Abstract
Background
Heart failure represents a major public health issue that impacts 26 million people globally. Currently, real-world data represents a key instrument for providing the verification of both internal and external validity, yet there is still a lack of understanding regarding its scope in complementing evidence of treatments for heart failure. This study aims to increase understanding of the utilisation of real-word data from heart failure registries in Organisation for Economic Co-operation and Development (OECD) countries.
Method
This was a systematic review of existing observational studies from heart failure registries in 35 OECD member countries. Studies from 2000 to March 2017 were identified through electronic databases (MEDLINE (Ovid), EMBASE, Web of Science Core Collection, CINAHL (Ebsco), Cochrane CENTRAL) and appraised according to eligibility criteria.
Results
Two-hundred and two studies met the inclusion criteria, in which the majority were published from 2013 to 2016. All 202 studies were observational, among which 98% were cohort studies (198). The median sample size of all studies was 5152 (2417 to 32,890) and median study period 55 months (33.0 to 72.0). Swedish heart failure registry had the most publications (24, 12%).
Conclusion
Since 2000 there has been an upward trend in the number of published observational studies on heart failure registries in OECD countries with increasingly diverse outcomes and advanced statistical methods to improve their validity and reliability. This indicates that the utilisation of real-world data has experienced a significant upsurge in complementing the findings of clinical trials for improved research of heart failure treatments.
Abbreviations: CONSORT, consolidated standards of reporting trials; HF, heart failure; OECD, Organisation for Economic Co-operation and Development; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; PROMS, patient reported outcome measures; RCT, randomised controlled trial; RRCT, registry-based randomised clinical trials; RWD, real-world data
Keywords: Systematic review, Heart failure, Registry, Real-world data, Observational study, OECD
Highlights
-
•
The utilisation of real-world data from heart failure registries has been gradually increasing since the 2000s
-
•
Survival, length of inpatient stay, and cost were defined as outcomes, complementing research of heart failure treatments.
-
•
An assessment of the quality of the published studies based on RWD would be needed in future research.
1. Introduction
Heart failure (HF) represents a major public health issue that impacts as many as 26 million people globally [1,2]. Treatments for HF are various, including lifestyle modification, medication and medical device implantation. Thus, it is important to investigate different treatment options and their impacts on patients' health. Randomised clinical trials (RCTs) deliver the highest level of evidence on the subject of safety and efficacy of HF treatments [3]. Importantly, randomization is the only reliable method to control for confounding factors when comparing treatment groups. However, RCTs are often associated with increasingly prohibitive costs of conducting adequately powered studies with sufficient follow period, and RCTs only involve selected patients who are treated according to protocols that might not represent real-world practice [[3], [4], [5]]. In contrast, studies based on real-world data such as observational studies based on quality registry or Registry-based randomised clinical trials (RRCT) [6] may also include patients that are not typically included in RCTs, and the follow-up periods are usually sufficiently long to assess delayed risks and long-term benefits of a treatment.
Real-world data (RWD) currently represent an instrument for providing the verification of both efficacy and effectiveness of investigated treatments, including those for HF. This particular type of evidence is widely acknowledged to be extracted from sources that cannot be incorporated in RCTs, for example patient HF registries that provide detailed information about treatment, drug compliance, clinical outcomes, adherence and costs, presenting the main source of evidence for various stakeholders in healthcare [7,8]. Relative to RCTs, they are cheaper and enable analysis on a large group of indicators e.g. resource utilisation, provider characteristics and patient socio-economic status [9]. Despite the acknowledged value of RWD there is still a lack of understanding of its scope in generating evidence for treatments in addition to RCTs. To our knowledge current usage of RWD for HF has not yet been systematically evaluated [[10], [11], [12]]. Organisation for Economic Co-operation and Development (OECD) member countries have the world's highest level of adherence to evidence-based chronic HF therapies, primarily in North America, Western Europe, and Japan [13] and are pioneering access and implementation of RWD for decision-makers and various stakeholders in healthcare [14]. Therefore, the aim of this study was to increase understanding of the utilisation of RWD from HF registries in OECD countries.
2. Methods
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and followed the systematic review PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) 2009 checklist for the reporting of the study [[15], [16], [17]].
2.1. Systematic search
A literature search was performed to identify studies based on HF registries in OECD countries in the following electronic databases: Medline (OVID), Embase.com, CINAHL (Ebsco), Web of Science Core Collection and Cochrane Library (Wiley). The MeSH-terms identified for searching Medline (OVID) were adapted in accordance to corresponding vocabularies in Embase and Cinahl. Each search concept was also complemented with relevant free-text terms: HF, cardiac failure, registry, database. The free-text terms were, if appropriate, truncated and/or combined with proximity operators. No language restriction was applied. Databases were searched from inception to March 2017. The searches were supported by two librarians at the Karolinska Institutet University Library in March 2017. Articles were also identified from additional sources, such as reference lists and HF registries website. The search strategies are available in Appendix A.
2.2. Inclusion & exclusion criteria
Inclusion criteria for studies were as follows: 1. registry-based only; 2. observational study or pragmatic clinical trial (RRCT); 3. abstract available for review; 4. conducted in any of the 35 OECD member-countries listed as of March 2017 (http://www.oecd.org/about/membersandpartners/list-oecd-member-countries.htm); 5. no time limitation. Exclusion criteria for studies were: 1. surveys; 2. abstracts from conferences, editorials or commentaries; 3. articles providing descriptive registry information; 4. study sample size less than 1000; 5. no medical outcomes; 6. no full-text available (no full-text online or paid ones). Furthermore, observational studies based on international HF registries were included in the analysis if they matched the specified inclusion criteria and were based on an international HF registry that had an OECD member country as one of the participatory centers.
2.3. Data extraction
The abstracts and full-texts of identified studies were reviewed by the two authors (XD, and AK) independently. A study flow chart adapted from Prisma was applied to record the reviewing process [15]. The following categories of data from each identified study were collected and extracted in a standardized form in Excel by the two authors (XD, and AK) independently: 1. general information, which included unique identifier number, author, year of publication, disease type, aim and main findings; 2. study population; 3. study design; 4. statistical analysis; 5. quality assessment; 6. limitations. Abstract review was undertaken using the Rayyan software for screening and coding of studies through use of tagging and filtering to code and organize a large amount of references efficiently [18].
2.4. Statistical analysis
Descriptive analyses were performed with a total of 87 baseline variables. Categorical variables were summarized using count and percentage (n, %). Continuous variables were summarized using the median with interquartile intervals. Analyses were undertaken in R studio version 1.0.136 (R foundation for Statistical Computing, Vienna, Austria) [19].
3. Results
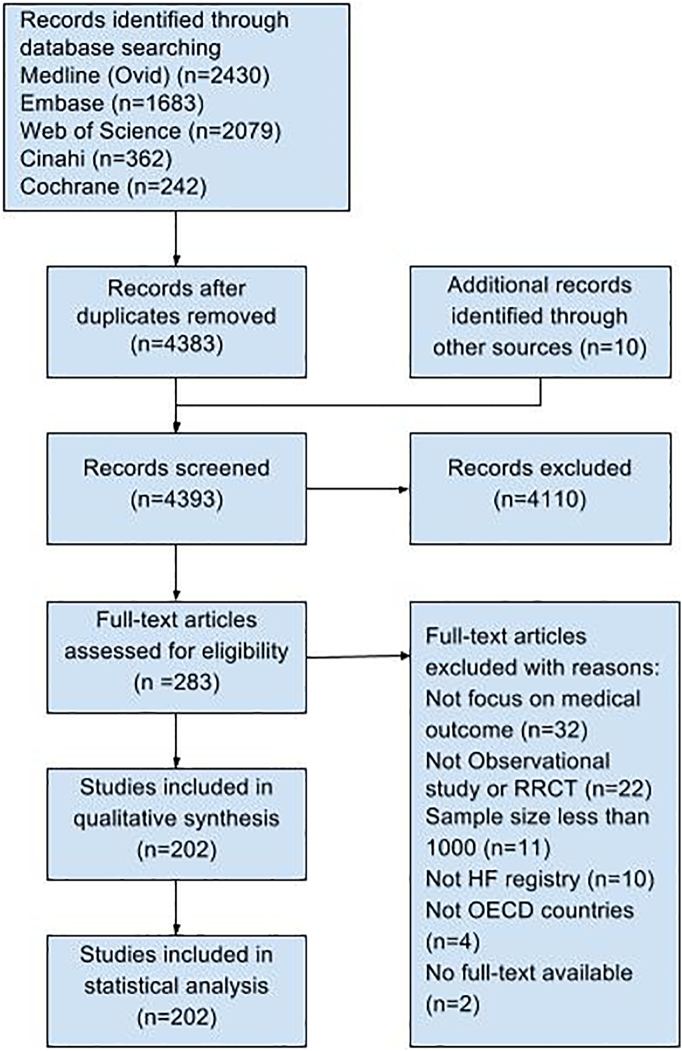
The total number of records in the five electronic databases was 6706. After excluding all duplicates and including 10 records identified through reference lists and HF registry website, the number of hits retrieved was 4393. Based on title and abstraction, after applying the inclusion criteria, 4110 records were excluded. Of the remaining 283 documents identified, 81 were excluded after full text review; 11 excluded due to a sample size of less than 1000 and 2 because full text versions were not available and no response from the first author. Two-hundred and two records met the criteria; 193 in English and 9 non-English articles (these were analysed with assistance from native speakers) (see Fig. 1).
Fig. 1.
PRISMA flow diagram showing study identification, selection, eligibility, and inclusion.
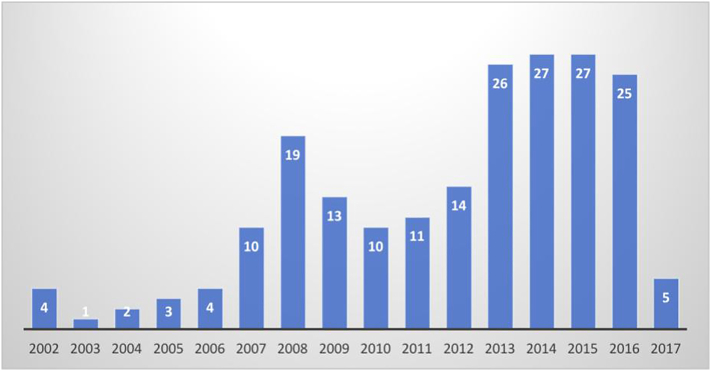
Within the 15-year period the majority of the studies were published between 2013 and 2016 (Fig. 2), showing an upward trend in the use of RWD. The Swedish heart failure registry (SwedeHF) had the most publications (n = 24) among all studies identified; this was followed by the Acute Decompensated Heart Failure National Registry (ADHERE) and Get With The Guidelines-HF Quality Improvement Registry (GWTG-HF), with 23 and 19 studies respectively.
Fig. 2.
Number of published observational studies based on HF registries In 35 OECD countries per year⁎.
⁎2002 was the first year observational studies on HR were published and met the inclusion criteria and 2017 includes only January to March.
Among the 202 included studies the median sample size was 5152 (2417, 32,890). The median study period was 55 months (33.0, 72.0). No pragmatic clinical trials were found. The majority were observational cohort studies (98%) while 4 studies were economic studies. One-hundred and sixty-nine (84%) studies stratified patient groups by age, sex, race or other variables. Over 90% studies did subgroup/sensitivity analysis to control for confounders. Some studies included multiple primary outcomes; most (91%) studies used mortality, followed by hospital admission (17%) and length of stay (15%). One fifth of the studies mentioned secondary outcomes as well, mostly mortality (8%), followed by hospital admission and survival (Table 1). Cost were reported by four economic studies from ARNO registry, ADHERE, and Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure registry. Seventy (35%) studies involved evaluation of interventions of drugs surgeries, or devices.
Table 1.
Summary characteristics of the observational studies.
| N = 202 | % | |
|---|---|---|
| Study type | ||
| Cohort | 198 | 98,0 |
| Case-control | 1 | 0.5 |
| Cross-sectional | 3 | 1.5 |
| Primary outcome | ||
| Mortality | 184 | 91,0 |
| Survival | 26 | 13,0 |
| PROMS | 0 | 0,0 |
| Cost | 4 | 2,0 |
| Hospital admission | 35 | 17,0 |
| Length of stay | 31 | 15,0 |
| Secondary outcome | 40 | 20,0 |
| Mortality | 16 | 8,0 |
| Survival | 10 | 5,0 |
| PROMS | 0 | 0,0 |
| Cost | 0 | 0,0 |
| Hospital admission | 13 | 6,0 |
| Length of stay | 7 | 3,0 |
| Intervention | 70 | 35,0 |
| Comparator | 46 | 23,0 |
| Stratification | 169 | 84,0 |
| Controlling confounder | 193 | 96,0 |
| Patient baseline characteristics | ||
| Age | 201 | 99,0 |
| Sex | 200 | 99,0 |
| Socio-economic | 66 | 33,0 |
| Life style factors | 91 | 45,0 |
| Comorbidities | 198 | 98,0 |
| Baseline health status | 197 | 98,0 |
| Median | IQR | |
| Sample size | 5152 | 2471,32,890 |
| Study period (month)⁎ | 55 | 33.0, 72.0 |
⁎53 studies had missing data for study period.
3.1. Patient characteristics
Age, sex, comorbidities and baseline health status, as patient baseline characteristics were documented in most studies (Table 1). Socio-economic status was input in 66 (33%) studies, in which 17 studies were based on GWTG-HF, 15 from SwedeHF and 13 studies from ADHERE. Life style factors were recorded in 91 (45%) studies, containing 16 studies from GWTG-HF, 16 studies from ADHERE, 15 studies from SwedeHF, 10 studies from Norwegian Heart Failure Registry, and 9 studies from Japanese Cardiac Registry of Heart Failure in Cardiology.
3.2. Statistical method
The Kaplan Meier survival curve was applied in 107 (53%) studies; the most common statistical test was the chi-test (155; 77%). Generalized linear models were performed in 191 (95%) studies (see Table 2). Multi-level model, mainly generalized estimating equation was used in 30 (15%) studies. Forty-nine (24%) studies applied a propensity score method complementary to regression models. Advanced machine learning was used in 8 (4%) studies from the USA and Japan. Only 4 studies (2%) mentioned quality assessment but 186 studies (92%) discussed study limitations (Table 2).
Table 2.
Statistical analysis and quality check of observational studies.
| N = 202 | % | |
|---|---|---|
| Descriptive analysis | 202 | 100,0 |
| Mean/median, SD/IQR, Min, Max | 196 | 97,0 |
| Percentage | 201 | 99,0 |
| Count | 136 | 67,0 |
| Kaplan Meier survival curve | 107 | 53,0 |
| Statistical tests | 182 | 90,0 |
| t-test | 76 | 38,0 |
| Wilcoxon rank test/Mann-Whitney U test | 72 | 36,0 |
| ANOVA | 46 | 24,0 |
| Kruskal-Wallis test | 42 | 23,0 |
| Chi-test | 155 | 77,0 |
| Fisher's exact test | 25 | 12,0 |
| Log-Rank test | 56 | 28,0 |
| Regression models | 191 | 95,0 |
| General linear regression | 12 | 6,0 |
| Generalized linear models | 191 | 95,0 |
| Logistic | 95 | 47,0 |
| Logit | 0 | 0,0 |
| Probit | 0 | 0,0 |
| Tobit | 0 | 0,0 |
| Quantile regression | 0 | 0,0 |
| Count regression | 10 | 5,0 |
| Survival models (time-to-event) | 137 | 68,0 |
| Multi-level model | 30 | 15,0 |
| Propensity score | 49 | 24,0 |
| Machine learning | 8 | 4,0 |
| Quality check | ||
| Quality assessment performed | 4 | 2,0 |
| Compliance with results from RCTs | 128 | 63,0 |
| Limitations addressed | 186 | 92,0 |
4. Discussion
This systematic review has shown an increase in the volume of identified studies over time, suggesting that the utilisation of RWD from HF registries has been gradually increasing since the 2000s, as well as the growth pattern of the number of published observational studies and the number of established HF registries. These findings are similar to those of a study by Moen F. et al. on the value of cancer treatments from RWD and a systematic review by Oyinlola J. et al. on RWD influencing practice across a number of disease areas, such as diabetes, obesity and mental illness [13,14]. The results of these studies illustrate an increasing, yet relatively small utilisation of RWD in healthcare research compared with the amount of available RWD sources since their inception at the end of the 20th century. Despite the increasing awareness of the importance of studies based on RWD, this type of research is often neglected or initiated late. This happens due to the fact that the proper utilisation of RWD requires a close cooperation between healthcare, industry, patients and authorities. Currently, with the growing demand for RWD, questions regarding the sustainability of patient registries and databases are being raised more frequently [[20], [21], [22]]. In addition, as the stratification of disease areas and treatments continues to expand, larger cohorts of patients are needed to provide more generalizable and sufficient data sets. For many complicated diseases, including HF, as well as for smaller countries, this will demand international collaboration.
This review also showed that the variability of the design of identified observational studies is low, with cohort studies accounting for 98% of all analysed studies based on the HF registries. Also, despite the growing application of in clinical and scientific communities, this systematic review identified no publications of RRCT according to inclusion criteria. The most prevalent main outcome of identified observational studies proved to be mortality. Survival, length of stay, admission, and cost were also defined to be among either primary or secondary outcomes. Furthermore, the findings of the present review showed that the existing observational studies from HF registries in OECD countries apply a number of advanced statistical methods to enable the minimization of bias and limitations of RWD, which in turn improve their validity and reliability. The findings of the present review have also demonstrated that RWD from HF registries has been employed primarily with the same purpose as RWD for cancer and rare diseases, which is to evaluate new treatments outside the defined protocol of clinical trials [23,24]. As such, the results of this review are generalizable to other studies based on RWD applications in the evaluation of treatments and health outcomes in other disease areas. However, the value of a particular HF treatment has not yet been done in a definitive way even with the application of RWD, since the value of the investigated treatment must be assessed in contrast with other treatment therapy that could have been employed instead. Such an estimate of the treatment value is required to support healthcare decision making and evaluate the cost and benefits of novel treatments, as has been shown with cancer treatment studies [25].
Moreover, this review has illustrated that the strength of registry-based studies in the field of HF lies not only in rapid collection of data in a large number of patients. Registry-based studies also prove applicability in population-based healthcare improvement by allowing hypothesis generation in estimation of mortality, morbidity and resource utilisation, which can serve as the basis for a clinical trial [26,27]. In addition, as the present findings indicate, such studies allow for the comparison of the disease management between several different countries. Yet, the limitations of HF registry-based studies include long-term data collection, high set-up and running costs as well as quality control enablement [28].
There are a number of strengths to this review. First, it was conducted with the application of the PRISMA methodology for performing systematic reviews [13] to ensure completeness in the reporting of results. Moreover, a comprehensive search of multiple bibliographic databases was applied using five different databases. The review also focused on identifying studies based on the HF registries of all 35 OECD member-countries, thus providing a cross-border comparison of the utilisation of RWD in the OECD region. In addition, the review employed no language or time restriction, thus enabling a broader coverage of pertinent observational studies. Also, the search strategies were supported by librarians at the Karolinska Institutet University Library and the researchers also sought help from experts in the cardiology field to ensure a proper clinical understanding of HF conditions for the purpose of the present review.
However, a number of limitations to this review should also be noted. Because the value of particular HF treatments has not yet been assessed using RWD at this time, it is not possible to currently assess how the studies have influenced the indications and recommendations in HF guidelines. Also, since the research question was relatively broad, various study design types were included which made comparisons between studies challenging. Therefore, no risk of bias was performed for each single study or across studies. Furthermore, it was not possible to do a meta-analysis for this review due to the absence of correlation of mutual exposures and outcomes of identified studies. Future research on the utilisation of RWD should include a more specific assessment of the quality of the published studies based on RWD, evaluation of risk of bias and the effect of research results on HF recommendation guidelines.
5. Conclusion
Since 2000 there has been an upward trend in the number of published observational studies on HF registries in OECD countries with increasingly diverse outcomes and advanced statistical methods to improve their validity and reliability. This indicates that the utilisation of RWD from HF registries has experienced a significant upsurge in complementing the findings of clinical trials for improved research of HF treatments.
Acknowledgments
Acknowledgements
The authors thank Synergus AB, and the Karolinska Institutet Library Support office, in particular Klas Moberg and Carl Gornitzki, for helping us with producing quality level research strategies for this systematic review and providing us with an extended number of search databases.
Conflicts of interest
None.
Footnotes
Acknowledgement of grant support
None.
Appendix A. Search strategies
Databases:
-
1.
Medline (Ovid)
-
2.
Embase (embase.com)
-
3.
Web of Science Core Collection
-
4.
Cinahl (Ebsco)
-
5.
Cochrane (Wiley)
Total number of hits:
-
•
Before deduplication: 6706
-
•
After deduplication: 4383
1. Medline (Ovid)
| Date of Search: 2017-03-30 Number of hits: 2,340 Comments: |
Field labels:
|
| 1. exp Heart Failure/ 2. ((heart* or cardia* or myocard*) adj3 (fail* or decompensat* or edema* or incompetence or insufficiency)).ti,ab,kf. 3. or/1-z 4. Registries/ 5. Databases, Factual/ 6. Databases as Topic/ 7. (registry or registries or register or registers).ti,ab,kf,au,fa. 8. database*.ti. 9. or/4-8 10. exp Australia/ 11. Austria/ 12. Belgium/ 13. exp Canada/ 14. Chile/ 15. Czech Republic/ 16. exp "Scandinavian and Nordic Countries"/ 17. Estonia/ 18. exp France/ 19. exp Germany/ 20. Greece/ 21. Hungary/ 22. Iceland/ 23. Ireland/ 24. Israel/ 25. exp Italy/ 26. exp Japan/ 27. exp Republic of Korea/ 28. Latvia/ 29. Luxembourg/ 30. Mexico/ 31. Netherlands/ 32. New Zealand/ 33. Poland/ 34. Portugal/ 35. Slovakia/ 36. Slovenia/ 37. Spain/ 38. Switzerland/ 39. Turkey/ 40. exp United Kingdom/ 41. exp United States/ 42. "Organisation for Economic Co-Operation and Development"/ 43. (oecd or australia* or austria* or belgium or belgian or canada or canadian or chile* or czech* or denmark or danish or estonia* or finland or finnish or france or french or german* or greece or greek or hungar* or iceland* or ireland or irish or israel* or italy or italian or japan* or korea* or latvia* or luxembourg* or mexico or mexican or netherlands or dutch or holland* or new zealand or norway or norwegian or poland or polish or portugal or portuguese or slovak* or slovenia* or spain or spanish or sweden or swedish or switzerland or swiss or turkey or turkish or united kingdom or great britain or wales or england or scotland or united states or uk or us or usa or america*).ti,ab,kf. 44. or/10-43 45. exp Clinical Trial/ 46. Observational Study/ 47. Observational Studies as Topic/ 48. exp Case-Control Studies/ 49. exp Cohort Studies/ 50. Cross-Over Studies/ 51. Cross-Sectional Studies/ 52. Multicenter Study/ 53. Matched-Pair Analysis/ 54. Epidemiologic Studies/ 55. Pragmatic Clinical Trial/ 56. Pragmatic Clinical Trials as Topic/ 57. (case control* or cohort* or cross over or cross sectional or follow up or followed or longitudinal or pragmatic or practical or random* or real world or clinical trial*).ti,ab,kf. 58. or/45-57 59. 3 and 9 and 44 and 58 60. remove duplicates from 59 61. limit 60 to (comment or editorial or letter) 62. 60 not 61 | |
2. Embase (embase.com)
| Date of Search: 2017-03-30 Number of hits: 1,683 Comments: |
Field labels:
|
| 'heart failure'/exp OR ((heart* OR cardia* OR myocard*) NEAR/3 (fail* OR decompensat* OR edema* OR incompetence OR insufficiency)):ti,ab AND 'register'/de OR 'disease registry'/de OR 'factual database'/exp OR registry:ti,ab,au OR registries:ti,ab,au OR register:ti,ab,au OR registers:ti,ab,au OR database*:ti AND 'australia'/exp OR 'austria'/de OR 'belgium'/exp OR 'canada'/exp OR 'chile'/de OR 'czech republic'/de OR 'scandinavia'/exp OR 'estonia'/de OR 'france'/exp OR 'germany'/exp OR 'greece'/de OR 'hungary'/de OR 'iceland'/de OR 'ireland'/de OR 'israel'/de OR 'italy'/exp OR 'south korea'/de OR 'latvia'/de OR 'luxembourg'/de OR 'mexico'/exp OR 'netherlands'/de OR 'new zealand'/de OR 'poland'/de OR 'portugal'/exp OR 'slovakia'/de OR 'slovenia'/de OR 'spain'/exp OR 'switzerland'/de OR 'turkey (republic)'/de OR 'united kingdom'/exp OR 'united states'/exp OR 'organisation for economic co-operation and development'/de OR oecd:ti,ab OR australia*:ti,ab OR austria*:ti,ab OR belgium:ti,ab OR belgian:ti,ab OR canada:ti,ab OR canadian:ti,ab OR chile*:ti,ab OR czech*:ti,ab OR denmark:ti,ab OR danish:ti,ab OR estonia*:ti,ab OR finland:ti,ab OR finnish:ti,ab OR france:ti,ab OR french:ti,ab OR german*:ti,ab OR greece:ti,ab OR greek:ti,ab OR hungar*:ti,ab OR iceland*:ti,ab OR ireland:ti,ab OR irish:ti,ab OR israel*:ti,ab OR italy:ti,ab OR italian:ti,ab OR japan*:ti,ab OR korea*:ti,ab OR latvia*:ti,ab OR luxembourg*:ti,ab OR mexico:ti,ab OR mexican:ti,ab OR netherlands:ti,ab OR dutch:ti,ab OR holland*:ti,ab OR 'new zealand':ti,ab OR norway:ti,ab OR norwegian:ti,ab OR poland:ti,ab OR polish:ti,ab OR portugal:ti,ab OR portuguese:ti,ab OR slovak*:ti,ab OR slovenia*:ti,ab OR spain:ti,ab OR spanish:ti,ab OR sweden:ti,ab OR swedish:ti,ab OR switzerland:ti,ab OR swiss:ti,ab OR turkey:ti,ab OR turkish:ti,ab OR united:ti,ab AND kingdom:ti,ab OR 'great britain':ti,ab OR wales:ti,ab OR england:ti,ab OR scotland:ti,ab OR 'united states':ti,ab OR uk:ti,ab OR us:ti,ab OR usa:ti,ab OR america*:ti,ab AND 'clinical trial'/exp OR 'observational study'/de OR 'case control study'/exp OR 'cohort analysis'/de OR 'crossover procedure'/de OR 'cross-sectional study'/de OR 'case control*':ti,ab OR cohort*:ti,ab OR 'cross over':ti,ab OR 'cross sectional':ti,ab OR 'follow up':ti,ab OR followed:ti,ab OR longitudinal:ti,ab OR pragmatic:ti,ab OR practical:ti,ab OR random*:ti,ab OR 'real world':ti,ab OR 'clinical trial*':ti,ab AND ('article'/it OR 'article in press'/it OR 'conference paper'/it OR 'review'/it) | |
3. Web of Science Core Collection
| Date of Search: 2017-03-30 Number of hits: 2,079 Comments: |
Field labels:
|
| #6 #4 AND #3 AND #2 AND #1 Refined by: [excluding]: DOCUMENT TYPES: (EDITORIAL MATERIAL OR LETTER OR NOTE OR MEETING ABSTRACT OR BOOK CHAPTER) DocType=All document types; Language=All languages; #5 #4 AND #3 AND #2 AND #1 DocType=All document types; Language=All languages; #4 TOPIC: ("case control*" OR cohort* OR "cross over" OR "cross sectional" OR "follow up" OR followed OR longitudinal OR pragmatic OR practical OR random* OR "real world" OR "clinical trial*") DocType=All document types; Language=All languages; #3 TOPIC: ((oecd OR australia* OR austria* OR belgium OR belgian OR canada OR canadian OR chile* OR czech* OR denmark OR danish OR estonia* OR finland OR finnish OR france OR french OR german* OR greece OR greek OR hungar* OR iceland* OR ireland OR irish OR israel* OR italy OR italian OR japan* OR korea* OR latvia* OR luxembourg* OR mexico OR mexican OR netherlands OR dutch OR holland* OR "new zealand" OR norway OR norwegian OR poland OR polish OR portugal OR portuguese OR slovak* OR slovenia* OR spain OR spanish OR sweden OR swedish OR switzerland OR swiss OR turkey OR turkish OR "united kingdom" OR "great britain" OR wales OR england OR scotland OR "united states" OR uk OR us OR usa OR america*)) DocType=All document types; Language=All languages; #2 TOPIC: (registry OR registries OR register OR registers) OR AUTHOR: (registry OR registries OR register OR registers) OR TITLE: (database*) DocType=All document types; Language=All languages; #1 TOPIC: ((heart* OR cardia* OR myocard*) NEAR/3 (fail* OR decompensat* OR edema* OR incompetence OR insufficiency)) DocType=All document types; Language=All languages; | |
4. Cinahl (Ebsco)
| Date of Search: 2017-03-30 Number of hits: 362 Comments: |
Field labels:
|
| S1 | (MH "Heart Failure+") |
| S2 | TI ((heart* or cardia* or myocard*) N3 (fail* or decompensat* or edema* or incompetence or insufficiency)) OR AB ((heart* or cardia* or myocard*) N3 (fail* or decompensat* or edema* or incompetence or insufficiency)) |
| S3 | S1 OR S2 |
| S4 | (MH "Registries, Disease") |
| S5 | (MH "Resource Databases") |
| S6 | TI (registry or registries or register or registers) OR AB (registry or registries or register or registers) OR AU (registry or registries or register or registers) |
| S7 | TI database* |
| S8 | S4 OR S5 OR S6 OR S7 |
| S9 | (MH "Australia+") |
| S10 | (MH "Austria") |
| S11 | (MH "Belgium") |
| S12 | (MH "Canada+") |
| S13 | (MH "Chile") |
| S14 | (MH "Czech Republic") |
| S15 | (MH "Scandinavia+") |
| S16 | (MH "Estonia") |
| S17 | (MH "France") |
| S18 | (MH "Germany+") |
| S19 | (MH "Greece") |
| S20 | (MH "Hungary") |
| S21 | (MH "Iceland") |
| S22 | (MH "Ireland") |
| S23 | (MH "Israel") |
| S24 | (MH "Italy") |
| S25 | (MH "Japan") |
| S26 | (MH "South Korea") |
| S27 | (MH "Latvia") |
| S28 | (MH "Luxembourg") |
| S29 | (MH "Mexico") |
| S30 | (MH "Netherlands") |
| S31 | (MH "New Zealand") |
| S32 | (MH "Poland") |
| S33 | (MH "Portugal") |
| S34 | (MH "Slovakia") |
| S35 | (MH "Slovenia") |
| S36 | (MH "Spain") |
| S37 | (MH "Switzerland") |
| S38 | (MH "Turkey") |
| S39 | (MH "United Kingdom+") |
| S40 | (MH "United States+") |
| S41 | (MH "Organisation for Economic Co-Operation and Development") |
| S42 | TI (oecd or australia* or austria* or belgium or belgian or canada or canadian or chile* or czech* or denmark or danish or estonia* or finland or finnish or france or french or german* or greece or greek or hungar* or iceland* or ireland or irish or israel* or italy or italian or japan* or korea* or latvia* or luxembourg* or mexico or mexican or netherlands or dutch or holland* or “new zealand” or norway or norwegian or poland or polish or portugal or portuguese or slovak* or slovenia* or spain or spanish or sweden or swedish or switzerland or swiss or turkey or turkish or “united kingdom” or “great britain” or wales or england or scotland or “united states” or uk or us or usa or america*) OR AB (oecd or australia* or austria* or belgium or belgian or canada or canadian or chile* or czech* or denmark or danish or estonia* or finland or finnish or france or french or german* or greece or greek or hungar* or iceland* or ireland or irish or israel* or italy or italian or japan* or korea* or latvia* or luxembourg* or mexico or mexican or netherlands or dutch or holland* or new zealand or norway or norwegian or poland or polish or portugal or portuguese or slovak* or slovenia* or spain or spanish or sweden or swedish or switzerland or swiss or turkey or turkish or “united kingdom” or “great britain” or wales or england or scotland or “united states” or uk or us or usa or america*) |
| S43 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 |
| S44 | (MH "Clinical Trials+") |
| S45 | (MH "Nonexperimental Studies") |
| S46 | (MH "Case Control Studies+") |
| S47 | (MH "Prospective Studies+") |
| S48 | (MH "Crossover Design") |
| S49 | (MH "Cross Sectional Studies") |
| S50 | (MH "Retrospective Design") |
| S51 | (MH "Multicenter Studies") |
| S52 | (MH "Matched-Pair Analysis") |
| S53 | (MH "Epidemiological Research") |
| S54 | TI (“case control*” or cohort* or “cross over” or “cross sectional” or “follow up” or followed or longitudinal or pragmatic or practical or random* or “real world” or “clinical trial*”) OR AB (“case control*” or cohort* or “cross over” or “cross sectional” or “follow up” or followed or longitudinal or pragmatic or practical or random* or “real world” or “clinical trial*”) |
| S55 | S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 |
| S56 | S3 AND S8 AND S43 AND S55 |
5. Cochrane (Wiley)
| Date of Search: 2017-03-30 Number of hits: 242 Comments: |
Field labels:
|
| #1 ((heart* or cardia* or myocard*) near/3 (fail* or decompensat* or edema* or incompetence or insufficiency)):ti,ab #2 (registry or registries or register or registers):ti,ab,au #3 (oecd or australia* or austria* or belgium or belgian or canada or canadian or chile* or czech* or denmark or danish or estonia* or finland or finnish or france or french or german* or greece or greek or hungar* or iceland* or ireland or irish or israel* or italy or italian or japan* or korea* or latvia* or luxembourg* or mexico or mexican or netherlands or dutch or holland* or "new zealand" or norway or norwegian or poland or polish or portugal or portuguese or slovak* or slovenia* or spain or spanish or sweden or swedish or switzerland or swiss or turkey or turkish or "united kingdom" or "great britain" or wales or england or scotland or "united states" or uk or us or usa or america*):ti,ab #4 #1 and #2 and #3 | |
References
- 1.Ponikowski P., Anker S.D., Alhabib K.F. Heart failure: preventing disease and death worldwide. Eur. Soc. Cardiol. 2014;373(9667):941–955. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Christ M., Störk S., Dörr M., Heppner H.J., Müller C., Wachter R. Heart failure epidemiology 2000–2013: insights from the German Federal health monitoring system for the trend HF Germany project. Eur. J. Heart Fail. 2016;18:1009–1018. doi: 10.1002/ejhf.567. http://onlinelibrary.wiley.com/store/10.1002/ejhf.567/asset/ejhf567.pdf?v=1&t=j2du2b4z&s=16a03f8bd9b5e07ec4c34414dc54520390d25f78 Available from. (cited 2017 May 7) [DOI] [PubMed] [Google Scholar]
- 3.Wollert K.C., Meyer G.P., Lotz J., Ringes Lichtenberg S., Lippolt P., Breidenbach C. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. http://linkinghub.elsevier.com/retrieve/pii/S0140673604166269 Available from: (Jul [cited 2017 Mar 7]) [DOI] [PubMed] [Google Scholar]
- 4.Agha R., Cooper D., Muir G. The reporting quality of randomised controlled trials in surgery: a systematic review. Int. J. Surg. 2007;5(6):413–422. doi: 10.1016/j.ijsu.2007.06.002. http://www.ncbi.nlm.nih.gov/pubmed/18029237 Available from. (Dec [Cited 2017 May 7]) [DOI] [PubMed] [Google Scholar]
- 5.Bolli R., Chugh A.R., D'Amario D., Loughran J.H., Stoddard M.F., Ikram S. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. http://linkinghub.elsevier.com/retrieve/pii/S0140673611615900 Available from: (Nov [cited 2017 Mar 7]) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lund L.H., Oldgren J., James S. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr. Heart Fail. Rep. 2017;1–12 doi: 10.1007/s11897-017-0325-0. http://link.springer.com/10.1007/s11897-017-0325-0 Available from: (Feb 28 [cited 2017 Mar 4]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman K.J. Real world data. Value Heal. 2007;10:322–323. doi: 10.1111/j.1524-4733.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 8.Filippatos D., Farmakis V., Bistola V., Karavidas A., Mebazaa A., Maggioni A.P. 2014. Temporal trends in epidemiology, clinical presentation and management of acute heart failure: results from the Greek cohorts of the Acute Heart Failure Global Registry of Standard Treatment and the European Society of Cardiology-Heart Failure pilot survey. (Eur. Hear J. Acute Cardiovasc Care). [Internet] 2048872614527012. Available from: https://doi.org/10.1177/2048872614527012 (Mar 4 [cited 2017 Mar 30]) [DOI] [PubMed] [Google Scholar]
- 9.Oyinlola J.O., Campbell J., Kousoulis A.A. Is real world evidence influencing practice? A systematic review of CPRD research in NICE guidances. BMC Health Serv. Res. 2016;16:299. doi: 10.1186/s12913-016-1562-8. http://www.ncbi.nlm.nih.gov/pubmed/27456701 Available from. (Jul 26 [cited 2017 Apr 23]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trullàs J.C., Miró Ò., Formiga F., Martín-Sánchez F.J., Montero-Pérez-Barquero M., Jacob J. The utility of heart failure registries: a descriptive and comparative study of two heart failure registries. Postgrad. Med. J. 2016;92(1087):260–266. doi: 10.1136/postgradmedj-2015-133739. http://www.ncbi.nlm.nih.gov/pubmed/26739849 Available from. (May [cited 2017 Mar 2]) [DOI] [PubMed] [Google Scholar]
- 11.European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT) 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2017;19(3):438. doi: 10.1002/ejhf.772. http://www.ncbi.nlm.nih.gov/pubmed/28251778 Available from. (Mar [cited 2017 Mar 4]) [DOI] [PubMed] [Google Scholar]
- 12.Hu C.-Y., Xing Y., Cormier J.N., Chang G.J. Assessing the utility of cancer-registry-processed cause of death in calculating cancer-specific survival. Cancer. 2013;119(10):1900–1907. doi: 10.1002/cncr.27968. http://doi.wiley.com/10.1002/cncr.27968 Available from: (May 15 [cited 2017 Mar 2]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggioni A.P., Van Gool K., Biondi N., Urso R., Klazinga N., Ferrari R. Appropriateness of prescriptions of recommended treatments in organisation for economic co-operation and development health systems: findings based on the long-term registry of the European society of cardiology on heart failure. Value Health. 2015;18(8):1098–1104. doi: 10.1016/j.jval.2015.08.005. https://doi.org/10.1016/j.jval.2015.08.005 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Montero-Perez-Barquero M., Manzano L., Formiga F., Roughton M., Coats A., Rodríguez-Artalejo F. Utility of the SENIORS elderly heart failure risk model applied to the RICA registry of acute heart failure. On behalf of the RICA investigators. Int. J. Cardiol. 2015;182:449–453. doi: 10.1016/j.ijcard.2014.12.173. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. https://doi.org/10.1371/journal.pmed.1000100 Available from: (Jul 21 [cited 2017 May 7]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://doi.org/10.1371/journal.pmed.1000097 Available from: (Jul 21 [cited 2017 May 7]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions Cochrane Book Series THE COCHRANE COLLABORATION ®. https://dhosth.files.wordpress.com/2011/12/cochrane-handbook-for-systematic-reviews-of-interventions.pdf Available from: ([cited 2017 May 7])
- 18.Ouzzani Mourad, Hammady Hossam, Fedorowicz Zbys, Elmagarmid Ahmed. Rayyan - a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. https://rayyan.qcri.org/ Available from. (cited 2017 May 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A language and environment for statistical computing.https://www.R-project.org/ Available from. [Google Scholar]
- 20.Mullins C.D. Using Real World Data for Coverage and Payment Decisions: The ISPOR Real World Data Tast Force Report. https://www.ispor.org/workpaper/RWD_TF/RWTFManuscript.pdf Available from: (cited 2017 Mar 4) [DOI] [PubMed]
- 21.Mahajan R. Real world data: additional source for making clinical decisions. Int. J. Appl. Basic Med. Res. 2015;5(2):82. doi: 10.4103/2229-516X.157148. http://www.ncbi.nlm.nih.gov/pubmed/26097811 Available from. (cited 2017 Feb 27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger M.L., Lipset C., Gutteridge A., Axelsen K., Subedi P., Madigan D. Optimizing the leveraging of real-world data to improve the development and use of medicines. Value Health. 2015;18(1):127–130. doi: 10.1016/j.jval.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Hartling L., Hamm M., Milne A., Vandermeer B., Santaguida P.L., Ansari M. Agency for Healthcare Research and Quality (US); 2012. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments [Internet]. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments.http://www.ncbi.nlm.nih.gov/pubmed/22536612 Available from. (cited 2017 May 8) [PubMed] [Google Scholar]
- 24.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. http://www.bmj.com/content/355/bmj.i4919 Available from: (cited 2017 May 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moen F., Svensson J., Steen Carlsson K. Assessing the value of cancer treatments from real world data—issues, empirical examples and lessons learnt. J. Cancer Policy. 2017;11:32–37. http://linkinghub.elsevier.com/retrieve/pii/S221353831630039X Available from: (Mar [cited 2017 May 7]) [Google Scholar]
- 26.Hickey Graeme L., Grant Stuart W., Cosgriff Rebecca, Dimarakis Ioannis, Pagano Domenico, Kappetein Arie P., Bridgewater Ben. Clinical registries: governance, management, analysis and applications. Eur. J. Cardiothorac. Surg. 2013;44(4):605–614. doi: 10.1093/ejcts/ezt018. https://doi.org/10.1093/ejcts/ezt018 Available from. [DOI] [PubMed] [Google Scholar]
- 27.Lund L.H., Oldgren J., James S. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr. Heart Fail. Rep. 2017;14(2):59–70. doi: 10.1007/s11897-017-0325-0. https://doi.org/10.1007/s11897-017-0325-0 Available from. (cited 2018 Feb 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoque D.M.E., Kumari V., Hoque M., Ruseckaite R., Romero L., Evans S.M. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS ONE. 2017;12(9):e0183667. doi: 10.1371/journal.pone.0183667. https://doi.org/10.1371/journal.pone.0183667 Available from. (cited 2018 Feb 10) [DOI] [PMC free article] [PubMed] [Google Scholar]