 10.2147/OARRR.S112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arriens C., Wren J.D., Munroe M.E., Mohan C. Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology. 2017;56:i32–i45. doi: 10.1093/rheumatology/kew407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvadori M., Bertoni E. Complement related kidney diseases: recurrence after transplantation. World J Transplant. 2016;6:632–645. doi: 10.5500/wjt.v6.i4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurman J.M., Nester C.M. All things complement. Clin J Am Soc Nephro. 2016;11:1856–1866. doi: 10.2215/CJN.01710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nisihara R.M., Magrini F., Mocelin V., Messias-Reason I.J. Deposition of the lectin pathway of complement in renal biopsies of lupus nephritis patients. Hum Immunol. 2013;74:907–910. doi: 10.1016/j.humimm.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Sato N., Ohsawa I., Nagamachi S., Ishii M., Kusaba G., Inoshita H. Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus. 2011;20:1378–1386. doi: 10.1177/0961203311415561. [DOI] [PubMed] [Google Scholar]
- 9.Tan Y., Song D., Wu L.H., Yu F., Zhao M.H. Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritis. BMC Nephrol. 2013;14:63. doi: 10.1186/1471-2369-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira J.E., Costa R.S., Lachmann P.J., Wurzner R., Barbosa J.E. CR1 stump peptide and terminal complement complexes are found in the glomeruli of lupus nephritis patients. Clin Exp Immunol. 1996;105:497–503. doi: 10.1046/j.1365-2249.1996.d01-776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F.M., Song D., Pang Y., Sony Y., Yu F., Zhao M.H. The dysfunctions of complement factor H in lupus nephritis. Lupus. 2016;25:1328–1340. doi: 10.1177/0961203316642307. [DOI] [PubMed] [Google Scholar]
- 12.Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 13.Stegert M., Bock M., Trendelenburg M. Clinical presentation of human C1q deficiency: how much of a lupus? Mol Immunol. 2015;67:3–11. doi: 10.1016/j.molimm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Sekine H., Reilly C.M., Molano I.D., Garnier G., Circolo A., Ruiz P. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol. 2001;166:6444–6451. doi: 10.4049/jimmunol.166.10.6444. [DOI] [PubMed] [Google Scholar]
- 15.El-Husseini A., Hannan S., Awad A., Jennings S., Cornea V., Sawaya B.P. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am J Kidney Dis. 2015;65:127–130. doi: 10.1053/j.ajkd.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Pickering M.C., Ismajli M., Condon M.B., McKenna N., Hall A.E., Lightstone L. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology. 2015;54:2286–2288. doi: 10.1093/rheumatology/kev307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sciascia S., Radin M., Yazdany J., Tektonidou M., Cecchi I., Roccatello D. Expanding the therapeutic options for renal involvement in lupus: eculizumab, available evidence. Rheumatol Int. 2017;37:1249–1255. doi: 10.1007/s00296-017-3686-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Hu Q.L., Madri J.A., Rollins S.A., Chodera A., Matis L.A. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. P Natl Acad Sci USA. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F., Chiu S.M., Motan D.A.L., Zhang Z., Chen L., Ji H.L. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang J., Zhang H.Y., Hua B.Z., Wang H., Lu L.W., Shi S.T. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–1429. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 21.Sun L.Y., Akiyama K., Zhang H.Y., Yamaza T., Hou Y.Y., Zhao S.N. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L.Y., Wang D.D., Liang J., Zhang H.Y., Feng X.B., Wang H. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 23.Wang D.D., Li J., Zhang Y., Zhang M.J., Chen J.Y., Li X. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu Z., Li Q., Bu H., Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19:1803–1809. doi: 10.1089/scd.2009.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Wang Y., Zhang X., Qin Y., Qu B., Wu L. MicroRNA-130b ameliorates murine lupus nephritis through targeting the type I. Interf Pathway Ren Mesangial Cells Arthritis Rheumatol. 2016;68:2232–2243. doi: 10.1002/art.39725. [DOI] [PubMed] [Google Scholar]
- 26.Collins E., Gilkeson G. Hematopoetic and mesenchymal stem cell transplantation in the treatment of refractory systemic lupus erythematosus - where are we now? Clin Immunol. 2013;148:328–334. doi: 10.1016/j.clim.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Hahn B.H., McMahon M.A., Wilkinson A., Wallace W.D., Daikh D.I., Fitzgerald J.D. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petri M., Kim M.Y., Kalunian K.C., Grossman J., Hahn B.H., Sammaritano L.R. Combined oral contraceptives in women with systemic lupus erythematosus. New Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 30.Weening J.J., D'Agati V.D., Schwartz M.M., Seshan S.V., Alpers C.E., Appel G.B. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 31.Li L., Chen L., Zang J., Tang X., Liu Y., Zhang J. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64:597–610. doi: 10.1016/j.metabol.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Kikawada E., Lenda D.M., Kelley V.R. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 33.Pechtl I.C., Kavanagh D., Mcintosh N., Harris C.L., Barlow P.N. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J Biol Chem. 2011;286:11082–11090. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins S.J., Nan R., Li K., Khan S., Miller A. Complement factor H-ligand interactions: self-association, multivalency and dissociation constants. Immunobiology. 2012;217:281–297. doi: 10.1016/j.imbio.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Lech M., Anders H.J. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357–1366. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foreman K.E., Glovsky M.M., Warner R.L., Horvath S.J., Ward P.A. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 37.Quigg R.J. Complement and autoimmune glomerular diseases. Curr Dir Autoimmun. 2004;7:165–180. doi: 10.1159/000075692. [DOI] [PubMed] [Google Scholar]
- 38.Pickering M.C., Warren J., Rose K.L., Carlucci F., Wang Y., Walport M.J. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci U S A. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumichika H., Sakata K., Sato N., Takeshita S., Ishibuchi S., Nakamura M. Identification of a potent and orally active non-peptide C5a receptor antagonist. J Biol Chem. 2002;277:49403–49407. doi: 10.1074/jbc.M209672200. [DOI] [PubMed] [Google Scholar]
- 40.Kolev M., Le Friec G., Kemper C. Complement—tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 41.Grossman T.R., Hettrick L.A., Johnson R.B., Hung G., Peralta R., Watt A. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology. 2016;221:701–708. doi: 10.1016/j.imbio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parente R., Clark S.J., Inforzato A., Day A.J. Complement factor H in host defense and immune evasion. Cell Mol Life Sci. 2016;74:1605–1624. doi: 10.1007/s00018-016-2418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laskowski J., Renner B., Le Quintrec M., Panzer S., Hannan J.P., Ljubanovic D. Distinct roles for the complement regulators factor H and Crry in protection of the kidney from injury. Kidney Int. 2016;90:109–122. doi: 10.1016/j.kint.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vernon K.A., Ruseva M.M., Cook H.T., Botto M., Malik T.H., Pickering M.C. Partial complement factor H deficiency associates with C3 glomerulopathy and thrombotic microangiopathy. J Am Soc Nephrol. 2016;27:1334–1342. doi: 10.1681/ASN.2015030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao L., Haas M., Quigg R.J. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22:285–295. doi: 10.1681/ASN.2010060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pouw R.B., Vredevoogd D.W., Kuijpers T.W., Wouters D. Of mice and men: the factor H protein family and complement regulation. Mol Immunol. 2015;67:12–20. doi: 10.1016/j.molimm.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y., Denton H., Davies O.R., Smith-Jackson K., Kerr H., Herbert A.P. An engineered complement factor H construct for treatment of C3 Glomerulopathy. J Am Soc Nephrol. 2018;29 doi: 10.1681/ASN.2017091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan L.A., Yang A.C., Kishore U., Sim R.B. Interactions of complement proteins C1q and factor H with lipid A and Escherichia coli: further evidence that factor H regulates the classical complement pathway. Protein Cell. 2011;2:320–332. doi: 10.1007/s13238-011-1029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan L.A., Yu B., Sim F.C., Kishore U., Sim R.B. Complement activation by phospholipids: the interplay of factor H and C1q. Protein Cell. 2010;1:1033–1049. doi: 10.1007/s13238-010-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birmingham D.J., Hebert L.A. The complement system in lupus nephritis. Semin Nephrol. 2015;35:444–454. doi: 10.1016/j.semnephrol.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Eggenhofer E., Hoogduijn M.J. Mesenchymal stem cell-educated macrophages. Transplant Res. 2012;28:12. doi: 10.1186/2047-1440-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material