Abstract
The prevalence of allergic rhinitis (AR) and obesity in children increased concurrently during recent decades. However, the molecular pathway involved in the interaction between obesity and AR is still unclear. We aimed to investigate the interaction between leptin and osteopontin (OPN) and their effect on T helper (TH) response in the development of AR in children. Thirty AR and 30 healthy children with or without obesity were enrolled. Serum leptin and OPN levels were measured and their relationship with TH1/2 cytokines was analyzed. TH cell differentiation and cytokine production in peripheral blood mononuclear cells (PBMCs) stimulated by leptin and/or OPN were analyzed by enzyme linked immunosorbent assay (ELISA). Obese AR mice models were established to verify the effect of obesity on leptin and OPN as well TH regulation. Immunoprecipitation was performed to confirm the interaction between OPN and leptin in CD4+ T cells. Our results showed elevated serum leptin and OPN in AR children correlated with TH2 cytokines expression. Leptin and OPN enhanced TH2 inflammation in house dust mite stimulated PBMCs from AR children synergistically. Obese AR mice showed as more severe inflammatory reaction, symptoms and expression of nasal leptin and OPN compared with other groups. Immunoprecipitation suggested that OPN and leptin may interact with each other and this process may be mediated by α4 integrin and PI3K/AKT pathway in CD4+ T cells. Our data provide evidence that leptin-mediated OPN upregulation promote TH2 inflammation in AR and this process is achieved through the α4 integrin and PI3K/AKT signaling pathways.
Keywords: Allergic rhinitis, Leptin, Osteopontin, Obesity, T helper cell
Research in context
-
•
Previous studies suggested that both leptin and osteopontin are increased in allergic rhinitis patients and related to the severity of disease. We also provide evidence that leptin and osteopontin contributes to TH2-skewed airway diseases. In the present study, our data suggested that obesity and allergic rhinitis interacted closely through leptin and OPN, and these cytokines may be used as potential biomarkers for disease severity of allergic rhinitis despite that more studies were needed.
1. Introduction
The prevalence of allergic rhinitis (AR) and obesity in children increased gradually during recent decades. Previous studies have shown that AR children with obesity often presented with more severe clinical symptoms, suggesting that obesity may enhance inflammation in AR [1]. AR is a chronic inflammatory disease of the airways characterized by eosinophilic airway inflammation and enhanced Type 2 T helper (TH2) immune response, whereas obesity is a low-grade systemic inflammatory state characterized by altered levels of adipokines and cytokines [2]. However, the molecular pathway involved in the interaction between obesity and AR is still unclear.
Leptin is one of the adipose-derived energy regulating hormones and circulating leptin is positively correlated with body fat percentage and body fat mass [3, 4]. Leptin also exerts biologic functions in angiogenesis, hematopoiesis and immune responses. For example, leptin is involved in apoptosis, proliferation and activation of T cells [5]. The overall effect of leptin on T memory cells is to increase TH1 responses and decrease TH2 and regulatory T cells responses [6]. Emerging evidence shows that leptin is also upregulated during allergic reactions in the airway and related to the severity of disease in AR [7, 8].
Osteopontin (OPN), originally discovered in bone as an extracellular matrix protein, was later identified in many cell types in the immune system where it may be involved in the pathogenesis of inflammatory and immune diseases such as sarcoidosis, tuberculosis, rheumatoid arthritis, Crohn's disease, and multiple sclerosis [9]. Previous studies supports an active role for OPN in TH2-linked inflammation in the pathogenesis of AR [10, 11]. For example, our previous study showed that upregulated OPN expression can be found in the nasal mucosa of AR patients and serum OPN levels are associated with migration and activation of eosinophils and IL-5 overproduction [11].
Moreover, it has been reported that serum OPN levels are correlated with obese state and may be reduced by loss of fat mass [12]. Additionally, leptin-mediated fibrogenesis may be reduced by OPN neutralization in methionine-choline deficient diets fed mice [13]. These above findings and the similar roles between OPN and leptin in regulation of TH balance and inflammatory cells suggested that OPN and leptin may interact with each other under different conditions. However, the interaction and pathway between OPN and leptin in AR is not well-established. Therefore, we aimed to investigate the correlation between leptin and OPN in the development of AR in children with obesity in this study.
2. Materials and Methods
2.1. Patients
Thirty AR children (7–12 years old) with or without obesity were recruited at the Department of Otolaryngology, Guangzhou Women and Children's Medical Center from January 2016 to October 2016. Our study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The study protocols were approved by local ethics committee boards. Written informed consent was obtained from the parents of all subjects. The diagnosis of perennial AR was made according to disease history, clinical examination and specific IgE measurement according to the Allergic Rhinitis and its Impact on Asthma guideline (2010) [14]. The atopic status was evaluated by the detection of IgE specific to common inhalant allergens (dust mites, pets, molds, cockroach, etc). Specific IgE values greater than or equal to 0.35 kIU/L were considered positive. For controls, 30 healthy children (7–12 years old) with or without obesity were enrolled.
Children were excluded if any of the following criteria were present: history of atopic dermatitis, asthma, nasal abnormalities, concurrent purulent nasal infection; use of systemic or topical corticosteroids or sodium cromoglycate within the past 4 weeks; use of histamine H1 antagonist within the past 7 days or a history of any infection within the past 2 weeks.
Body weight and height were measured, with the subject lightly clothed and bare-foot, using an electronic weighing scale (Tanita Corp., Tokyo, Japan) and a Harpenden stadiometer. Obesity was defined as a body mass index (BMI) greater than the 95th percentile. These percentile standards were established by a local population survey [15].
2.2. Symptom Scores
A total nasal symptom score was calculated for the evaluation of severity in children as described previously [11]. Individual nasal symptoms including sneezing, rhinorrhea, itchy nose, and nasal congestion were recorded on a 0 to 3 scale (0, none; 1, mild; 2, moderate; and 3, severe) and the total nasal symptom score (0−12) was summed. The nasal symptoms were recorded and averaged in an 8-week observation period.
2.3. Blood Sample Collection
Venous blood samples were obtained into Vacuette tubes between 6 am and 8 am after an overnight fast. After centrifuging at 1000g for 15 min at 4 °C, serum samples were separated and stored at −80 °C until assay.
2.4. TH2 Cell Differentiation and Cytokine Production in Peripheral Blood Mononuclear Cells (PBMCs) Stimulated by Leptin and OPN
Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep density-gradient centrifugation from heparinized leukocyte-enriched buffy coats, which were obtained from ten AR (sensitive to HDM) and ten control children, as described early [16]. PBMCs were cultured at 37 °C in 5% CO2 for 3 days in 1 mL medium (2*106 cells/mL) with phorbol myristate acetate (PMA) (50 ng/mL) and ionomycin (500 ng/mL) (Sigma-Aldrich, St. Louis, MO, USA). The house dust mite extract (20 μg/mL HDM), recombinant leptin and OPN or other stimulators or inhibitors were also added (R&D system, Minneapolis, MN, USA). TH cell differentiation was detected by analyzing cytokines expression in supernatant by enzyme linked immunosorbent assay (ELISA). Each sample was assayed and measured in triplicate, and all experiments were repeated at least three times.
2.5. Animal Models
All animal experiments comply with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments. Four-week-old male C57BL6/J mice were housed three per cage on a 12 h light–dark cycle, and raised for ten weeks with either a standard chow diet (carbohydrate: 70%; protein: 20%; fat: 10%) or a high-fat diet that induces obesity (carbohydrate: 29%; protein: 16%; fat: 55%) (BioServ, Frenchtown, NJ, USA). A total of 54 mice were randomly divided into the control (15 mice), AR (15 mice), and intervention groups (24 mice).
All the mice were actively sensitized by subcutaneous injection (0.4 mL) of 100 mg of ovalbumin (OVA) mixed with 1.6 mg Al(OH)3 in 0.9% NaCl (day 0) (Sigma-Aldrich Co., St Louis, MO, USA). On day 7, the mice received a second subcutaneous injection of 100 mg ovalbumin and non-sensitized mice received only a subcutaneous injection of Al(OH)3. Then the sensitized mice were challenged nasally with 10 mg of OVA in 40 mL of PBS (20 mL per nostril) or saline for controls from day 14 to day 18. Twenty-four hours after the last challenge, the mice were anaesthetized and killed, after which a sample of peripheral blood was collected from the abdominal vena cava. The measurements of total cholesterol (TC), high-density lipoprotein (HDL) and triglycerides (TGs) in serum were carried out using commercially available kits. Epididymal fat mass was also collected and weighed. Nose tissues were collected for morphological study, as detailed below.
In order to define the role of leptin or OPN in the challenge phase, 100 μg of recombinant mouse leptin, OPN, anti-leptin, anti-OPN protein or anti-α4 integrin (R&D Systems, Minneapolis, MN, USA) in 40 mL of PBS was administered intranasally 2 h before each challenge, respectively. Each sample was assayed and measured in triplicate, and all experiments were repeated at least three times.
2.6. Elisa
The serum, nasal lavage and supernatant levels of leptin, OPN, IFN-γ, IL-12, IL-4, IL-5, IL-10, IL-13 and TGF-beta were measured in duplicate using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All experiments were repeated at least three times.The detection limits of the assays were as follows: leptin (22 pg/mL), OPN (312.5 pg/mL), IFN-γ (12.5 pg/mL), IL-12 (5 pg/mL), IL-4 (1.56 pg/mL), IL-5 (7.8 pg/mL), IL-10 (3.9 pg/mL), IL-13 (93.8 pg/mL), and TGF-beta (14.5 pg/mL).
2.7. Morphological Study
Nasal turbinate sections of 4- to 5-μm thickness were deparaffinized in xylene,
rehydrated in alcohol and incubated in 0.3% H2O2 to block endogeneous peroxidase activity. For immunohistochemistry (IHC), the slides were incubated with rabbit polyclonal antibody against OPN (Zhongshan Goldenbridge, Beijing, China) (1: 200) or rabbit polyclonal antibody against leptin (Thermo Fisher Scientific, Fremont, CA, USA) overnight at 4 °C. At the second day, the samples were then incubated with secondary biotinylated goat anti-mouse/rabbit IgG antibody, followed by avidin-peroxidase complex (Thermo Fisher Scientific, Fremont, CA, USA). After additional washing, the slides were stained with 3% diaminobenzidine chromogen, counter-stained with hematoxylin, and affixed with a coverslip. Isotype-matched IgG was used in place of the primary antibody as the negative control.
2.8. Tissue Lysis and Immunoprecipitation
For Th2 polarization experiments, CD4+ T cells were negatively prepared from PBMCs by magnetic activated cell sorting (Miltenyi Biotec). The cells were seeded with a density of 1.5*106 cells/mL in 24-well plates coated with anti-CD3 and anti-CD28 (1 μg/mL) (R&D Systems, Minneapolis, MN, USA). IL-4 (1000 U/mL), IL-2 (10 U/mL), and anti-IFN-γ (10 μg/mL) were added at day 0 and maintained throughout the experiment (R&D Systems, Minneapolis, MN, USA).
Cell protein extracts from differentiated Th2 cells were prepared using modified RIPA buffer. Protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad). Immunoprecipitation was performed with anti-leptin or anti-OPN antibody (Santa Cruz Biotechnology) for co-immunoprecipitation assay and then analyzed by SDS-PAGE (Invitrogen, Paisley, UK) and detected with Western blot analysis by chemiluminescence (ECL Plus; GE Healthcare, Chalfont St. Giles, UK). Protein expression levels of leptin or OPN were expressed relative to beta-actin expression used as a control for protein loading. Each sample was assayed and measured in triplicate, and all experiments were repeated at least three times.
2.9. Statistical Analysis
All data are expressed as the medians and interquartile ranges and analyzed by using the Kruskal-Wallis H test or the nonparametric Mann-Whitney U test except additional note. Correlations between the various parameters were assessed by the Spearman rank correlation analysis. A P value of <0.05 was defined as statistically significant.
3. Results
3.1. Elevated Serum Leptin and OPN in AR Children and its Relationship with Th Cytokines and Disease Severity
The characteristics of participants are summarized in Table 1. The total nasal symptom score were significantly higher in obese children with AR than in non-obese children with AR (P < .05) (Table 1).
Table 1.
Demographic characteristic of AR children and controls.
| Groups | AR without obesity | AR with obesity | Obesity control | Control |
|---|---|---|---|---|
| Number | 15 | 15 | 15 | 15 |
| Sex (Male:Female) | 8:7 | 7:8 | 9:6 | 7:8 |
| Age (months) | 90.1 ± 33.0 | 84.5 ± 22.1 | 88.2 ± 32.0 | 91.0 ± 30.8 |
| BMI | 16.2 ± 1.3 | 23.4 ± 1.5⁎ | 24.2 ± 1.6⁎ | 16.7 ± 1.8 |
| TNSS score | 7.2 ± 1.3 | 10.5 ± 1.4# | – | – |
AR, allergic rhinitis, BMI, body mass index, TNSS, total nasal symptom score.
Compared with control group, p < .05.
Compared with AR without obesity group, p < .05.
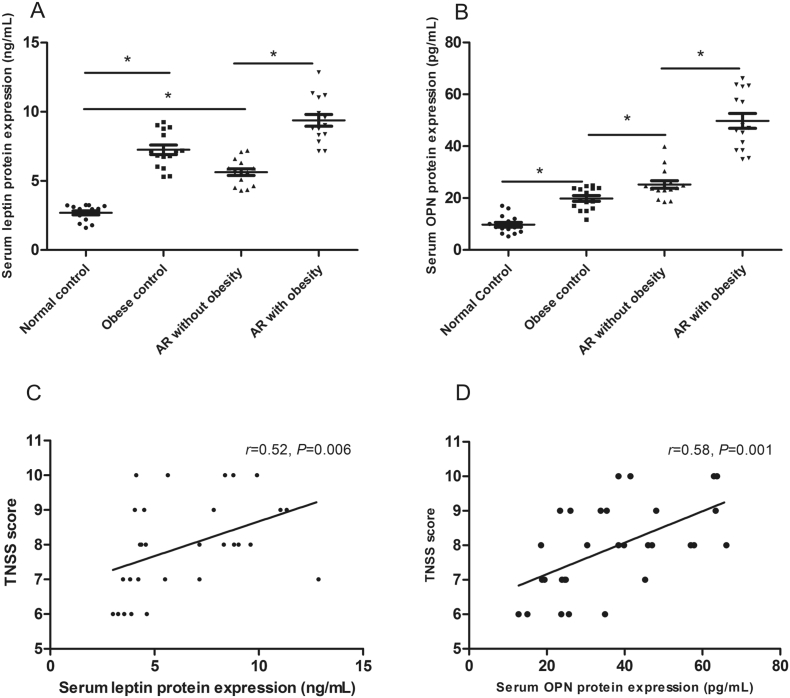
Our results showed that both serum leptin and OPN concentrations were significantly increased in children with AR compared with normal controls, especially in patients with obesity (P < .05) (Fig. 1). The elevated serum leptin (r = 0.52, P = .006) and OPN (r = 0.58, P = .001) levels were also significantly correlated with total nasal symptom score in all children with AR (including obese and non-obese AR), suggesting that serum leptin and OPN levels were related to disease severity (P < .05) (Fig. 1).
Fig. 1.
Serum leptin (A) and OPN (B) expression in AR children and the correlation between serum leptin (C) and OPN (D) concentration and TNSS score in patients with AR. *P < .05, TNSS, total nasal symptom score.
We also found that the elevated serum leptin concentration was significantly correlated with upregulated IL-4, IL-5, IL-13, IL-10 and TGF-beta levels in AR patients (P < .05), and the elevated serum OPN concentration was significantly correlated with leptin, IL-4, IL-5, IL-13, IFN-γ, IL-12, IL-10 and TGF-beta levels in AR patients (P < .05) (Table 2).
Table 2.
Relationship between serum OPN/leptin and Th cytokines in AR children.
| Serum | Leptin |
OPN |
||
|---|---|---|---|---|
| r | P | r | P | |
| OPN | 0.78 | 0.01 | – | – |
| Leptin | – | – | 0.78 | 0.01 |
| IL-4 | 0.68 | 0.01 | 0.72 | 0.03 |
| IL-5 | 0.65 | 0.02 | 0.63 | 0.01 |
| IL-13 | 0.55 | 0.01 | 0.72 | 0.03 |
| IL-12 | – | – | −0.63 | 0.01 |
| IFN-γ | – | – | −0.58 | 0.02 |
| IL-10 | −0.62 | 0.02 | −0.66 | 0.02 |
| TGF-beta | −0.56 | 0.01 | −0.57 | 0.01 |
AR, allergic rhinitis, OPN, osteopontin.
3.2. Th2 Cell Differentiation and Cytokine Expression by PBMC Cells Stimulated by Leptin/OPN
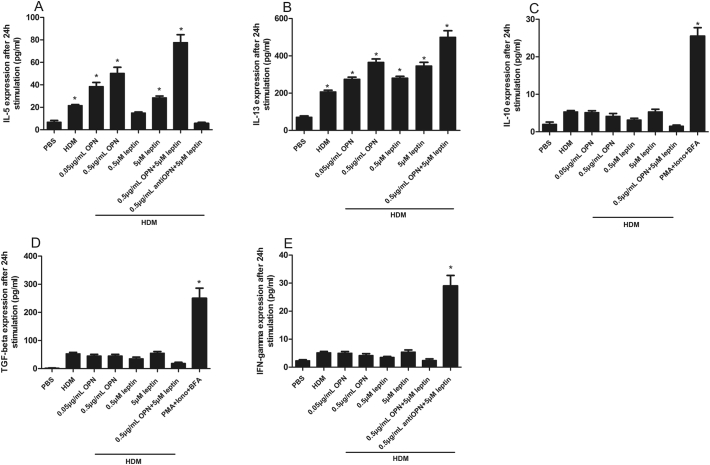
PBMCs from the healthy control group did not produce IL-4/5/13 in response to house dust mite (HDM). However, after stimulation with OPN + HDM, PBMCs from AR children produced significant upregulation of IL-5, IL-13 and inhibited IL-10 and TGF-beta and these effects were enhanced when leptin was added. Interestingly, pure leptin did not enhance Th1 differentiation, however, IFN-γ expression was upregulated significantly when anti-OPN was added (Fig. 2). We also found that IL-4 production by PBMCs from AR children was not detectable after stimulation with OPN + HDM (Data not shown). Besides, PBMCs from obese or non-obese AR children showed similar results in all stimulation experiments (Data not shown).
Fig. 2.
The expression of IL-5 (A), IL-13 (B), IL-10 (C), TGF-beta (D), and IFN-γ (E) by peripheral blood mononuclear cells (PBMCs) from AR children after leptin and/or OPN stimulation. HDM, house dust mite, PMA, phobol myristate acetate, Iono, Ionomycin, BFA, Brefeldin A.
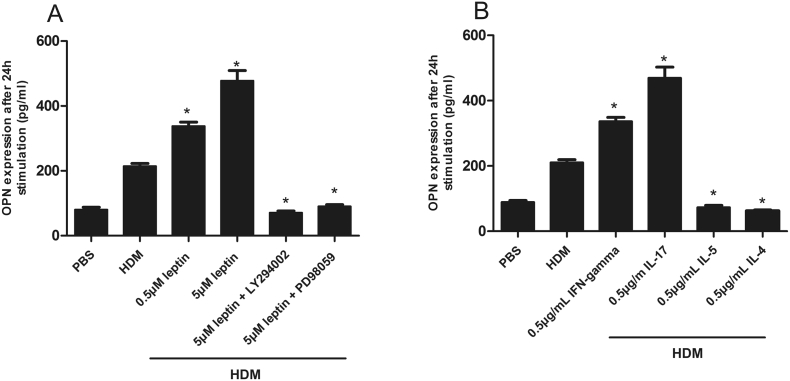
Additionally, leptin also induces OPN expression in PBMCs dose dependently and this process can be inhibited by phosphatidylinositol 3-kinase (PI3K) and serine/threonine-protein kinases (AKT) inhibitors (Fig. 3), whereas OPN has no direct effect on leptin production by PBMC cells (Data not shown).
Fig. 3.
The expression of OPN by peripheral blood mononuclear cells (PBMCs) from AR children after leptin stimulation (A). The effect of TH cytokines on the expression of OPN was shown in Fig. B. HDM, house dust mite.
Next, we also detected the effect of TH cytokines on the expression of leptin or OPN. Our results showed that IFN-gamma and IL-17 is able to induce OPN, while two Th2 cytokines, IL-4 and IL-5, suppress its expression (Fig. 3). We also found that no TH cytokines has effect on leptin expression (Data not shown).
3.3. HFD Augments Allergic Airway Inflammation and Nasal Leptin/OPN Expression
Mice fed with high-fat diets showed a significant increase in body weight, subcutaneous and visceral fat pad weight compared with control groups (Table 3). The obese mice also presented increased serum levels of TC and LDL compared to the lean mice, but no significant differences in TGs and HDL levels between both groups were found (Table 3).
Table 3.
Effect of a high-fat diet on mice anthropometric and lipid profiles.
| Control mice | Obese mice | |
|---|---|---|
| Body weight (g) | 26.2 ± 0.26 | 38.1 ± 0.29⁎ |
| Epididymal fat (g) | 0.26 ± 0.03 | 1.37 ± 0.05⁎ |
| TC (mg/L) | 923 ± 54 | 1388 ± 78⁎ |
| LDL (mg/L) | 223 ± 39 | 573 ± 65⁎ |
| HDL (mg/L) | 521 ± 28 | 565 ± 32 |
| TGs (mg/L) | 745 ± 25 | 701 ± 34 |
TC, total cholesterol, LDL, low-density lipoprotein, HDL, high-density lipoprotein, TGs, triglycerides.
Compared with control group, p < .05.
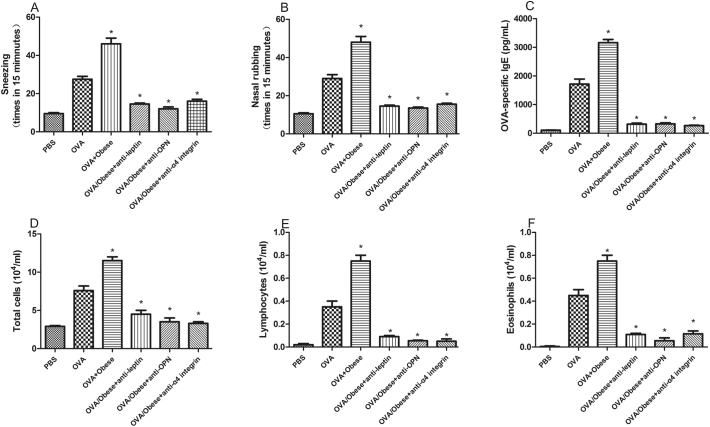
The OVA specific IgE, the count of lymphocytes and eosinophils, the times of nasal rubbing and sneezing were significantly higher in obese AR mice than that of non-obese AR mice (Fig. 4). Our results also showed that the anti-leptin, anti-OPN or anti-α4 integrin attenuate inflammation in obese AR mice, manifested as lower symptom score and less inflammatory infiltration.
Fig. 4.
The times of nasal rubbing and sneezing (A,B), OVA specific IgE (C), the count of total cells (D), lymphocytes (E) and eosinophils (F) were significantly higher in obese AR mice and the anti-leptin, anti-OPN or anti-α4 integrin attenuate inflammation in obese AR mice.
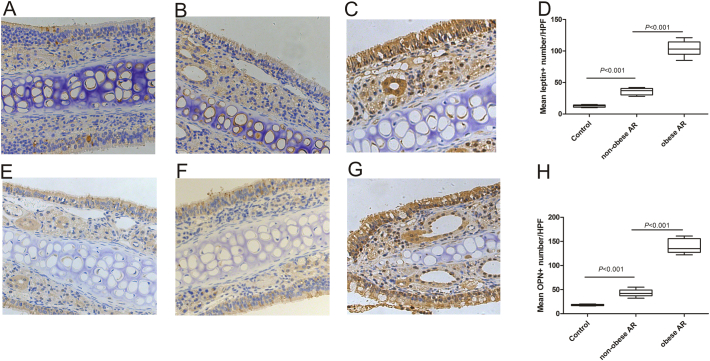
In nasal tissues from obese AR mice, epithelial cells, interstitial cells, glandular cells and endothelial cells were positive for leptin and OPN staining, while the number of OPN and leptin positive cells in non-obese AR mice and normal controls reduced significantly (Fig. 5).
Fig. 5.
The expression and cellular sources of leptin and OPN in the nasal mucosa of OVA-challenged obese mice (C, G) was significantly higher than in OVA-challenged non-obese (B, F) and control mice (A, E). The mean number of leptin-positive cells (D) and OPN-positive cells (H) in nasal tissues and normal control were compared among groups.
3.4. Interaction between Leptin and OPN by Co-Immunoprecipitation
Whole protein was extracted from differentiated Th2 cells, and leptin were co-immunoprecipitaed with OPN. As shown in Fig. 5, leptin were detected in OPN immunoprecipitates, suggesting that leptin and OPN were in same complex. Similarly, co-immunoprecipitation with leptin or OPN was conducted at 48 h after anti-OPN, anti-leptin, anti-α4 integrin treatments, the results suggested that all these antibodies can block the interaction between leptin and OPN (Fig. 6).
Fig. 6.
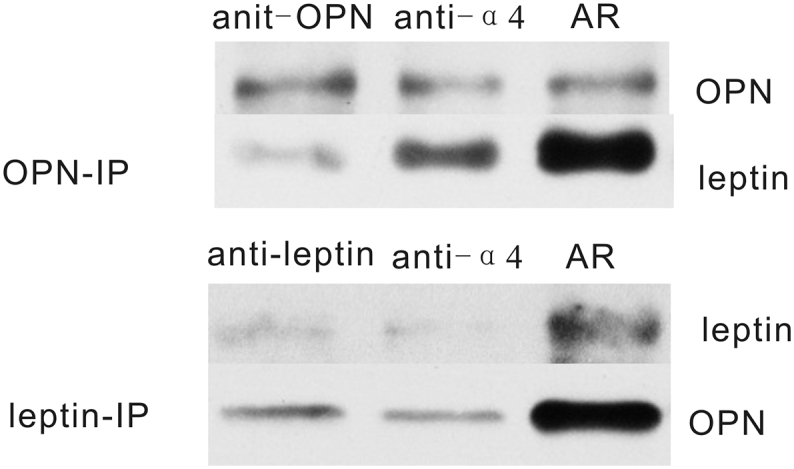
Interaction between leptin and OPN by co-immunoprecipitation in Th2 cells. The anti-OPN, anti-leptin or anti-α4 integrin treatment may block the interaction between OPN and leptin.
4. Discussion
In this study, our results showed that significant upregulation of leptin and OPN in obese children with AR. We also found that increased expression of leptin and OPN were correlated with TH2 cell differentiation in PBMCs. The role of leptin and OPN with TH2 regulation was also proved in HFD allergic mice model used in our study.
Leptin, which is released by adipocytes and other cells and was first described to regulate body weight, also exerts other biologic functions in angiogenesis, hematopoiesis and the immune responses. Our previous data provides evidence to show that leptin is involved in TH2/17 inflammation and that the accumulation and activation of inflammatory cells in obese children with AR is related to disease severity [17]. Recent studies also suggested that the expression of leptin is increased in AR patients and related to the severity of disease [7]. Therefore, we supposed that leptin may be deeply involved in TH response regulation.
OPN is known to function both as an extracellular matrix molecule and as a cytokine that contributes to TH2-skewed airway diseases. Our previous study has provided evidence that elevated serum OPN level is related to TH2 systemic inflammation in AR. Moreover, it has previously been demonstrated that OPN plays a crucial role in allergic inflammation by regulating distinct dendritic cell subsets in a time-related manner according to allergen exposure in an asthma mouse model [18]. Kurokawa et al. showed that the ovalbumin (OVA)- induced specific IgE levels are higher in OPN-deficient mice when compared to those of the control group [19]. Different roles of OPN in different diseases or even the different process of the same disease suggests that the function of OPN may be regulated through different pathway and thus play different roles. In this study, we found that upregulation of OPN in obese children with AR may contribute to enhanced TH2 inflammation compared with non-obese children with AR, suggesting that obesity may be involved in regulation of OPN. However, the detailed mechanism of significant increased OPN expression in obese children with AR was not clear.
Our in vitro results showed that OPN promotes significant Th2 differentiation and inhibited Treg and these effects were enhanced when leptin was added, suggesting that OPN and leptin may have synergistic action. We also found that the role of leptin in inhibition of Th1 differentiation as observed in other disease was shown only when anti-OPN was added. These results suggested that leptin-mediated TH2 cytokine production in AR may be achieved through increased OPN expression induced by leptin rather than through a direct effect of leptin on TH2 response. OPN and HDM activated PBMCs did not express elevated leptin, suggesting the interaction between leptin and OPN may be one-way. Our results also showed that leptin exerts its action on OPN through the PI3K and AKT signaling pathways.
Our results showed that IFN-gamma and IL-17 is able to induce OPN, while two Th2 cytokines, IL-4 and IL-13, suppress its expression, which is consistent with previous reports [20, 21]. However, we did not found any effect of TH cytokine on leptin expression, suggesting that leptin had not direct regulation on TH response.
Next, we established obese-OVA mouse model to prove our hypothesis. Similarly, we found that upregulation of leptin/OPN in obese-OVA mouse was accompanied by higher symptom score and more severe inflammatory infiltration. However, after treated with anti-leptin or anti-OPN or anti-α4 integrin, nasal inflammation reduced significantly, confirming the importance of lepin/OPN axis in AR.
To prove the interaction between leptin and OPN, leptin were co-immunoprecipitaed with OPN in Th2 cells. Our results suggested that leptin and OPN may interact with each other directly throughα4 integrin, which providing strong evidence of leptin/OPN axis in allergic inflammation. However, more definitive experiments should be necessary to provide evidence for interaction between leptin/OPN and α4-integrin.
In sum, our data provide evidence that in an obese state, leptin-mediated OPN upregulation regulate TH balance and this process is achieved through the PI3K and AKT signaling pathways through in vivo and in vitro studies.
Acknowledgments
Acknowledgements
Not applicable.
Funding Source
This study was supported by grants from the National Natural Science Grant of China (No.81500772,81470673, 81725004), the Guangdong Province Natural Science Grant (No.2014A030310216) and the Pearl River S&T Nova Program of Guangzhou and a grant from the Ministry of Hygiene (No. 201202005).
Conflicts of Interest
The authors declare that they have no competing interests.
Author Contributions
Dr. Wenlong Liu and Huabin Li conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Qingxiang Zeng, Xi Luo, Miaomiao Han collected the sample, performed the experiment, data collection and statistics, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Contributor Information
Wenlong Liu, Email: lwl20103@163.com.
Huabin Li, Email: allergyli@163.com.
References
- 1.Hsueh K.C., Lin Y.J., Lin H.C., Lin C.Y. Serum leptin and adiponectin levels correlate with severity of allergic rhinitis. Pediatr Allergy Immunol. 2010;21:e155–e159. doi: 10.1111/j.1399-3038.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [quiz 920] [DOI] [PubMed] [Google Scholar]
- 3.Conde J., Scotece M., Abella V. An update on leptin as immunomodulator. Expert Rev Clin Immunol. 2014;10:1165–1170. doi: 10.1586/1744666X.2014.942289. [DOI] [PubMed] [Google Scholar]
- 4.Mai X.M., Chen Y., Krewski D. Does leptin play a role in obesity-asthma relationship? Pediatr Allergy Immunol. 2009;20:207–212. doi: 10.1111/j.1399-3038.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S., Danzaki K., MacIver N.J. Nutritional effects on T-cell immunometabolism. Eur J Immunol. 2017;47:225–235. doi: 10.1002/eji.201646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol. 2010;108:744–753. doi: 10.1152/japplphysiol.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciprandi G., Filaci G., Negrini S. Serum leptin levels in patients with pollen-induced allergic rhinitis. Int Arch Allergy Immunol. 2009;148:211–218. doi: 10.1159/000161581. [DOI] [PubMed] [Google Scholar]
- 8.Ciprandi G., De Amici M., Tosca M.A., Marseglia G. Serum leptin levels depend on allergen exposure in patients with seasonal allergic rhinitis. Immunol Invest. 2009;38:681–689. doi: 10.3109/08820130903107965. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto J., Kon S., Matsui Y., Uede T. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets. 2010;11:494–505. doi: 10.2174/138945010790980321. [DOI] [PubMed] [Google Scholar]
- 10.Samitas K., Zervas E., Vittorakis S. Osteopontin expression and relation to disease severity in human asthma. Eur Respir J. 2011;37:331–341. doi: 10.1183/09031936.00017810. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Xia W, Fan Y, et al. Elevated serum osteopontin level is associated with blood eosinophilia and asthma comorbidity in patients with allergic rhinitis. J Allergy Clin Immunol 2012;130:1416–8.e6. [DOI] [PubMed]
- 12.You J.S., Ji H.I., Chang K.J. Serum osteopontin concentration is decreased by exercise-induced fat loss but is not correlated with body fat percentage in obese humans. Mol Med Rep. 2013;8:579–584. doi: 10.3892/mmr.2013.1522. [DOI] [PubMed] [Google Scholar]
- 13.Coombes J.D., Choi S.S., Swiderska-Syn M. Osteopontin is a proximal effector of leptin-mediated non-alcoholic steatohepatitis (NASH) fibrosis. Biochim Biophys Acta. 2016;1862:135–144. doi: 10.1016/j.bbadis.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brozek J.L., Bousquet J., Baena-Cagnani C.E. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Liu W., Lin R. Socioeconomic determinants of childhood obesity among primary school children in Guangzhou, China. BMC Public Health. 2016;8:482. doi: 10.1186/s12889-016-3171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holvoet S., Zuercher A.W., Julien-Javaux F., Perrot M., Mercenier A. Characterization of candidate anti-allergic probiotic strains in a model of th2-skewed human peripheral blood mononuclear cells. Int Arch Allergy Immunol. 2013;161(2):142–154. doi: 10.1159/000343703. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Zeng Q., Zhou L., Luo R., Dong H. Association of leptin with disease severity and inflammation indicators in Chinese obese children with allergic rhinitis. Pediatr Allergy Immunol. 2018 Jan 4 doi: 10.1111/pai.12856. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Xanthou G., Alissafi T., Semitekolou M. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurokawa M., Konno S., Takahashi A. Regulatory role of DC-derived osteopontin in systemic allergen sensitization. Eur J Immunol. 2009;39:3323–3330. doi: 10.1002/eji.200838970. [DOI] [PubMed] [Google Scholar]
- 20.Konno S., Eckman J.A., Plunkett B. Interleukin-10 and Th2 cytokines differentially regulate osteopontin expression in human monocytes and dendritic cells. J Interferon Cytokine Res. 2006;26:562–567. doi: 10.1089/jir.2006.26.562. [DOI] [PubMed] [Google Scholar]
- 21.Li X, O'Regan AW, Berman JS. IFN-gamma induction of osteopontin expression in human monocytoid cells. J Interferon Cytokine Res nnn23:259–265. [DOI] [PubMed]