Abstract
Introduction:
As the incidence of liver cancer continues to increase in the setting of cirrhosis, parenchyma-sparing liver resection is increasingly necessary. A technique is described that involves using a sling made from 1-inch-wide packing gauze to retract and rotate the liver to divide the right triangular and coronary ligaments and mobilize segment 7. The right lobe is rotated anteriorly and counterclockwise, allowing access and parenchymal transection of segment 7 under ultrasonographic guidance.
Case Presentation:
Seven patients with tumors in segment 7 underwent resection with the technique described above: 4 had Child's A cirrhosis and hepatocellular carcinoma (HCC), 1 had metastatic colon cancer, 1 had an adenoma, and 1 had a symptomatic hemangioma. Tumor size ranged between 2.5 and 7.7 cm. Blood loss during resection was between 150 and 500 mL. No patients required transfusion as a result of surgery. With the exception of 1 patient with Clostridium difficile colitis, the average hospital stay was 3.8 days.
Management and Outcome:
Parenchyma-sparing laparoscopic resection of segment 7 is feasible and can be safely performed using a sling for intracorporal hepatic retraction, manipulation, and positioning. Given the risk of HCC recurrence, laparoscopic liver resection may also be better suited for subsequent salvage liver transplant because of less perihepatic adhesions.
Keywords: Cirrhosis, Laparoscopy, Liver cancer, Liver resection, Segment 7
INTRODUCTION
Advances in laparoscopic surgery, intraoperative ultrasound, hepatic parenchymal transection, and vascular stapling have increased the feasibility of laparoscopic liver resection (LLR).1,2 LLR has been shown to compare favorably with open liver resection for primary and metastatic cancers of the liver.3,4 LLR has also been shown to have acceptable safety in patients with cirrhosis and to facilitate salvage liver transplant in the event the patient develops recurrent inoperable liver cancer.5,6 In 2009, the International Position on Laparoscopic Liver Surgery did not support resection of segment 7.7 However, several reports have since described the feasibility of LLR for tumors in the posterior segments of the liver. For tumors in segment 7, segment 6 is often included as part of a posterior sectionectomy.8–10 Given the increasing incidence of HCC in patients with cirrhosis, parenchyma-sparing techniques are needed to minimize postoperative hepatic insufficiency. We describe the prospective development of a technique to resect tumors in segment 7 with preservation of segment 6.
METHODS
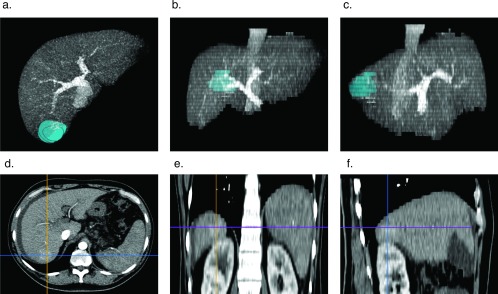
Patients were evaluated with multiphase contrast abdominal imaging (contrast computed tomographic [CT] scan and magnetic resonance imaging [MRI] with gadolinium or Eovist; Bayer Schering Pharma, Berlin, Germany). Patients deemed acceptable surgical candidates and physiologically acceptable providing consent for surgery included those with hepatoma and Child's class A cirrhosis with a platelet count greater than 100,000/mm3. Surgical planning for all patients was performed with the Horos Digital Imaging and Communications in Medicine (DICOM) viewer.11 The tumor was rendered as a volume and placed within a 3-dimensional (3D) maximum projection intensity (MIP) reconstructed sequence in the venous phase (Figure 1). The retrohepatic vena cava was inspected for short hepatic veins greater than 5 mm in diameter and marked for display. At the time of surgery, images were displayed on a 55-inch LED display within the operative suite.
Figure 1.
3D MIP reconstruction of tumor rendered as a volume for surgical planning. The tumor is rendered as a volume within reconstructed 3D MIP DICOM of the liver in the venous phase: axial (A), coronal (B), and right sagittal (C) views. DICOMs were read with the Horos open-source medical image viewer.11 Axial (D), coronal (E), and right sagittal (F) arterial phase images are of a segment 7 hepatoma.
For exploration, a 5-mm infraumbilical port was placed with a Veress needle. A 5-mm, 30° laparoscope was placed in the abdomen. An upper midline 12-mm abdominal port was placed for laparoscopic ultrasound of the liver. Two right-side abdominal (5- and 12-mm) ports were placed to dissect and manipulate the liver (Figure 2). A diagnostic laparoscopy was performed to rule out extrahepatic disease in patients with cancer.
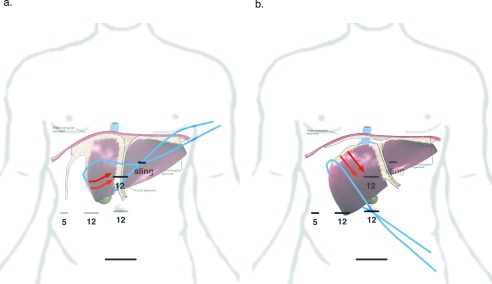
Figure 2.
Port and sling placement to expose right triangular ligament. (A) The blue line represents sling placement and direction of tension to initiate exposure of the right triangular ligament. The red arrows represent the anticipated rotation of the liver. (B) The sling after being slid over the dome of the liver. This maneuver allows the liver to be pulled away from the diaphragm, enabling access to the dorsal hepatic ligament and release of segment 7. Sites of 5- and 12-mm ports are shown as horizontal lines.
The round and falciform ligaments were divided, followed by division of the right and left coronary ligaments and separation of the bare area until the anterior aspect of the right, middle, and left hepatic veins were visualized. Using a fan retractor, the inferolateral aspect of the right triangular ligament was exposed and divided until the right lateral wall of the vena cava was visualized. A sling made of 1-inch packing tape was wrapped around segment 6, and externalized through a 3–4-mm stab in the left upper abdomen (Figure 2A). The right lobe of the liver was gently pulled anterior and cephalad to the left, further exposing the right triangular ligament. As the right triangular ligament was divided, the sling was slid cephalad toward segment 8. Once the sling was over segment 8, it was repositioned and externalized along the right side of the infraumbilical midline port to pull the liver farther away from the diaphragm to continue dividing the right triangular ligament (Figures 2B and Figure 3).
Figure 3.
Use of the sling to retract the liver and expose the right triangular ligament. (A) Sling exposing the posterior lateral aspect of the right triangular ligament. (B) Division of the posterior superior right triangular ligament. (C) Sling pulling the dome of the liver away from the diaphragm, exposing the right coronary ligament anteriorly and laterally. Note the right phrenic vein draining into the vena cava or right hepatic vein.
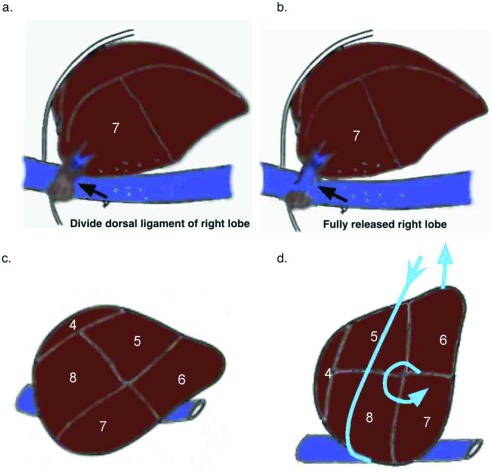
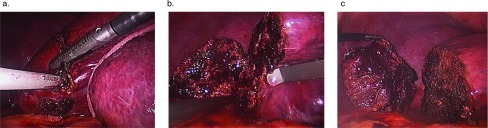
The sling was then repositioned back to the left upper abdomen and externalized, and the liver was rotated anteriorly, exposing the short hepatic veins, which were doubly clipped and ligated between. Depending on the size of the liver with respect the chest wall, if sufficient room was not available to rotate the right lobe anteriorly, the left triangular ligament was divided, allowing the left lateral segment to slide over the spleen providing additional room to rotate the right lobe of liver anteriorly. To fully release segment 7, the dorsal hepatic ligament of the liver was divided. Particular attention should be given to this step, as the dorsal hepatic ligament may contain a sizable vein and is near the right hepatic vein. Division of the dorsal hepatic ligament exposed the right hepatic vein and allowed full mobilization of the right lobe of the liver (Figure 4A and 4B). Care was taken not to pull up too hard at this point, as there may have been additional short hepatic veins adjacent to the right hepatic vein, which could avulse. This approach was also used for laparoscopic right hepatic lobectomy.
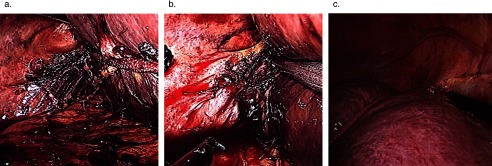
Figure 4.
Release of the dorsal hepatic ligament and rotation of the liver. (A) Right liver mobilized after division of short hepatic vein with exposure of dorsal hepatic ligament. (B) Liver released after division of dorsal hepatic ligament. (C) Natural position of the liver. (D) Rotation of the liver using 1-inch packing wrapped around the dome of the liver.
Once adequate division of the right triangular ligament and short hepatic veins were complete, the sling was positioned overlying segment 8, running posterior between segments 5 and 6, and was externalized through the left upper abdominal stab wound. The sling was used to rotate the liver by releasing the arm overlying segment 8 and pulling on the arm posterior to segments 5 and 6 (Figure 4C and 4D).
To apply inflow control, an umbilical tape was wrapped around the hepatoduodenal ligament. To do so, a bariatric golden finger was fed through the foramen of Winslow with the umbilical tape at the tip. Once through the foramen, the hepatogastric ligament was divided, and the umbilical tape was grasped with Maryland dissecting forceps. The ends were externalized through the upper midline 12-mm port and fed through a 10-French Rummel tourniquet. A laparoscopic bulldog clamp was cinched down along the umbilical tape on the hard plastic tube (Figure 5A and 5B).
Figure 5.
Use of umbilical tape for laparoscopic inflow control of hepatoduodenal ligament (Pringle maneuver). (A) Umbilical tape around the hepatoduodenal ligament. (B) The hepatoduodenal ligament with bulldog clamp cinched down to limit inflow to the liver.
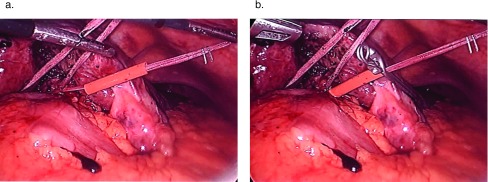
Using ultrasonographic guidance, the borders of the lesion were marked and then were marked again at a 2-cm margin on the surface of the liver with an argon beam. Inflow occlusion was initiated, and parenchymal transection was commenced with Enseal, Harmonic Scalpel (both from Ethicon, Summerville, New Jersey, USA) or, more recently, a Cavitron ultrasonic surgical aspirator (CUSA; Cavitron, York, Pennsylvania, USA) depending on the vessels identified by ultrasonography (Figure 6).
Figure 6.
Rotated liver and tissue transection for hepatoma. (A) Sling being used to rotate liver and segment 7 inferiorly and anteriorly. The harmonic scalpel is used to transect liver after lesion is identified and the borders are marked using laparoscopic ultrasonography. (B) Hepatic transection plane. (C) Resected specimen in segment 7, which is then removed through an infraumbilical incision.
RESULTS
Seven patients underwent laparoscopic segment 7 resection. Four patients had HCC in the setting of well-compensated cirrhosis. Table 1 shows patient characteristics, liver status, tumor type, and the dimensions of the right lobe and chest cavity. Table 2 shows operative details, adverse events, hospital length of stay, and surgical margins.
Table 1.
Patient Demographics, Tumor Etiology and Dimensions
| Patient | Age | Sex | Height (in.) | Weight (lb) | BMI | Liver Type | Tumor | Size (cm) | Size Right Lobe CC × AP × Lat | Dimension of Chestb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 66 | 203 | 32.8 | HCV cirrhosis | HCC | 3 | 10.5 × 14.8 × 10.4 | 28 × 11.2 |
| 2 | 65 | M | 72 | 197 | 25.3 | HCV cirrhosis | HCC | 3.5 | 15 × 17.5 × 12.2 | 31.3 ×11.5 |
| 3 | 56 | M | 63 | 159 | 28.2 | HCV cirrhosis | HCC | 4 | 12.8 × 15.7 × 9.7 | 26.1 × 12 |
| 4 | 30 | F | 63 | 133 | 23.6 | Normal | Aden | 3 | 16 × 12 × 9.1 | 24.2 × 6.2 |
| 5a | 39 | F | 66 | 233 | 37.6 | Fatty | Hem | 7.7 | 18 × 18 × 8.0 | 28 × 10.4 |
| 6a | 69 | M | 62 | 140 | 25.6 | Fatty | mCRC | 3 | 15.1 × 17.1 × 9.7 | 29.5 × 10.7 |
| 7 | 39 | M | 71 | 210 | 27.7 | HBV cirrhosis | HCC | 2.5 | 18.2 × 16.6 × 10.1 | 29.4 × 9 |
Aden, adenoma; AP, anterior–posterior; CC, craniocaudad; Hem, hemangioma; Lat, from vena cava to right lateral chest wall; mCRC, metastatic colorectal cancer.
aRequired hand-assisted surgery because of limited space to rotate liver.
bDistance across chest at the level of the bifurcation of the portal vein and from posterior sternum to anterior aorta.
Table 2.
Operative and Postoperative Events
| Patient | OR Time (min) | Blood Loss (mL) | Blood Transfusion (n) | Surgical Margins (cm) | Adverse Events | LOS (days) |
|---|---|---|---|---|---|---|
| 1 | 182 | 200 | 0 | 1–2 | None | 5 |
| 2 | 360 | 200 | 0 | 1–2 | Atrial fibrillation | 6 |
| 3 | 340 | 150 | 0 | 2 | Ascites | 5 |
| 4 | 200 | 150 | 0 | 1 | None | 3 |
| 5 | 246 | 400 | 0 | 1 | Right pleural effusion | 5 |
| 6 | 320 | 500 | 1a | 2 | Right pleural effusion, C. difficile | 21 |
| 7 | 270 | 200 | 0 | 2 | None | 2 |
| Average | 274 | 257 | 7 (4)b |
LOS, length of stay; OR, operative time.
aStarting hemoglobin level 9.2 g/dL; patient transfused at beginning of case.
b(4) LOS excluding patient with C. difficile colitis.
Although the sample of patients is too small to draw any definitive conclusions, of the 2 patients requiring hand assist, patient 5 had a body mass index of 37.6, a fatty liver, and the largest right lobe of the 7 patients resected. Because of the fragile nature of the fatty liver, use of the sling caused several tears in the liver capsule. To reduce any risk of significant blood loss, hand assisted laparoscopy was used. Patient 6 had undergone right hemicolectomy for a 7-cm colon cancer at the hepatic flexure. A preoperative abdominal CT scan showed a segment 7 mass consistent with metastasis. The general surgeon intended to resect the liver mass at the time of the colon surgery, but realized that it was not possible. A hepatobiliary consult was obtained. A CT scan of the chest was negative, and the patient was scheduled for laparoscopic liver resection 7 days later. Because of the patient's small body frame and adhesions, a hand assisted technique was used. The patient also sustained postoperative Clostridium difficile colitis, which extended his hospital length of stay.
Only 1 patient received blood transfusion before surgery; otherwise, no blood products were required. Operative times ranged from 182 to 360 minutes. There were no deaths. Two patients required thoracentesis for right pleural effusion. One patient with a history of open heart surgery for atrial myxoma developed atrial fibrillation on postoperative day 3. One patient developed postoperative ascites and was discharged home on diuretics. With the exception of the patient who developed C. difficile colitis, the average hospital length of stay was 3.8 days.
All the patients were alive with normal liver function at last follow-up. Patient 3, with treated hepatitis C virus (HCV) cirrhosis developed a new 2.2-cm hepatoma of the left lateral segment 18 months after surgery.
DISCUSSION
The increasing incidence of liver cancer in the setting of cirrhosis carries a risk of postoperative hepatic dysfunction and failure. Sparing of hepatic parenchyma is crucial while ensuring sufficient surgical margins to achieve R0 resection. We describe a systematic laparoscopic technique to mobilize and rotate the right lobe of the liver, providing access to segment 7. Aided by the use of laparoscopic ultrasonography, the tumor can be identified, mapped, and safely resected with sufficient margins.
Although 4 of 7 patients underwent resection for HCC in the setting of cirrhosis, the mobilization technique was similar for all the patients. Intercostal trocars were not needed to divide the right coronary or triangular ligaments, as the sling enabled retraction of the liver away from the diaphragm.12 This retraction was also useful when exposing and dividing the dorsal hepatic ligament, which is crucial to the release of segment 7. It should be noted, to best visualize and divide the lateral right triangular and dorsal hepatic ligaments required switching the 30° laparoscope from the midline infraumbilical port to the lower right lateral 12-mm port. In addition, to visualize the superior aspect of the right triangular ligament, the 30° laparoscope was switched from the midline infraumbilical port to the upper midline 12-mm one.
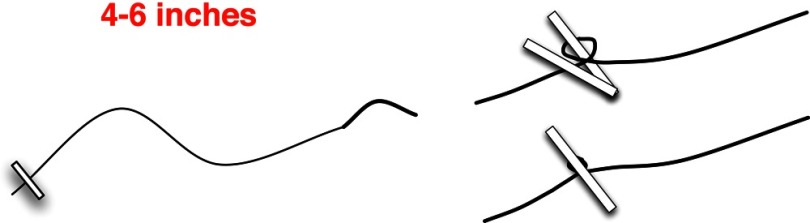
Consistent with prior laparoscopic liver resections, the most variability associated with this technique was during parenchymal transection. Using inflow control and laparoscopic guidance, cirrhotic hepatic parenchyma was divided satisfactorily with the Harmonic Scalpel. For the fatty liver, we used an Enseal tissue sealer. For the normal or slightly fibrotic liver, we used a laparoscopic CUSA and Enseal tissue sealer. Use of a laparoscopic stapling device with ultrasound guidance was useful with the fatty livers, but was avoided with cirrhotic livers.2 Contrary to the anvil's easily traversing fatty hepatic parenchyma, with cirrhotic parenchyma there is significant resistance in passing the anvil into the liver, as well as closing the stapling device. For instances when parenchymal bleeding cannot be controlled with cautery, Argon beam, or pressurized cautery, a 4-inch 2.0 silk suture on a small half-circle (SH) needle was used to place a figure-of-eight stich into the hepatic parenchyma. After the suture is placed, the needle is gently pulled with the laparoscopic needle driver. Once hemostasis is obtained, a laparoscopic clip is placed on the needle side of the suture. These sutures should be made at the beginning of the case, with the silk wrapped around 1 arm of a large hemoclip before being compressed (Figure 7). This technique has been used in over 150 laparoscopic liver resections, without a single instance of postoperative parenchymal bleeding. As with most laparoscopic surgery, having stereo-optics may be particularly useful in the event a suture needs to be placed. Although robotic liver resection has been described for posterior segment tumors, the startup cost maybe prohibitively high.9 In addition, given the need to change the laparoscope to 3 different ports, using a robot may prove cumbersome.
Figure 7.
Construction of suture for quick control of hepatic parenchymal bleeding. A 2.0 silk suture with an SH needle is used. The anchor is made by wrapping the 2.0 suture around 1 of the arms of a large hemoclip. The hemoclip is then compressed and the anchor is secured.
Routine inflow control for 15 minutes, followed by 5 minutes of inflow release was used repeatedly as needed until parenchymal transection was complete. Increases in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were no more than 5 times the upper limit of normal and quickly normalized. The 2 patients who developed a right pleural effusion did not have cirrhosis, although 1 of the patients with cirrhosis developed postoperative ascites. All 3 patients with HCV cirrhosis underwent resection after HCV treatment, and were without detectable virus at the time of surgery and 6 months later. Patient 3 developed a new hepatoma 18 months after surgery. Given his young age, diuretic dependence, and the HCC being his second one, he is being evaluated for local–regional treatment and salvage liver transplant. Although the HCC is technically resectable, further liver surgery would be likely to increase risk during liver transplantation.6
In summary, laparoscopic resection of tumors in segment 7 is feasible and safe. This report provides a detailed technical description that we hope will expand its use and lead to acceptable reproducible outcomes.
Contributor Information
Igor Mashchenko, Department of Surgery, St Georges University School of Medicine, Grenada, West Indies..
Anna Trtchounian, Department of Surgery, St Georges University School of Medicine, Grenada, West Indies..
Christopher Buchholz, Department of Bariatric Surgery, Hackensack Medical Center, Hackensack, New Jersey..
Andrew N. de la Torre, Department of Surgery, New Jersey Medical School, Rutger's University, Newark, New Jersey..
References:
- 1. Cuesta MA, Meijer S, Borgstein PJ, Sibinga Mulder L, Sikkenk AC. Laparoscopic ultrasonography for hepatobiliary and pancreatic malignancy. Br J Surg. 80:1993;1571–1574. [DOI] [PubMed] [Google Scholar]
- 2. Gumbs AA, Gayet B, Gagner M. Laparoscopic liver resection: when to use the laparoscopic stapler device. HPB (Oxford) 2008;10:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937–1943. [DOI] [PubMed] [Google Scholar]
- 4. Langella S, Russolillo N, D'Eletto M, Forchino F, Lo Tesoriere R, Ferrero A. Oncological safety of ultrasound-guided laparoscopic liver resection for colorectal metastases: a case-control study. Updates Surg. 2015;67:147–155. [DOI] [PubMed] [Google Scholar]
- 5. Memeo R, de'Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014;38:2919–2926. [DOI] [PubMed] [Google Scholar]
- 6. Laurent A, Tayar C, Andréoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310–314. [DOI] [PubMed] [Google Scholar]
- 7. Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. [DOI] [PubMed] [Google Scholar]
- 8. Coles SR, Besselink MG, Serin KR, et al. Total laparoscopic management of lesions involving liver segment 7. Surg Endosc. 2015;29:3190–3195. [DOI] [PubMed] [Google Scholar]
- 9. Boggi U, Caniglia F, Vistoli F, Costa F, Pieroni E, Perrone VG. Laparoscopic robot-assisted resection of tumors located in posterosuperior liver segments. Updates Surg. 2015;67:177–183. [DOI] [PubMed] [Google Scholar]
- 10. Casaccia M, Andorno E, DiDomenico S, et al. Laparoscopic right posterior sectionectomy for hepatocellular carcinoma using a modified liver-hanging maneuver. J Laparoendosc Adv Surg Tech A. 2012;22:488–491. [DOI] [PubMed] [Google Scholar]
- 11. Horos DICOM Medical Image Viewer v. 2.2.0 (2017). Available at https://horosproject.org.
- 12. Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci. 2014;21:E65–E684. [DOI] [PubMed] [Google Scholar]