Abstract
Heterotopic pancreas is the presence of pancreatic tissue outside its normal location. It can develop similar pathological conditions that develop in the normal pancreas, including adenocarcinoma and its precursor lesions. Due to the rarity of the condition, the diagnosis can be challenging, and treatment is not well established. We present a 47-year-old female patient referred for endoscopic resection of a 2 cm polyp in the second part of her duodenum. Complete endoscopic mucosal resection (EMR) was performed, with pathology revealing low-grade pancreatic intraepithelial neoplasia (PanIN) in heterotopic pancreatic tissue. To the best of our knowledge, this is the first case of heterotopic pancreas with low-grade PanIN in the duodenum to be incidentally diagnosed and treated with EMR.
Keywords: endoscopy, pancreas and biliary tract, small intestine, EMR, endoscopic mucosal resection, duodenal adenoma
Background
We present an alternative management approach for a heterotopic pancreatic tissue in the duodenum. To the best of our knowledge, this is the first case of heterotopic pancreas with low-grade pancreatic intraepithelial neoplasia (PanIN) in the duodenum to be incidentally diagnosed and treated with endoscopic mucosal resection (EMR).
Case presentation
A 47-year-old woman was referred for endoscopic resection of a 2 cm polyp in the second part of duodenum found during a recent oesophagogastroduodenoscopy (EGD). Her medical history was significant for ulcerative colitis well controlled on oral mesalamine therapy. She denied smoking, alcohol or illicit drug use. There was no history of jaundice or acute pancreatitis. The patient’s family history was significant for coronary artery disease in her father and type II diabetes mellitus in her mother.
Investigations
Physical examination was unremarkable. Initial biopsies of the duodenal polyp showed duodenal adenoma, and she was referred to our centre for further endoscopic management. Laboratory tests including complete blood count, serum electrolytes, lipase, amylase and liver function tests were within normal range.
Treatment
Repeat EGD was performed and revealed a 2 cm sessile polyp in the proximal second portion of the duodenum. The lesion was examined with white light and narrow band imaging (figure 1A,B). The polyp was located 2 cm proximal to the major ampulla and was distinct from the minor papilla. Complete EMR was performed using submucosal saline and methylene blue injection followed by a piecemeal hot snare polypectomy. The postpolypectomy edges were treated with argon plasma coagulation (effect: 2; flow: 0.5 L/min; power: 15 W) for ablation of any residual polyp tissue, and the post-EMR mucosal defect was effectively closed with placement of five endoclips (figure 1C,D). The patient was discharged home after the procedure.
Figure 1.
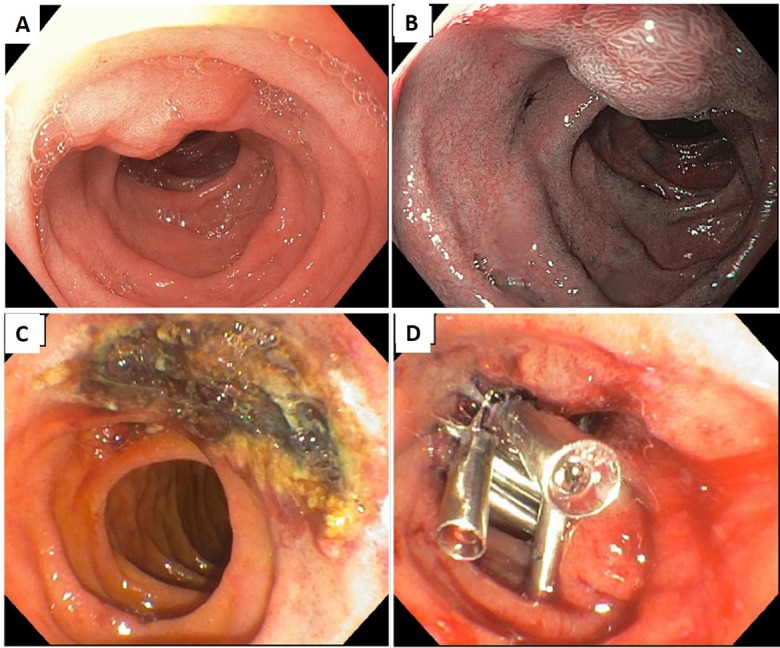
A 47-year-old woman was referred for endoscopic resection of a 2 cm polyp. (A) Under white light. (B) Under narrow band imaging. (C) Endoscopic mucosal resection was performed, and edges were treated with argon plasma coagulation. (D) The mucosal defect was closed with endoclips.
Histopathological examination showed a duodenal submucosal lesion involving the duodenum. The periphery of lesion largely comprised pancreatic acini and small pancreatobiliary glandular units, whereas the central aspect largely contained dilated and tortuous pancreatic ducts with low-grade PanIN. No high-grade dysplasia or invasive carcinoma was identified. In addition, there was no evidence of a pancreatic mucinous neoplasm (eg, intraductal papillary mucinous neoplasm). Histological findings are shown in figures 2–4.
Figure 2.

Overlying duodenal mucosa (top left) and underlying ectopic pancreas with low-grade pancreatic intraepithelial neoplasia involving ducts (bottom right) (magnification 2×).
Figure 3.

Low-power view of pancreatic acini (top) and dilated ducts (below) (magnification 4×).
Figure 4.

Pancreatic acini are seen (left), with adjacent dilated pancreatic ducts with mucinous cytoplasm (right). Rare papillary/micropapillary projections are present (magnification 10×).
Outcome and follow-up
Repeat EGD at 6-month follow-up showed no endoscopic evidence of recurrence or residual polyp at the site of duodenal EMR. The patient remains asymptomatic and continues to do well 9 months after the procedure.
Discussion
The presence of pancreatic tissue outside its normal location has been reported in the literature as ectopic or heterotopic pancreas. It is thought to be a congenital anomaly, where some portions detach during the foregut rotation in fetal life and develop in the aberrant location.1 Pancreatic heterotopia is composed of ductal cells, acinar cells, islet cells or a combination thereof.2 This heterotopic pancreatic tissue can be present in different sites, most commonly the stomach, duodenum and jejunum. It lacks the anatomical and vascular continuity with the pancreas, but it shares the physiological function and genetic make-up of the pancreas, which makes it susceptible to developing similar pathological conditions.3 Pancreatitis, pseudocysts, cysts and malignant transformation of the heterotopic pancreas are rare complications that have been reported.4 These lesions are mostly asymptomatic; however, they can present clinically with varying symptoms including nausea, vomiting, abdominal pain and ulceration based on the pathological condition.5 In one large study including 165 patients with heterotopic pancreas, symptoms were found to be associated with younger age, larger size, gastric location and lymphoid cuffs.6
The diagnosis of heterotopic pancreas remains challenging. Transabdominal ultrasonography and CT are not very specific and can have limited utility in the evaluation of smaller lesions.7 On endoscopy, heterotopic pancreas usually appears as small well-circumscribed lesions, sometimes with central umbilication, and covered with normal mucosa.8 They are mostly located in the submucosa, but can also be found in the muscularis propria or subserosal layer.9 Superficial biopsies are usually inadequate for diagnosis of these lesions due to the overlying normal mucosa. The definitive diagnosis of heterotopic pancreas is mostly reached on histopathological examination following surgical resection of the symptomatic or suspicious lesion.8 10 More recently, with the increasing use of endoscopic ultrasound (EUS) in the evaluation of submucosal gastrointestinal lesions,11 12 successful diagnosis of ectopic pancreas in the stomach and gastro-oesophageal junction has been reported with EUS-guided fine-needle aspiration (EUS-FNA).13–15 EUS was not performed on our patient given initial biopsy results showing an adenoma and the overall appearance of the lesion on endoscopy. However, we believe EUS/EUS-FNA prior to endoscopic resection could have been beneficial by providing more details for diagnosis and guiding optimal endoscopic management.
Adenocarcinoma arising from the heterotopic pancreatic tissue has been reported in the literature in fewer than 30 cases.3 16 PanIN is the most common precursor lesion of pancreatic ductal adenocarcinoma.17 These PanINs are histologically well-defined precursors and have been recognised in the heterotopic pancreatic tissue as well. They are larger, more common in stomach, have deeper wall location, and show infiltrative growth and lymphoid cuffs.6 PanIN-1A and PanIN-1B (low-grade PanIN) have a flat and papillary structure, but lack nuclear abnormality. PanIN-3 shows carcinoma in situ, and PanIN-2 is an intermediate category between both of them.18 The low-grade PanIN group represents a combination of neoplastic and non-neoplastic lesions with an unclear clinical significance.19 When PanINs arise in the normal pancreatic tissue, they involve the pancreatic ducts and are usually 2 mm to 4 cm in size. They are typically found incidentally during resections,17 and the best way to diagnose them is EUS-FNA.20–22 However, the presence of PanINs in a heterotopic pancreatic tissue remains more challenging, depending on the location of these lesions.
There is no established treatment for PanIN arising in ectopic pancreas. Surgical resection is the commonly used treatment given the good prognosis following radical resection.4 Macedo et al23 described a patient who had a pancreatic mass and a left renal mass, and during the surgical resection of these masses a small bowel neoplasm was incidentally found. It was surgically resected and biopsy was consistent with ectopic pancreatic tissue containing PanIN-2. Lee et al4 reported a case with ectopic pancreatic tissues in the stomach, complicated by PanIN-3, in which laparoscopic gastric wedge resection was used. Most of the reported cases of PanIN arising in ectopic pancreas were in the stomach and surgical resection has been used in most of them.9 16 24 The size and location of these lesions might determine the best treatment option.
Due to the rarity of PanIN arising in a heterotopic pancreas, it remains unclear which lesions need to be followed or treated. However, given that some of these lesions have the potential to develop malignancy, resection might be warranted. Surgical resection has been the only option in the past, but now with the advancement of endoscopic techniques, endoscopic resection via endoscopic submucosal dissection (ESD) or EMR might offer an alternative management approach for selected lesions. EMR is widely accepted as an effective and safe minimally invasive therapy for management of ampullary and non-ampullary duodenal adenomas. Yamamoto et al25 carried out a study on the therapeutic outcomes of EMR in 17 patients with superficial non-ampullary duodenal tumours, and satisfactory therapeutic outcomes were achieved for lesions <20 mm. Deeper lesions such as submucosal lesions can be effectively managed with ESD or endoscopic full-thickness resection. Follow-up for these lesions is not well established. We suggest endoscopic follow-up to ensure absence of residual polyp or recurrence. A 6-month follow-up was performed for our patient.
Our patient had ectopic pancreatic tissue in the duodenum, which is reported to occur in 9%–36% of cases of ectopic pancreas.2 The pathology examination of the resected specimen from EMR revealed an incidental finding of heterotopic pancreas with low-grade PanIN. Therefore, EMR enabled the diagnosis as well as the treatment in our case. EMR was attempted for our patient with successful resection of the lesion. To the best of our knowledge, this is the first case of heterotopic pancreas with low-grade PanIN in the duodenum to be incidentally diagnosed and treated with EMR.
Learning points.
Ectopic or heterotopic pancreas is the presence of pancreatic tissue outside its normal location and can be at risk for adenocarcinoma or its precursor lesions.
Diagnosis remains challenging and requires histopathological examination.
Treatment is determined based on the size and location of these lesions.
Surgical resection has been the only option in the past, but now EMR might offer an alternative management approach for selected lesions.
Footnotes
Contributors: SS, DRM and TR have all contributed significantly to planning and writing this case. TR performed the endoscopic procedure, DRM provided the pathology images, and all authors drafted and revised the article. They all agreed on the final version submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bini R, Voghera P, Tapparo A, et al. Malignant transformation of ectopic pancreatic cells in the duodenal wall. World J Gastroenterol 2010;16:1293–5. 10.3748/wjg.v16.i10.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DU, Lubner MG, Mellnick VM, et al. Heterotopic pancreatic rests: imaging features, complications, and unifying concepts. Abdom Radiol 2017;42:216–25. 10.1007/s00261-016-0874-9 [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Sanderson SO, Lloyd RV, et al. Pancreatic intraepithelial neoplasia in heterotopic pancreas: evidence for the progression model of pancreatic ductal adenocarcinoma. Am J Surg Pathol 2007;31:1191–5. 10.1097/PAS.0b013e31806841e1 [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Cho BS, Park JS, et al. Premalignant lesion of heterotopic pancreas combined with gastritis cystica profunda in gastric fundus. J Gastrointestin Liver Dis 2013;22:337–40. [PubMed] [Google Scholar]
- 5.Eisenberger CF, Gocht A, Knoefel WT, et al. Heterotopic pancreas–clinical presentation and pathology with review of the literature. Hepatogastroenterology 2004;51:854–8. [PubMed] [Google Scholar]
- 6.Jun SY, Son D, Kim MJ, et al. Heterotopic Pancreas of the Gastrointestinal Tract and Associated Precursor and Cancerous Lesions: Systematic Pathologic Studies of 165 Cases. Am J Surg Pathol 2017;41:833–48. 10.1097/PAS.0000000000000850 [DOI] [PubMed] [Google Scholar]
- 7.Gurocak B, Gokturk HS, Kayacetin S, et al. A rare case of heterotopic pancreas in the stomach which caused closed perforation. Neth J Med 2009;67:285–7. [PubMed] [Google Scholar]
- 8.Gokhale UA, Nanda A, Pillai R, et al. Heterotopic pancreas in the stomach: a case report and a brief review of the literature. JOP 2010;11:255–7. [PubMed] [Google Scholar]
- 9.Christodoulidis G, Zacharoulis D, Barbanis S, et al. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol 2007;13:6098–100. 10.3748/wjg.v13.45.6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mönig SP, Selzner M, Raab M, et al. Heterotopic pancreas. A difficult diagnosis. Dig Dis Sci 1996;41:1238–40. [DOI] [PubMed] [Google Scholar]
- 11.Yamao K, Sawaki A, Mizuno N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol 2005;40:1013–23. 10.1007/s00535-005-1717-6 [DOI] [PubMed] [Google Scholar]
- 12.Caletti GC, Brocchi E, Ferrari A, et al. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy 1991;23:251–4. 10.1055/s-2007-1010679 [DOI] [PubMed] [Google Scholar]
- 13.Kanayama K, Imai H, Yoneda M, et al. Cytological findings of an ectopic pancreas of the stomach obtained at endoscopic ultrasound-guided fine needle aspiration, differential diagnosis from acinar cell carcinoma: a case report. Cytopathology 2016;27:379–81. 10.1111/cyt.12302 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez FJ, Abraham SC, Allen MS, et al. Fine-needle aspiration cytology findings from a case of pancreatic heterotopia at the gastroesophageal junction. Diagn Cytopathol 2004;31:175–9. 10.1002/dc.20066 [DOI] [PubMed] [Google Scholar]
- 15.Jang KY, Park HS, Moon WS, et al. Heterotopic pancreas in the stomach diagnosed by endoscopic ultrasound-guided fine needle aspiration cytology. Cytopathology 2010;21:418–20. 10.1111/j.1365-2303.2009.00727.x [DOI] [PubMed] [Google Scholar]
- 16.Emerson L, Layfield LJ, Rohr LR, et al. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol 2004;87:53–7. 10.1002/jso.20087 [DOI] [PubMed] [Google Scholar]
- 17.Noë M, Brosens LA. Pathology of Pancreatic Cancer Precursor Lesions. Surg Pathol Clin 2016;9:561–80. 10.1016/j.path.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Takaori K, Hruban RH, Maitra A, et al. Pancreatic intraepithelial neoplasia. Pancreas 2004;28:257–62. 10.1097/00006676-200404000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003;16:996–1006. 10.1097/01.MP.0000087422.24733.62 [DOI] [PubMed] [Google Scholar]
- 20.Reid MD, Lewis MM, Willingham FF, et al. The Evolving Role of Pathology in New Developments, Classification, Terminology, and Diagnosis of Pancreatobiliary Neoplasms. Arch Pathol Lab Med 2017;141:366–80. 10.5858/arpa.2016-0262-SA [DOI] [PubMed] [Google Scholar]
- 21.György L, Gábor B, Fiore N, et al. [Pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasms (IPMN)]. Magy Seb 2006;59:12–19. [PubMed] [Google Scholar]
- 22.Yasuda K, Nakajima M, Kawai K. Endoscopic ultrasonography in the diagnosis of submucosal tumor of the upper digestive tract. Scand J Gastroenterol Suppl 1986;123:59–67. 10.3109/00365528609091864 [DOI] [PubMed] [Google Scholar]
- 23.Macedo FI, Taggarshe D, Makarawo T, et al. Pancreatic intraepithelial neoplasia arising from an ectopic pancreas in the small bowel. Hepatobiliary Pancreat Dis Int 2014;13:658–61. 10.1016/S1499-3872(14)60273-3 [DOI] [PubMed] [Google Scholar]
- 24.Papaziogas B, Koutelidakis I, Tsiaousis P, et al. Carcinoma developing in ectopic pancreatic tissue in the stomach: a case report. Cases J 2008;1:249 10.1186/1757-1626-1-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Yoshizawa N, Tomida H, et al. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc 2014;26(Suppl 2):50–6. 10.1111/den.12273 [DOI] [PubMed] [Google Scholar]


