Abstract
A 26-year-old woman presented with a 5-day history of fever after returning from Bali. She denied sexual contact abroad. On examination, there was suprapubic tenderness and a widespread maculopapular rash. Malaria serology was negative and blood tests were normal except for an elevated C reactive protein. Treatment was initially with ceftriaxone, metronidazole and doxycycline, but her symptoms failed to improve. A CT pelvis suggested a possible tubo-ovarian abscess, a suspected inferior vena cava (IVC) anomaly and left internal iliac/femoral venous thrombosis. A gynaecology review demonstrated left tubo-ovarian tenderness and fullness. An MRI suggested pelvic inflammatory disease and thrombophlebitis affecting the pelvic veins; deep vein thrombosis (DVT) treatment was commenced. Further family history revealed thrombosis throughout multiple generations. Further imaging analysis demonstrated agenesis of the IVC with compensatory dilation of pelvic collaterals and an acute DVT of the deep pelvic venous system. The patient was discharged with direct oral anticoagulant therapy.
Keywords: venous thromboembolism, travel medicine, tropical medicine (infectious disease), radiology, sexual health
Background
The presentation of fever in a young returning traveller may be a result of sexually transmitted disease. Clinical examination and initial imaging supported a diagnosis of pelvic inflammatory disease (PID). However, the subsequent failure of the patient to respond to treatment prompted more detailed pelvic imaging. This demonstrated a developmental abnormality in the inferior vena cava (IVC) with consequent hypertrophy of collaterals and a venous thrombosis. IVC abnormalities increase the risk of deep vein thrombosis (DVT) and are present in 0.3%–0.5% of the general population.1 In those patients with a strong thrombotic family history, it is therefore important to consider structural vascular abnormalities and consequent thrombosis as a potential differential diagnosis in fever of unknown origin cases.
Case presentation
A 26-year-old woman returned from Bali with a 5-day history of tiredness and back pain. It was noticed by family that she was shivering with a high temperature. No cough or dysuria was reported nor weight loss. At the time of presentation, she was menstruating, on the combined contraceptive pill/oral contraceptives and denied sexual contact while on holiday.
No medical history or regular medications were reported. The patient was up to date with her vaccinations and normally independent prior to travel.
On examination, the patient was feverish with right upper quadrant and suprapubic tenderness, with a maculopapular rash over the body—thought initially to be secondary to bed bugs that the patient had noticed in the last hostel she stayed in. The remainder of the clinical examination including cardiovascular and respiratory systems were normal and her calves were soft and non-tender.
Investigations
Bloods: C reactive protein 388, sodium 140 mmol/L, potassium 4.1 mmol/L, urea 2.8 mmol/L, creatinine 63 μmol/L, eGFR (estimated Glomerular Filtration Rate) >90 mL/min/1.73 m2, albumin 32 g/L, ALP (alkaline phosphatase) 61 IU/L, ALT (alanine aminotransferase) 12 IU/L, bilirubin 6 μmol/L, INR (international normalised ratio) 1.1, white cell count 8.45×109/L, neutrophils 5.85×109/L, haemoglobin 126 g/L, platelets 291×109/L.
Malaria screen negative.
Dengue negative.
Differential diagnosis
PID.
Pyelonephritis.
Hepatitis.
Dengue.
Treatment
The patient was started on ceftriaxone initially as appropriate broad-spectrum antibiotic cover for fever of unknown origin. Malaria and dengue screening tests were negative early in the course of investigation, and the clinical presentation and laboratory findings were not in keeping with these travel associated infections. Metronidazole and doxycycline were added for treatment of PID once imaging findings were reported.
Treatment dose low molecular weight heparin therapy was started on radiological diagnosis of pelvic DVT. This was switched to direct oral anticoagulant therapy following discussion with haematology.
Outcome and follow-up
The patient’s persistent symptoms prompted a CT abdomen/pelvis for investigation for a possible psoas abscess. This demonstrated a complex soft-tissue density in the left side of the pelvis/tubo-ovarian region—possibly a tubal ovarian abscess and associated lymphadenopathy—and mild hydronephrosis. There was a left internal iliac/femoral venous thrombosis. Incidental note was made of a poorly visualised IVC with multiple smaller veins in the retroperitoneum suggestive of an IVC anomaly.
The gynaecology review following the CT abdomen/pelvis report noted the tenderness to the left iliac fossa and vague mass. White non-offensive discharge was found on vaginal examination, with no cervical excitation. Tenderness and fullness was found to the left tubo-ovarian area only.
A subsequent MRI pelvis showed anomalous venous structures within the abdomen and pelvis with likely thrombosis and inflammation of grossly dilated varices within the left side of the pelvis. The scan also demonstrated wall enhancement post contrast associated with moderate inflammatory change in the left side of the pelvis.
She was maintained on PID treatment. The patient’s symptoms failed to resolve and she reported increased pain. A second gynaecology consultant reviewed the images and determined that the ongoing pain was likely due to inflammation secondary to thrombotic varices in the pelvis. Further review of previous imaging demonstrated agenesis of the IVC (AIVC) with compensatory dilation of pelvic collaterals and an acute DVT of the deep pelvic venous system (see figures 1 and 2). A more detailed family history revealed that her father had had three previous DVTs and other family members had multiple pulmonary embolisms.
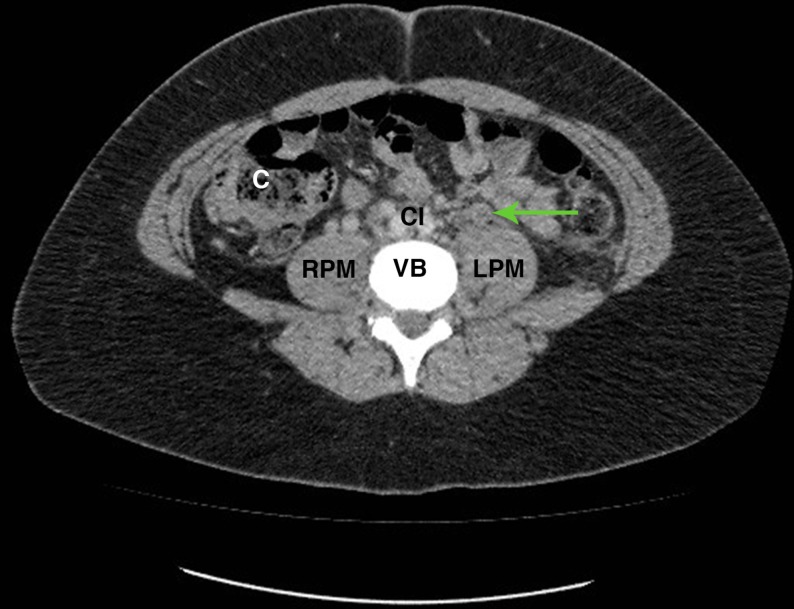
Figure 1.
Axial CT slice at the level of L4 with arrow demonstrating thrombosis in the left ovarian vein anterior to the left psoas muscle. C, ascending colon; CI, bifurcation of aorta to common iliac arteries; LPM, left psoas major; RPM, right psoas major; VB, vertebral body.
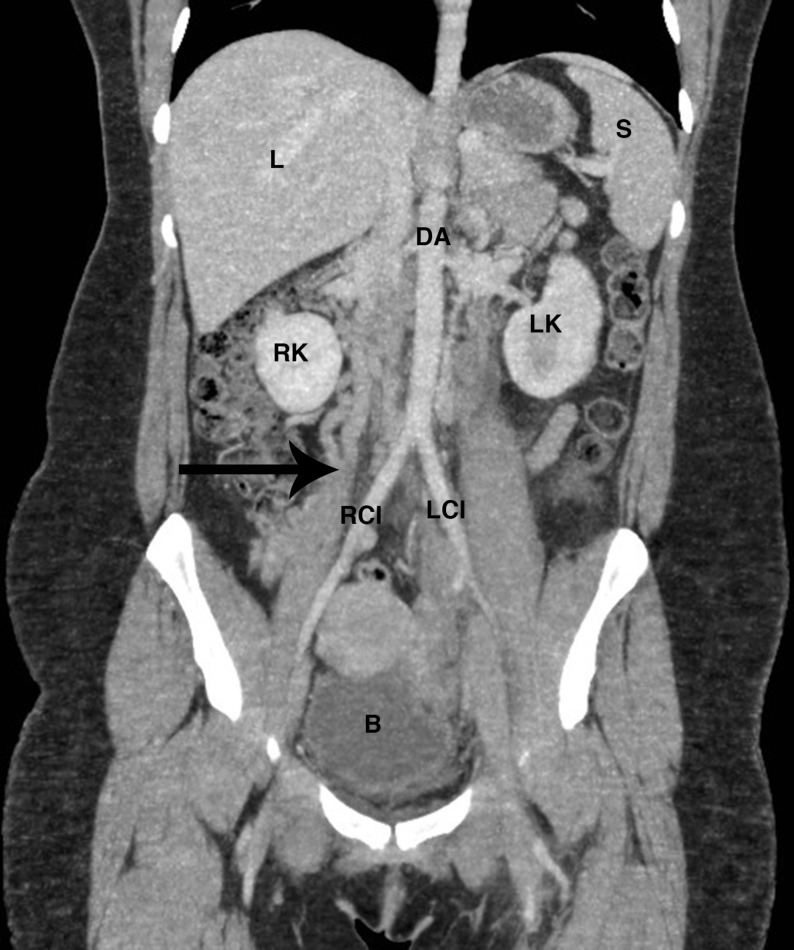
Figure 2.
Coronal CT slice with arrow demonstrating the expected location of an inferior vena cava; in this patient replaced with distended collateral veins. B, bladder; DA, descending aorta; L, liver; LCI, left common iliac artery; LK, left kidney; RCI, right common iliac artery; RK, right kidney; S, spleen.
The patient was increased to treatment dose (1.5 mg/kg) low molecular weight heparin. A filter in the larger collateral vein was discussed with haematology, but not recommended. She was discharged on rivaroxaban and followed up by haematology in thrombosis clinic.
A repeat MRI pelvis was carried out, which showed multiple bilateral pelvic varicosities. There was some resolution of the thrombosis, and she will continue under gynaecology follow-up.
Discussion
IVC abnormalities are present in 0.3%–0.5% of the general population,2 rising to 0.6%–2% in those with cardiac abnormalities. The incidence of AIVC in the general population is estimated to be 0.0005%–1%3–6; however, this may rise to as high as 9% in young patients presenting with thrombosis investigated with CT/MRI.7
DVT associated with IVC abnormalities are well described in the literature attributed to poor collateral venous drainage often resulting in proximal and bilateral DVTs.7 8
Our case highlights the potential for misdiagnosing pelvic DVT in this atypical population presenting with features such as pelvic pain and menorrhagia in young women.9 In addition, while AIVC is usually asymptomatic and often diagnosed incidentally, first presentations are often with venous thrombosis though fever and rigours have been described as well as subsequent lower-limb oedema.10
Due to the increased risk of DVT in the collateral vessels,3–5 previous case reports endorse screening of the IVC in young patients presenting with idiopathic DVT11 with either ultrasound or CT venography, though MRI has also been described as an effective imaging technique for IVC abnormalities.4 6 10 12 The network of collateral vessels in these patients can cause diagnostic issues in non-contrast CT scanning due to a tumour-like appearance.13
The authors are not aware of an increased risk to infections as a result of this congenital abnormality; however, there are case reports suggesting a higher risk of DVT if there is a concomitant infection, with one reporting pelvic thrombosis in a 14-year-old boy with Mycoplasma pneumoniae infection.7
Treatment generally involves the acute treatment of the thrombotic presentation with thrombolytics or heparinoids followed by longer-term preventative therapy including mechanical compression and lifelong anticoagulation.7 10 14–17 Thrombolytic therapy for proximal DVTs has also been delivered through a catheter-directed approach.8 Additionally, multiple surgical techniques have been described.18 19
Stereotypical presentations can be misleading and it is important to review a diagnosis if the clinical course or response to treatment does not proceed as expected. In this case, imaging by a different modality was key to establishing the cause.
Learning points.
Congenital malformation of the inferior vena cava is a rare cause of pelvic and other deep vein thromboses and should be considered in young people with thromboses, particularly those with paediatric presentations.
Review a diagnosis if the clinical course or response to treatment does not proceed as expected.
Footnotes
Contributors: OST: drafted report and obtained consent from patient. DABM: reviewed literature and drafted discussion points. EM: reviewed report and identified case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sitwala PS, Ladia VM, Brahmbhatt PB, et al. Inferior vena cava anomaly: a risk for deep vein thrombosis. N Am J Med Sci 2014;6:601–3. 10.4103/1947-2714.145486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolocan A, Ion D, Ciocan D, et al. Congenital agenesis of the inferior vena cava—cause of deep vein thrombosis. Chirurgia 2014;109:832–6. [PubMed] [Google Scholar]
- 3.Simon RW, Amann-Vesti BR, Pfammatter T, et al. Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. J Vasc Surg 2006;44:416 10.1016/j.jvs.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Salgado Ordóñez F, Gavilán Carrasco JC, Bermúdez Recio FJ, et al. Absence of the inferior vena cava causing repeated deep venous thrombosis in an adult—a case report. Angiology 1998;49:951–6. 10.1177/000331979804901113 [DOI] [PubMed] [Google Scholar]
- 5.Takehara N, Hasebe N, Enomoto S, et al. Multiple and recurrent systemic thrombotic events associated with congenital anomaly of inferior vena cava. J Thromb Thrombolysis 2005;19:101–3. 10.1007/s11239-005-1380-z [DOI] [PubMed] [Google Scholar]
- 6.García-Fuster MJ, Forner MJ, Flor-Lorente B, et al. [Inferior vena cava malformations and deep venous thrombosis]. Rev Esp Cardiol 2006;59:171–5. 10.1016/S1885-5857(06)60127-8 [DOI] [PubMed] [Google Scholar]
- 7.Kalicki B, Sadecka M, Wawrzyniak A, et al. Absence of inferior vena cava in 14-year old boy associated with deep venous thrombosis and positive Mycoplasma pneumoniae serum antibodies—a case report. BMC Pediatr 2015;15:40 10.1186/s12887-015-0357-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randrianarisoa E, Rittig K, Brechtel K, et al. [Thrombosis of the pelvic and leg—conservative procedure or thrombolytic therapy?]. Dtsch Med Wochenschr 2013;138:1130 10.1055/s-0033-1343102 [DOI] [PubMed] [Google Scholar]
- 9.Nichols JL, Gonzalez SC, Bellino PJ, et al. Venous thrombosis and congenital absence of inferior vena cava in a patient with menorrhagia and pelvic pain. J Pediatr Adolesc Gynecol 2010;23:e17–e21. 10.1016/j.jpag.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Baeshko AA, Zhuk GV, Orlovskiĭ I, et al. [Congenital anomalies of the inferior vena cava: diagnosis and medical treatment]. Angiol Sosud Khir 2007;13:91–5. [PubMed] [Google Scholar]
- 11.Gil RJ, Pérez AM, Arias JB, et al. Agenesis of the inferior vena cava associated with lower extremities and pelvic venous thrombosis. J Vasc Surg 2006;44:1114–6. 10.1016/j.jvs.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 12.Gaber Y, Schmeller W, Römer C, et al. [Pelvic and leg vein thrombosis in azygous and hemi-azygous vein continuity syndrome and complete agenesis of the inferior vena cava]. Vasa 1998;27:187–91. [PubMed] [Google Scholar]
- 13.Parsa P, Lane JS, Barleben AR, et al. Congenital agenesis of inferior vena cava: a rare cause of unprovoked deep venous thrombosis. Ann Vasc Surg 2015;29:1017.e15–1017.e18. 10.1016/j.avsg.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Halcín A, Kovácová E, Mikla F, et al. [Symmetrical phlebothrombosis of lower extremities resulting from congenital malformation of vena cava inferior]. Vnitr Lek 2009;55:1189–92. [PubMed] [Google Scholar]
- 15.Klessen C, Deutsch HJ, Karasch T, et al. [Thrombosis of the deep leg and pelvic veins in congenital agenesis of the vena cava inferior]. Dtsch Med Wochenschr 1999;124:523–6. 10.1055/s-2007-1024354 [DOI] [PubMed] [Google Scholar]
- 16.Körber T, Petzsch M, Placke J, et al. [Acute thrombosis of pelvic and leg veins in agenesis of the renal segment of the inferior vena cava]. Z Kardiol 2001;90:52–7. [PubMed] [Google Scholar]
- 17.Rühlmann C, Engelmann L, Scheel H, et al. [Thrombolysis of an extensive venous thrombosis of the lower body in an anomaly of the vena cava inferior]. Dtsch Med Wochenschr 1996;121:124–8. 10.1055/s-2008-1042982 [DOI] [PubMed] [Google Scholar]
- 18.Mirzaie M, Schorn B, Meyer T, et al. [Deep leg and pelvic vein thrombosis in aplasia of the inferior vena cava]. Vasa 1999;28:293–5. 10.1024/0301-1526.28.4.293 [DOI] [PubMed] [Google Scholar]
- 19.Over LM, van der Laan JG, Gökemeijer JD. [Deep venous thrombosis as a complication of congenital absence of vena cava inferior]. Ned Tijdschr Geneeskd 2001;145:2280–3. [PubMed] [Google Scholar]