Abstract
Astrocytomas are responsible for 30% of all primitive intramedullary tumors with cervicothoracic predominance. However, only about one hundred cases of intramedullary pilocytic astrocytomas were described. The authors described the case of a 69 year-old patient presenting with a broad-base gait, bilateral pain and dysesthesia of inferior limbs with a diagnosis of an intra-axial cystic lesion centered to the conus medullaris, diagnosed as pilocytic astrocytoma of conus medullaris (PACM) after surgery. To the best of our knowledge, only two previous reports concerning PACM were made. As benign lesions associated with long survivals, function should be the mainstay of treatment of PACM. Surgical resection should be performed. Adjuvant radiotherapy or chemotherapy has no establish role in pilocytic astrocytic lesions.
KEYWORDS : chemotherapy, conus medullaris, intramedullary spinal cord tumor, pilocytic astrocytoma, radiotherapy
Practice points.
Even though the majority of the conus medullaris tumors are myxopapillary ependymomas, astrocytomas should also be considered in the differential diagnosis.
Surgery is the mainstay of management for intramedullary spinal cord tumors (Class I evidence, except astrocytoma – Class IIb for biopsy).
No clear role for adjuvant radiotherapy or chemotherapy is established in the literature for intramedullary pilocytic astrocytomas.
Considering the long survivals and the ill-defined impact of surgery in patients with intramedullary astrocytomas, neurological function preservation should always guide the treatment approach.
Adult pilocytic astrocytoma (PA) of conus medullaris (PACM) is a rare and unsuspected pathology in the preoperative setting. In the adult population, only 5% of spine tumors are intramedullary lesions, the majority of the conus medullaris tumors diagnosed as myxopapillary ependymomas (ME) [1].
PA and ME are WHO Grade I lesions but with different clinical and therapeutic implications. Although the resection rates seem to be comparable between conus and the remaining spinal cord, histopathology affects the surgeon's ability to remove the lesion (90% complete resection rate for ependymomas vs 14–76% for low-grade astrocytomas) [2,3]. Intra-operative cleavage plan is the most important prognostic factor for gross total resection among all histopathological tumor types [4]. However, it is not clear if gross total resection improves overall survival in low-grade astrocytoma patients.
Surgery is the mainstay of management for intramedullary spinal cord tumors (IMSCT; Class I evidence, except astrocytoma – Class IIb for biopsy) [5]. Adjuvant therapy as radiotherapy and chemotherapy has been explored in the context of IMSCT. Nevertheless, the evidence for their role is low [5] and they are usually reserved for systemic metastatic lesions or high-grade (WHO III and IV) tumors as they produce important side effects and have little impact in overall survival [7,8].
According to the paucity of cases of reported adult PACM, no direct conclusions can be drawn from the previous stated literature. Therefore, we present the following case report supported by a clinical review and therapeutic considerations.
Case report
A 69-year-old woman was admitted in the neurosurgical outpatient clinic for bilateral lower limb pain and gait disturbance. She was previously submitted to posterior–lateral fusion with internal fixation at L4–L5 with transpedicular screws for Grade I Degenerative Spondylolisthesis (Meyerding Classification) in a clinical background of chronic lumbago. The neurological examination showed bilateral lower limb pain with concomitant hypo/dysesthesia with no single-root distribution, no signs of motor weakness, broad-base gait with normal knee reflexes and diminished ankle reflexes.
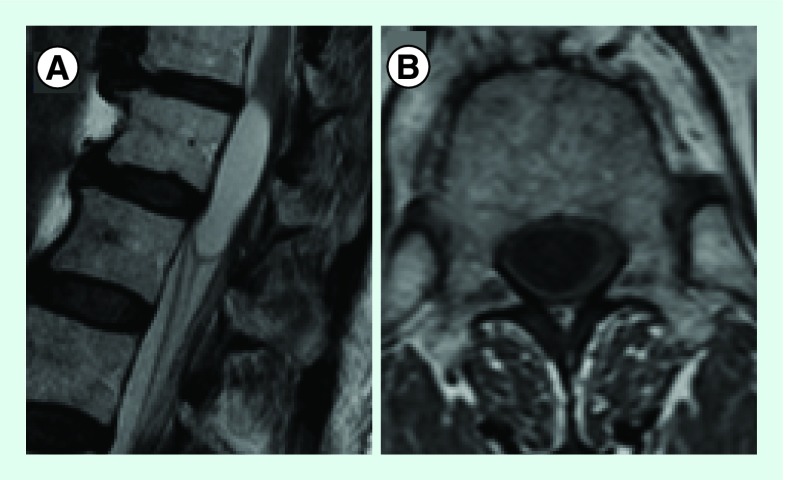
A thoracolumbar MRI was performed showing a cystic lesion centered to the conus medullaris (Figure 1). No contrast was administered according to patient refusal. A McCormick Grade II tumor was admitted.
Figure 1. . T2 Sagital (A) and T1 Axial (B) images showing a cystic lesion centered to the conus medullaris, hypointense in T1 and hyperintense in T2.
No contrast enhancement images are available as patient refused contrast injection.
A posterior–dorsal approach was performed. A D10–D11 laminectomy was done and the dura-mater was opened through a posterior midline incision. The lesion was identified with ultrasound support. It had a cleavage plan on its left side but was indistinguishable of neural parenchyma on its right side. A partial removal and an extent communication to the subarachnoid space were accomplished.
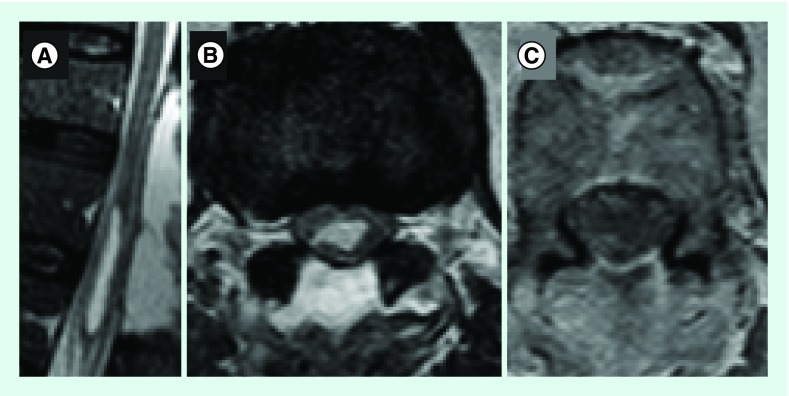
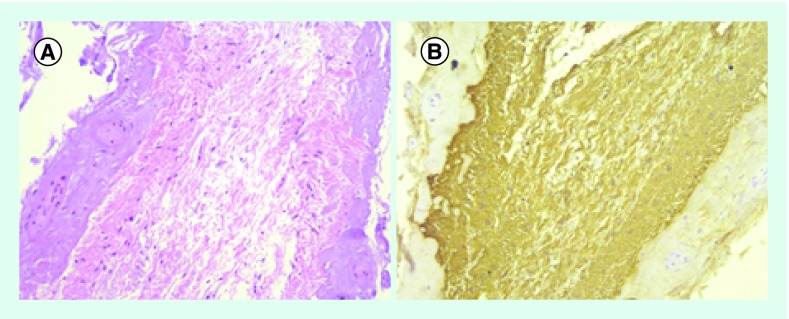
After surgery, the urinary catheter was left in place regarding urinary retention with preserved sensation. The patient was discharged 7 days after surgery with no further events and referred to a rehabilitation unit. One month after surgery, the urinary catheter was successfully removed and no gait disturbance was observed with only mild dysesthesia. The post-op MRI showed decreased mass effect of the residual cyst (Figure 2). Neuropathological examination disclosed a cystic tumor of low cellularity showing bipolar astrocytic elements with long thin processes. Anaplastic features were not elicited, and the proliferative index (Ki67) was less than 1%. ATRX and IDH1 were not mutated and there was translocation in BRAF. The diagnostic of PA was made (Figure 3).
Figure 2. .
T2 Sagital (A), T2 Axial (B) and T1 post-Gad Axial (C) images showing reduction of mass effect from cystic lesion and no contrast uptake after Gadolinium infection.
Figure 3. . Histology.
(A) moderately compact bipolar neoplastic astrocyts with long, hair-like processes. No signs of anaplasia (H&E x40); (B) strongly glial fibrillary acidic protein immunopositivity (x40).
No further treatment was performed and clinical surveillance is maintained with no recurrence at 12 months.
Discussion
IMSCT enclose one of the most striking trade-offs in spinal neurosurgery: maximal surgical resection for a better oncology outcome versus immediate postoperative neurosurgical outcome. The debate is even stronger when the insufficient clinical information precludes a formal recommendation about the impact of larger resections in oncology prognosis. This is particularly true when considering low-grade astrocytomas, and among this group, WHO Grade I PA.
To the best of our knowledge, only two previous reports concerning PACM were made [1,8] (Table 1). Our patient was older than the previous reported (69 vs 20 and 44 years old). All patients had the same clinical presentation – lumbago and conus medullaris syndrome. Regarding the surgical approach, our patient had no clear cleavage plan preventing a complete resection either en bloc or piece meal (Table 1). Regardless the histology, we choose to perform a subtotal resection considering the preservation of neurological function is of paramount importance in the outcome of patients with intramedullary lesions. No further treatment was performed as no role for adjuvant radio or chemotherapy is established. Some authors even reported a worst outcome with adjuvant radiotherapy or chemotherapy. However, this might have been related with selection bias toward adjuvant treatment to worst clinical cases [3].
Table 1. . Published case reports of adult pilocytic astrocytoma of conus medullaris.
| Case report (year) | Gender | Age (years) | Symptoms | Treatment | Follow-up | Ref. |
|---|---|---|---|---|---|---|
| Baréa et al. (1999) | H | 20 | Lumbago and conus medullaris syndrome | Surgical excision (en bloc) Preserved cleavage plan between tumor and neural tissue |
No adjuvant treatment | [8] |
| Kumar et al. (2012) | F | 44 | Lumbago and conus medullaris syndrome | Surgical excision (piece-meal) Preserved cleavage plan between tumor and neural tissue |
No adjuvant treatment | [1] |
| Present case report | F | 69 | Lumbago and conus medullaris syndrome | Subtotal removal and cyst marsupialization for subarachnoid space No preserved cleavage plan between tumor and neural tissue |
No adjuvant treatment No recurrence at 12 months |
|
Although maximal surgical resection with neurological preservation should be attempt with proper neurophysiologic monitoring (not available in this case), the prognostic significance of the extent of resection in intramedullary low-grade spinal astrocytomas (IMLGSA) is still to be shown. Some authors reported no influence either in low- or high-grade lesions [4,9,10]. Nevertheless, some epidemiologic data support radical resections for high-grade spinal cord astrocytomas as they are related with or showed a trend toward longer survivals [11,12].
Radiotherapy for IMLGSA has been a matter of discussion. Minehan et al. [13] published one of the largest series about IMLGSA and showed no benefit of postoperative radiotherapy for PA despite the type of surgical resection performed. Other series support these findings [10,14]. Indeed, radiotherapy showed benefit only in infiltrative IMLGSA [13]. However, even in high-grade lesions the benefit of adjuvant radiotherapy is not consensual as some series revealed no impact on survival [15].
The use of chemotherapy is a recent considered option for IMLGSA. Fakhreddine et al. showed that chemotherapy improved progression-free survival in IMLGSA (no differences seen between temozolamide – the most frequent used – and other regimens), whereas the extent of resection and adjuvant radiotherapy had no effect [16]. It has also been used in children to delay the adjuvant radiotherapy treatment [17]. Nevertheless, its role is limited and still waiting for being determined [18].
Therefore, adjuvant radiotherapy and/or chemotherapy are usually reserved for high-grade intramedullary astrocytomas and should only be considered for low-grade tumors on particular scenarios [6]. In this current case and according to the more recent literature, no further therapy was address and the residual tumor was small and mainly cystic and there is no recurrence to date.
Conclusion
Adult PACM is a rare tumor. The mainstay of intramedullary PA is surgical resection. This should be accomplished according to maximal resection-neurological preservation trade-off as it is related with longer survivals. No role for adjuvant radiotherapy or chemotherapy is established in the literature for PA in other anatomic locations within the spinal cord, so we believe these same treatment principles should be applied to PACM.
Future perspective
Surgery is the mainstay for intramedullary pilocytic astrocytomas treatment. Therefore, the increasing reliability of intraoperative neuromonitoring may imbalance the previous mentioned trade-off towards a more aggressive surgical approach with greater resections while preserving the neurological function.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
- 1.Kumar A, Shah RM, Gupta N. Rare tumor of conus medullaris in an adult with a favorable outcome. J. Surg. Tech. Case Rep. 2012;4(1):67–68. doi: 10.4103/2006-8808.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han IH, Kuh SU, Chin DK, et al. Surgical treatment of primary spinal tumors in the conus medullaris. J. Korean Neurosurg. Soc. 2008;4:72–77. doi: 10.3340/jkns.2008.44.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karikari IO, Nimjee SM, Hodges TR, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2015;76(Suppl. 1):S4–13. doi: 10.1227/01.neu.0000462073.71915.12. discussion S13. [DOI] [PubMed] [Google Scholar]
- 4.Garcés-Ambrossi GL, McGirt MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J. Neurosurg. Spine. 2009;11(5):591–599. doi: 10.3171/2009.4.SPINE08159. [DOI] [PubMed] [Google Scholar]
- 5.Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg. Focus. 2015;39(2):E14. doi: 10.3171/2015.5.FOCUS15158. [DOI] [PubMed] [Google Scholar]
- 6.Harrop JS, Ganju A, Groff M, et al. Primary intramedullary tumors of the spinal cord. Spine (Phila. PA 1976). 2009;34(22 Suppl.):S69–S77. doi: 10.1097/BRS.0b013e3181b95c6f. [DOI] [PubMed] [Google Scholar]
- 7.Juthani RG, Bilsky MH, Vogelbaum MA. Current management and treatment modalities for intramedullary spinal cord tumors. Curr. Treat. Options Oncol. 2015;16(8):39. doi: 10.1007/s11864-015-0358-0. [DOI] [PubMed] [Google Scholar]
- 8.Baréa D, Richez P, Gueguen E, et al. Pilocytic astrocytoma of the conus medullaris. J. Radiol. 1999;80(7):736–738. [PubMed] [Google Scholar]
- 9.Lam S, Lin Y, Melkonian S. Analysis of risk factors and survival in pediatric high-grade spinal cord astrocytoma: a population-based study. Pediatr. Neurosurg. 2012;48(5):299–305. doi: 10.1159/000353135. [DOI] [PubMed] [Google Scholar]
- 10.Robinson CG, Prayson RA, Hahn JF, et al. Long-term survival and functional status of patients with low-grade astrocytoma of spinal cord. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:91–100. doi: 10.1016/j.ijrobp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Adams H, Avendaño J, Raza SM, et al. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a population-based analysis from 1973 to 2007. Spine (Phila. PA 1976) 2012;37(12):E727–E735. doi: 10.1097/BRS.0b013e31824584c0. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGirt MJ, Goldstein IM, Chaichana KL, et al. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63(1):55–60. doi: 10.1227/01.NEU.0000335070.37943.09. discussion 60–61. [DOI] [PubMed] [Google Scholar]
- 13.Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and treatment of spinal cord astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:727–733. doi: 10.1016/j.ijrobp.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 14.Shirato H, Kamada T, Hida K, et al. The role of radiotherapy in the management of spinal cord glioma. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:323–328. doi: 10.1016/0360-3016(95)00179-3. [DOI] [PubMed] [Google Scholar]
- 15.Wong AP, Dahdaleh NS, Fessler RG, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J. Neurooncol. 2013;115(3):493–503. doi: 10.1007/s11060-013-1251-y. [DOI] [PubMed] [Google Scholar]
- 16.Fakhreddine MH, Mahajan A, Penas-Prado M, et al. Treatment, prognostic factors, and outcomes in spinal cord astrocytomas. Neuro. Oncol. 2013;15(4):406–412. doi: 10.1093/neuonc/nos309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khaw SL, Coleman LT, Downie PA, et al. Temozolomide in pediatric low-grade glioma. Pediatr. Blood Cancer. 2007;49:808–811. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 18.Goyal S, Puri T, Julka PK. Holocord low grade astrocytoma – role of radical irradiation and chemotherapy. J. Egypt Natl Canc. Inst. 2015;27(2):105–108. doi: 10.1016/j.jnci.2015.01.001. [DOI] [PubMed] [Google Scholar]