Abstract
Introduction
Cerebral palsy (CP) is the most common childhood physical disability, with 80% estimated to be in low-middle-income countries. This study aims to (1) determine the accuracy of General Movements (GMs)/Hammersmith Infant Neurological Examination (HINE) for detecting CP at 18 months corrected age (CA); (2) determine the effectiveness of a community-based parent-delivered early intervention for infants at high risk of CP in West Bengal, India (Learning through Everyday Activities with Parents for infants with CP; LEAP-CP).
Methods
This study comprises two substudies: (1) a study of the predictive validity of the GMs and HINE for detecting CP; (2) randomised, double-blinded controlled trial of a novel intervention delivered through peer trainers (Community Disability Workers, CDW) compared with health advice (15 fortnightly visits). 142 infants at high risk of CP (‘absent fidgety’ GMs; ‘high risk score’ on HINE) aged 12–40 weeks CA will be recruited to the intervention substudy, with infants randomised based on a computer-generated sequence. Researchers will be masked to group allocation, and caregivers and CDWs naïve to intervention status. Visits will include therapeutic modules (goal-directed active motor/cognitive strategies and LEAP-CP games) and parent education. Health advice is based on the Integrated Management of Childhood Illness, WHO. Infants will be evaluated at baseline, post intervention and 18 months CA. The primary hypothesis is that infants receiving LEAP-CP will have greater scaled scores on the Pediatric Evaluation of Disability Inventory—Computer Adaptive Test (mobility domain) at 18 months compared with health advice. Secondary outcomes include infant functional motor, cognitive, visual and communication development; infant growth; maternal mental health.
Ethics and dissemination
This study is approved through appropriate Australian and Indian ethics committees (see in text) with families providing written informed consent. Findings from this trial will be disseminated through peer-reviewed journal publications and conference presentations.
Trial registration number
12616000653460p; Pre-results.
Keywords: community child health, public health, rehabilitation medicine
Strengths and limitations of this study.
This study is an adequately powered randomised double-blinded controlled trial of a novel parent-delivered early intervention for infants at risk of cerebral palsy in a low-middle-income country.
Outcomes are evaluated with standardised measures and evaluate a range of infant and family domains, including functional motor, cognitive, visual and communication developmental outcomes; infant growth and maternal mental health.
This is a pragmatic Randomised Controlled Trial; as such the potential contamination of other ‘care as usual’ interventions and other ‘real world’ factors may influence the data and its interpretation.
Introduction
One in seven people globally has a disability, forming the world’s largest and most disadvantaged minority.1 Cerebral palsy (CP) is among the most common childhood physical disabilities with epidemiological studies in low-middle-income countries (LMIC) estimating rates from 2.9 to 4.0 per 1000 live births,2 3 up to twice as common as estimates from high-income countries. With higher rates and larger populations, it is proposed that 80% of CP cases globally are in LMICs, where individuals and their families are frequently trapped in the negative downward cycle of disability and poverty.4 Individuals with a disability and their families in LMIC have increased rates of premature mortality and associated morbidities and are economically disadvantaged by productivity loss, costs of interventions and equipment as well as consequences of social stigma.1 Significant gains have been made in the past decade in reducing infant mortality in LMIC.5 With a renewed global international development strategy pledged by the United Nations Sustainable Development Goals (2015–2030), it is pertinent to broaden attention from infant survival and to improved quality of life and developmental outcomes for children with a disability.6
In many LMICs, there is a shortage of specialised health workers to attend to the often larger populations and greater disease/disability rates.4 7 A recent multisite early intervention study (spanning Africa and Asia) for infants with birth asphyxia advocated for the need to train non-professional individuals to address this workforce gap.7 Lay health workers have been used as effective change agents across Asia, sub-Saharan Africa and Latin America, to improve outcomes for both communicable and non-communicable diseases.8 The current project aims to adapt this model for an early disability intervention for infants at risk of CP. The Indian National Rural Health Mission has been instrumental in establishing a community health worker programme to meet primary health needs in both urban and rural areas across the country (known as USHA/ASHA, Urban/Accredited Social Health Activists).9 West Bengal is an easterly state in India and with a population of 90 million people is considered one of the most densely populated geographies in the world. These factors collectively make West Bengal an important location for piloting and implementing innovative interventions and service delivery models that are highly scalable and transposable for disability intervention in other LMICs.
Recently published International Clinical Practice Guidelines advocate for CP detection from as young as 3 months using the General Movements (GMs) Assessment,10 11 and there is growing evidence to support the effectiveness of early cognitive and early active motor interventions in the first year of life for infants at high risk of CP.12 13 Despite this state of science, many children in LMICs only receive their diagnosis at the age of school entry, missing a significant window of opportunity for improved outcomes.14 Children with CP in these settings also face economic, geographical and social barriers to accessing medical and rehabilitation interventions.1 15 16 In addition to the impact of disability on the child, there is strong evidence of higher prevalence of poor mental health in mothers of children with CP,17 for which there are demonstrated improvements consequent to early intervention for their child.18 In order to impact on the social inclusion and workforce productivity of individuals with a disability in these contexts, it is essential to first establish innovative, accessible and feasible means to detect infants at risk of CP and subsequently develop early intervention programmes that are accessible for families of high-risk infants in these settings, are cost-effective and can be widely delivered. The GMs Assessment and Hammersmith Infant Neurological Examination (HINE) are two gold standard early detection tools based on systematic review, with demonstrated predictive validity for identifying later CP (GMs sensitivity: 95%–100%, specificity: 96%–98%; HINE sensitivity: 90%–96%, specificity: 85%–91%).11 19 Both have simple administration (the GMs a 3 min video taken on a smart phone, the HINE a truncated 26-item neurological examination) which we believe can be feasibly administered in the community context in LMIC and scored by expert certified raters.20 A community-based intervention of active goal-directed strategies and environmental enrichment delivered peer to peer in the home also presents a viable solution for accessible and scalable intervention in this context. The aims of this study are twofold; to determine the predictive validity of a community-based early detection programme for infants at risk of CP in LMIC and to determine the effectiveness of a home-based peer delivered early intervention.
Methods and analysis
Study design
This study consists of two substudies: (1) a study of the validity of an early community-based detection programme for identifying infants at high risk of CP and (2) a randomised, double-blinded controlled trial of a novel intervention (LEAP-CP: Learning through Everyday Activities with Parents for infants at high risk of Cerebral Palsy) compared with health advice (standard care). Field work will be conducted from March 2017 to March 2019.
Aims and hypotheses
Early intervention for infants at high risk of cerebral palsy in low-middle-income countries
-
To determine the effectiveness of a community-based parent-delivered intervention on infant’s developmental outcomes for those at high risk of CP.
Hypothesis 1: Infants with CP who receive the LEAP-CP intervention will have higher scores on the mobility domain of the Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) compared with infants of caregivers receiving health advice.
-
To determine the effectiveness of a community-based parent-delivered intervention on caregiver’s mental health outcomes.
Hypothesis 2: Caregivers who receive the LEAP-CP intervention will have reduced depression and anxiety scores compared with caregivers receiving health advice.
Early detection of infants at high risk of CP in LMIC
To determine the predictive validity of GMs assessment administered at 12–17 weeks for detecting CP at 18 months in high-risk infants in West Bengal.
-
To determine the predictive validity of the HINE when administered from 18 to 40 weeks for detecting CP at 18 months in high-risk infants in West Bengal.
Hypothesis 3: The GMs and HINE assessments will have predictive validity equivalent to that in high-income countries to detect CP at 18 months.
Recruitment
Participants
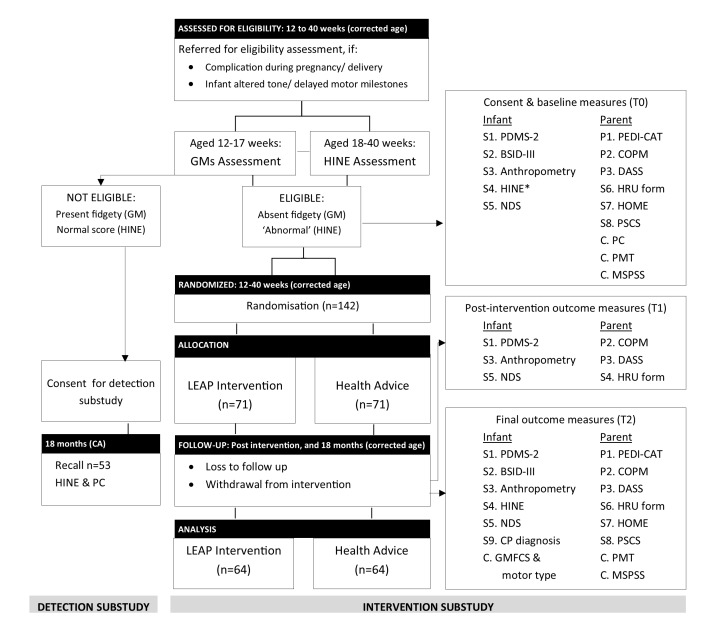
A total of 142 infants at high risk of CP aged 12–40 weeks corrected age (CA) will be recruited to the intervention trial. Infants will be screened until the target recruitment for the intervention substudy is achieved (recruitment pathways are shown in figure 1). The caregiver and their infant will be referred to the detection substudy by health professionals or Community Health Workers, from regional and tertiary hospitals, community health centres, routine immunisation clinics or in the community. Three implementing partner organisations were selected based on their existing expertise in the field of childhood disability, community-based maternal child health or neonatology. Corresponding geographical areas were selected based on the geographical catchment of the three partner organisations.
Asha Bhavan Centre is a community-based non-government (non-profit) organisation providing multidisciplinary rehabilitation and support to underprivileged children with a disability in rural West Bengal. The catchment for this site is Uluberia I, Bagnan II, Shyampur II (Howrah District), reaching a population of 575 961 and 240 villages (Census of India 2011).21
Child In Need Institute is a community-based non-government (non-profit) organisation that works in deprived communities to improve maternal and child nutrition, health, education and rights in urban and rural Kolkata. The catchment for this site is Ward 56, 57, 58, 65, 66 (Kolkata Municipality). This is a highly transitory population with high rates of urban migration from communities across India. Owing to this, accurate population estimates are difficult to ascertain, but officially recorded as 352 394, with majority living in slums.
Dr BC Roy Postgraduate Institute of Paediatric Sciences is a government hospital providing specialist paediatric services (including a 200 bed neonatal intensive care unit) for the 24 Parganas rural district in West Bengal. This site primarily targets a 1-hour radius surrounding the communities of Rajarhat, Berachampa, Ghatakpukur, Barisat and Bongaon (24 Parganas Districts).21
Figure 1.
CONSORT flowchart for infants in the LEAP-CP Study. *If this has not been administered for eligibility assessment. BSID, Bayley Scales of Infant Development; C, covariate; COPM, Canadian Occupational Performance Measure; CP, cerebral palsy; DASS, Depression, Anxiety, Stress Scale; GM, General Movements; GMFCS, Gross Motor Function Classification System; HINE, Hammersmith Infant Neurological Examination; HOME, Home Observation for Measurement of the Environment; HRU, Health Resource Use; LEAP, Learning through Everyday Activities with Parents; MSPSS, Multidimensional Scale of Perceived Social Support; NDS, Near Detection Scale; P, primary measures; PC, Physician Checklist; PDMS, Peabody Developmental Motor Scales; PEDI-CAT, Pediatric Evaluation of Disability Inventory—Computer Adaptive Test; PMT, Poverty Measurement Tool; PSCS, Parenting Sense of Competence Scale; S, secondary measures; T0, baseline; T1, postintervention; T2, final outcome.
Inclusion/exclusion criteria
To participate, infants must live in one of the study geographical areas and be 12–40 weeks CA. Infants with known or suspected congenital or chromosomal abnormalities which are likely to affect their neurodevelopmental outcome; those diagnosed with neurodegenerative conditions and those that are considered medically fragile will be excluded. Infants must also have one or more risk factors:
Maternal infection (antenatal).
Low birth weight (<2.5 kg).
Preterm delivery (<37 weeks).
Hypoxic ischaemic encephalopathy.
Perinatal asphyxia.
Neonatal jaundice requiring treatment.
Prolonged hypoglycaemia.
Seizures after birth.
Admission to neonatal intensive care unit or special newborn care unit.
Postneonatal complications in infant (infection, head injury, near drowning).
Altered tone or delayed motor milestones for the infant.
To be eligible for the intervention substudy, infants must be assessed in the detection substudy to be at ‘high risk’ of CP based on the GMs or HINE, as follows:
At 12–17 weeks CA infants with absent/abnormal fidgety movements on GMs assessment are considered high risk.22 The diagnostic accuracy of the GMs has been well documented, with sensitivity ranging from 95% to 100% and specificity 96% to 98%19; with the measure also shown to have robust reliability (inter-rater kappa=0.91–0.92, average agreement 90%).23 GMs will be videoed by trained Community Coordinators/Community Disability Workers (CDWs) and videos rated independently by two GMs Trust accredited scorers, of whom there are >20 advanced and >100 basic raters in India alone. In the case of disagreement, a third accredited rater will provide consensus.
At 18–40 weeks, CA infants scoring below the established HINE cut-points will be considered high risk of CP (<56 points at 3 months (SE 96%; sp 85%), <59 points at 6 months (SE 90%; sp 89%), <62 points at 9 months (SE 90%; sp 91%).24 The HINE has been shown to have excellent intrarater and inter-rater reliability for the global scores (Intra-Class Corrleation Coefficient=0.97 for both) when administered to high-risk 12-month-old infants in India.25 The scoring form provides simple instructions for administration and scoring and is accessible for all clinicians, regardless of experience.26 All HINE examinations will be videoed and conducted by trained Community Coordinators (Indian trained therapists). Videos will be rated independently by two HINE trained scorers. In the case of disagreement (ie, of ‘high risk’ status), a third rater will provide consensus.
Sample size calculation
Detection substudy
The primary hypothesis of the detection substudy is that the GMs and HINE will have equivalent predictive validity to that in Western literature. With estimates of the GM sensitivity=0.95 and specificity=0.96,19 a total of 19 infants identified as high risk of CP and 15 identified as low risk of CP (ie, rated present fidgety on the GMs) will be needed to demonstrate equivalent diagnostic accuracy to 10% precision either side (and 95% confidence). Similarly, with estimates of the HINE sensitivity=0.90 and specificity=0.89,19 a total of 35 infants identified as high risk of CP and 38 infants identified as low risk of CP (ie, ‘normal’ scores using the HINE) will be needed to demonstrate equivalent diagnostic accuracy to 10% precision either side (and 95% confidence).
Intervention substudy
The primary hypothesis of the intervention substudy is that infants receiving the LEAP-CP intervention will have significantly greater scaled scores on the mobility domain of the PEDI-CAT at 18 months compared with those receiving health advice. The PEDI-CAT mobility domain has been shown to detect a 1SD change in scaled scores following 3 or more months of physiotherapy in young children (aged 1 month–3 years).27 With 64 children per group, a difference of at least 0.5SD between groups will be detectable (α=0.05, β=0.8). Accounting for 10% attrition, this equates to 142 infants (71 in each group).
Detection substudy
Infants who have been screened as ‘low risk’ on the GMs and HINE will not be eligible to participate in the intervention substudy. A random sample of 53 infants with negative case status on the screening tests at 12–40 weeks CA (15 GMs screened, 38 HINE screened) will be contacted when the child is 18 months CA for CP diagnosis (as described in the outcome measures). Infants screened as ‘high risk’ on the GMs or HINE will be invited to participate in the intervention substudy and CP diagnosis provided at 18 months CA. Data from all 142 case infants will be used to calculate sensitivity.
Intervention substudy
The LEAP-CP intervention is a multidomain family-centred best practice intervention consisting of infant goal-directed therapeutic strategies and learning games and caregiver educational modules. It is based on effectiveness shown in systematic reviews13 28 29 and early intervention trials.30–33 In the development phase of LEAP-CP, in-depth consultation was held with the developers of GAME (Goals-Activity-Motor Enrichment). LEAP-CP includes many of the key ingredients of GAME but adapted for low-income settings and have been used with permission. The components shown necessary for effective interventions for infants with CP include (1) goal-directed tasks; (2) home-based delivery and include (3) active motor learning and (4) strategies to enrich the home environment. LEAP-CP is based on principles of parent coaching which promote caregiver problem solving and self-determination. Specifically, LEAP-CP includes:
Activity-based motor and cognitive skills training, based on goals identified by parents.28–30 Practice is structured using motor-learning principles of repetition and variation.34 Functional motor skills, such as reach/grasp and attaining independent mobility, will be coached and parents given visual supports (photo/video) for ongoing practice through the week.
Enrichment, which facilitates enhanced cognitive, motor and multisensory learning (eg, visual and auditory), will be encouraged within the home environment using resources based on the Abecedarian Learning Games curriculum modified for CP and adapted for the context.28 35 36 The Abecedarian approach has strong empirical evidence from >16 Randomised Controlled Trials (RCTs) in at-risk children.35 This includes early play-based learning and literacy activities and promotes use of materials readily and cheaply available in the home and community.
The parent educational modules (table 1) are evidence-based discussion topics which cover three broad areas: (1) ‘learn’—enabling active play and learning for babies with CP37; (2) ‘grow’—feeding, nutrition (breastfeeding, complementary feeding, balanced diet) and health38; (3) ‘love’—caregiver mental health based on acceptance and commitment therapy and responsive parenting.31
Table 1.
Parent educational modules in the LEAP-CP programme
| Learn28 73–75 | Grow38 53 76 77 | Love73 78 79 |
|
|
|
*Infants who are not breastfeeding at the time of this module will receive education discussing hydration.
LEAP-CP, Learning through Everyday Activities with Parents-Cerebral Palsy.
Dose
The LEAP intervention will commence at 3–9 months CA at a dose of 20 min per day for 5 days per week (1.6 hours) up to 6 months CA (total dose 19.2 hours); then graduate to 30 min per day for 5 days per week (2.5 hours per week) from 6 to 9 months CA (total 30 hours); then 40 min per day for 5 days per week (3.3 hours per week) from 9 to 12 months CA (total 40 hours). In addition, there will be approximately 15 hours of direct intervention administered during home visits by either the parent or CDW. The overall dose will be 104.2 hours for the entire intervention up to 18 months CA.
Peer to peer service delivery
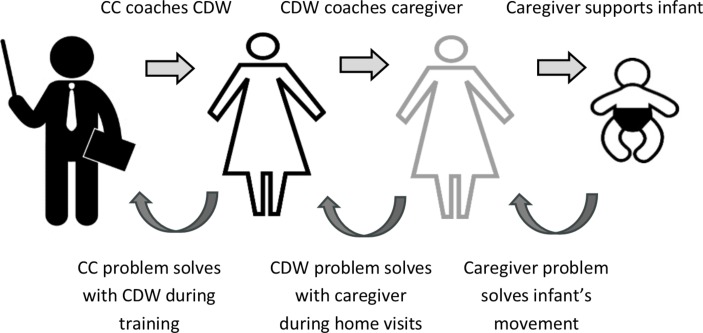
The service delivery model for each geographical catchment is represented in figure 2. The programme adopts an iterative coaching model as represented visually in figure 3. The Community Coordinator (health professional) oversees each site and coaches the CDW on new goals and activity targets for the infant at a fortnightly training session (forward loop) as well as providing supported problem solving of previous goals and activities via video recorded sessions (back loop). CDWs are peer trainers from their local communities, with priority for employment given to mothers of a child with a disability. CDWs will coach the caregiver (or other significant people in the infant’s life) on the goal or activity target at fortnightly home visits (forward and back loops). Caregivers are the primary change agent for their infant.
Figure 2.
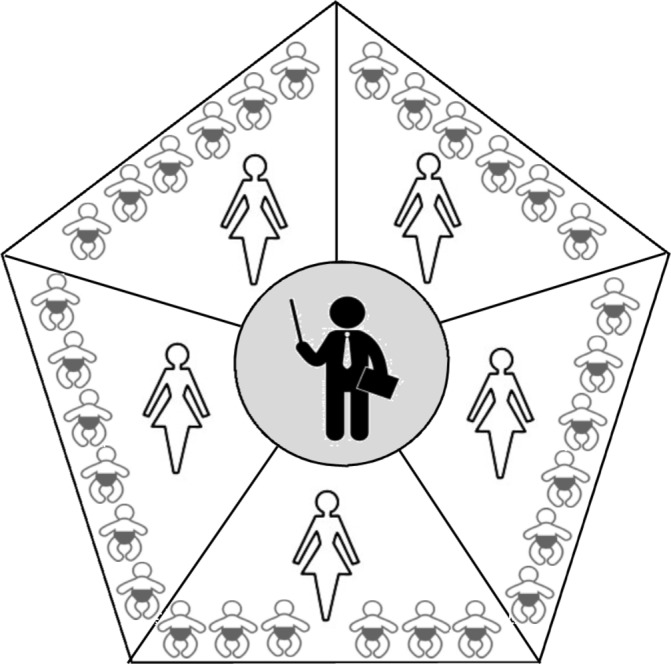
Service delivery model for LEAP-CP Study. Infographic represents one geographical area, with centralised community coordinator who trains approximately five Community Disability Workers, each working with approximately six infant-caregiver dyads. LEAP-CP, Learning through Everyday Activities with Parents-Cerebral Palsy.
Figure 3.
Coaching model for supporting goals in the LEAP-CP Study. CC, Community Coordinator; CDW Community Disability Worker; LEAP-CP, Learning through Everyday Activities with Parents-Cerebral Palsy.
The CDWs will receive a 3-day training package at the onset of the programme (with the Chief Investigator, an Australian Government funded Post-Doctoral Fellow and speech pathologist). This will include topics such as:
Building rapport and a positive therapeutic relationship with caregivers.
Exploring customs, beliefs and family culture.
Using everyday opportunities and routines to encourage infant development.
Observation skills and coaching.
Motor training and therapeutic principles.
Understanding typical development and development in children with CP.
Ethics and research practices.
Health advice (standard care)
The health advice is based on the WHO’s Integrated Management of Childhood Illness Key Family Practices.39 This includes counselling on breastfeeding and introduction of complementary nutrition, hygiene practices, vaccination counselling and management of the sick child. It also includes clinical signs indicating the need for referral to existing health services. It was considered necessary to provide nutrition and health advice to all families in the study, as undernutrition in conjunction with CP has been shown to increase mortality within this context.40 The same service delivery model and visiting schedule will be used as for the intervention arm (a fortnightly home visit for 15 visits), with a different CDW visiting standard care group families to avoid contamination. There will not be a direct intervention dose delivered to infants in this study arm.
Concurrent therapies (care as usual)
Infants from both study arms are able to continue to access medical and therapy support as per their family’s preference. Frequency and duration of access to local medical/therapy services will be recorded fortnightly on the Health Resource Use Form and included in the analysis.
Randomisation and blinding
Infants will be randomised to the LEAP-CP intervention arm or health advice (standard care) arm using simple randomisation based on computer-generated sequences, generated and stored centrally. If twins are both eligible, they will be randomised as a single family unit. Allocation concealment will be ensured by using the REDCap V.8.0.2 database (Vanderbilt University, USA 2009) for group assignment at the time of randomisation. The clinician completing the eligibility assessment will be unaware of group allocation. Caregivers receiving the intervention and CDWs administering the intervention will be naïve to intervention status. Researchers assessing the outcomes and analysing the data will be masked to group allocation.
Fidelity
Intervention fidelity will be monitored at each level of programme delivery, including its delivery to the community coordinator, their delivery to the Community Disability Worker, the CDW delivery to the caregiver and finally implementation of the intervention with the infant (table 2). These levels of fidelity will be monitored to ensure consistency within and across intervention sites for (1) study design (active ingredients); (2) training providers; (2) delivery of treatment; (4) receipt of treatment; (5) patient enactment.41
Table 2.
Fidelity evaluation plan for the LEAP-CP trial
| Data source | Monitoring frequency | Components of fidelity | ||||
| Study design | Training providers | Delivery of treatment | Receipt of treatment | Patient enactment | ||
| Coordinator to CDW | ||||||
| CDW training: 15 times per cycle, 2–3 hours | Fortnightly | X | ||||
| CDW training checklist: written record of content delivered at each CDW training session | Fortnightly | X | ||||
| CDW training log: all training is audio-recorded and a random selection rated | Quarterly | X | X | |||
| CDW to caregiver | ||||||
| Home visit: 15 visits per child, 2 hours | X | X | X | |||
| Progress notes: written record of content delivered at each home visit | Fortnightly | X | X | |||
| Home visit observation* | 3/programme | X | X | X | ||
| Caregiver to infant | ||||||
| Intervention dose (calendar or knotted string method, as per literacy—a knot/mark representing 10 min of intervention) | 3/programme | X | ||||
| Video-recorded 10 min activity observation (Caregiver-infant) | 3/programme | X | ||||
*Checklist based on fidelity tool developed by Sakzewski, Boyd and Ziviani for the REACH trial.80
LEAP-CP, Learning through Everyday Activities with Parents-Cerebral Palsy.
Adverse events
Any serious adverse events such as injury, prolonged hospitalisation or mortality occurring during programme delivery will be monitored by the Data Safety Monitoring Representative, a non-treating senior medical professional from the Indian context. They will review study retention, compliance/quality of treatment and monitor any adverse or unintended effects on a 12 monthly basis and advise the Chief Investigators regarding whether the adverse events are likely related to the intervention provided in the trial.
Outcome measurement
Baseline, postintervention and final outcome assessments (at 18 months CA) will be conducted by an assessor masked to intervention status, as shown in figure 1. If the postintervention outcome is within 1 month of the final outcome, this assessment point will be excluded. All questionnaires will be translated to Bengali to ensure a single consistent translation is used. All written translations have been back-translated (English to Bengali and back) to ensure translation accuracy.
The literature lacks consensus for the use of Western developmental norms in an Indian/Asian population. Some suggest that cognitive and physical milestones are appropriate, but that self-care skills such as toilet training, washing and dressing are considered to have greater cultural determination.42 Others propose that using western norms will significantly overestimate delayed development in socialisation and motor domains, but underestimate delay for communication and daily living skills.43 Raw scores will be compared in secondary analysis to account for these possible cultural and ethnic differences from standard scores.
Primary infant outcome measures
The infant’s functional outcomes will be assessed using the Pediatric Evaluation of Disability Inventory-Computer Adaptive Test (PEDI-CAT). This is a parent-reported measure of their child’s independence in self-care, mobility and social function (aged birth–20 years).44 The PEDI-CAT has been Rasch-analysed in both children with disabilities and those with typical development. The computerised adaptive version, based on Item-Response Theory, has been shown to increase accuracy and efficiency of administration.45 The raw scores will be converted to standardised scores using normative data (0–100) to measure change in function. The PEDI has been used in non-Western cultural contexts46 and has undergone cultural adaptation in conjunction with the present study based on the PEDI-CAT author’s guidelines (with n=13 Bengali clinicians, results not yet published). Most items relevant to children aged less than 18 months required minimal modification. The mobility domain was selected as the primary outcome owing to CP being primarily a physical disability and parents’ emphasis on their child’s physical development during the first year. The PEDI-CAT is administered using computer-based software (available on PC or iPad) through an annual license. No additional training or kits are required.
Secondary infant outcome measures
The Canadian Occupational Performance Measure (COPM) will be used to assist caregivers in setting and prioritising goals and measuring parent-perceived change of their infant’s performance of the goal and their own satisfaction with progress. This assessment will be administered by the Community Disability Worker (trained in its administration) in a semistructured interview as part of Educational Module 2 (goal setting). Postintervention assessment will be administered by an independent rater at 18 months CA. The COPM is administered using either a paper-based on online form, with training videos for its administration available online.
The infant’s motor outcomes will be assessed using the Peabody Developmental Motor Scales—Second edition (PDMS-2), a commonly used measure of motor skills in infants and children aged birth to 6 years. It has demonstrated validity (discriminative and concurrent with the Bayley47 and Gross Motor Function Measure48) and responsiveness in infants with CP.49 50 The PDMS-2 requires a semistandardised manipulatives kit and standard forms for its administration and should be administered by an allied health professional or special education professional.
The infant’s cognitive and communication outcomes will be assessed using the Bayley Scales of Infant Development III (BSID-III), the gold standard norm-referenced assessment of infant development (0–3 years).51 The BSID-III requires an extensive kit of manipulatives and standard forms, with test administrators with a high level of expertise in standardised testing.
Near Detection Scale is a 10-point vision assessment of visual fixation on graded standardised lures viewed at near distance (30 cm), ranging from no light perception (0) to 1.2 cm ‘lure’ (yellow candy presented on a dark green/black cloth) (10).52 This screener is simple and fast and requires no additional training.
Nutritional status will be determined using length/height and weight which will be converted to z scores using the WHO age and gender referenced data.53 Head circumference and mid-upper arm circumference will also be recorded.
The Health Resource Use Form will provide health usage outcome data as well as being included as a covariate in analyses. It was developed for a large population-based study in Australia54 and has been previously modified and used by our team in Bangladesh.55
Hammersmith Infant Neurological Examination (HINE) will be used to assess infant neurological status and CP severity. The HINE at 3–6 months has been shown to have strong and significant correlations with the GMFCS at 2 years.56 For infants who had the HINE administered for their eligibility assessment, only a postintervention HINE will be administered.
Home Observation for Measurement of the Environment (HOME) Inventory: Infant and Toddler Version is a measure of the quality and quantity of parent and home stimulation, covering six domains of parent responsivity, acceptance and involvement and the home physical environment including availability of learning materials and variety of stimulation.57 The HOME has been used in contexts of disability and low-income countries, including Bangladesh.58 59–61
Parenting Sense of Competence Scale (PSCS) is a commonly used measure of perceived parenting competence and self-esteem (Gilbaud-Wallston and Wanderson, 1978, cited in62). It consists of 17 items rated by parents on a 6-point response scale from ‘strongly agree’ to ‘strongly disagree’.
Differential diagnosis of cerebral palsy at 18 months CA will be provided by an Australian qualified paediatrician according to published guidelines,63 based on clinical history (on the Physician Checklist) and videoed HINE and Gross Motor Function Classification System (GMFCS) semistructured play session. Children will be classified as ‘definite CP’, ‘suspected CP’ or ‘no CP’. This method has been used in our previous research.55 Confirmed or suspected diagnoses other than CP will be identified by the physician, based on the clinical examination and medical history.
Primary caregiver outcome measure
Caregiver outcomes will be assessed using the Depression, Anxiety, Stress Scale—Short Form (DASS), a self-reported norm-referenced measure of depression, anxiety and stress.64 An official Bengali translation is available on the measure website.65
Covariates and descriptive measures
Physician checklist (PC) was developed for a large population-based study in Australia54 and used by the CIA in Bangladesh.55 It gathers birth and developmental history from the caregiver. Questions include preterm status, birth complications, presence of seizures and medications.
GMFCS is a five-level classification of children’s functional gross motor function. The <2 year old age band will be used to classify gross motor function at the completion of the study (18 months CA).66 This will be classified by an Australian physiotherapist from video, as per our previous work in Bangladesh.55
Motor type (spasticity, dyskinesia, hypotonia) and distribution (number of limbs) will be classified by an Australian physiotherapist from video according to the Surveillance of CP in Europe.67 This methodology has been used in this research team’s previous research in Bangladesh.55
Poverty Measurement Tool will provide a measure of poverty/economic status.68 This scale was developed in rural Bangladesh to provide a measure of poverty, defined as ‘inadequate fulfilment of basic needs, such as food, clothing, shelter, health, education and social involvement’. Scores range from 24 to 72 with increasing values indicating increasing poverty. The scoring cut-points were validated against wealth rankings of households using participatory rural appraisal methods. It has excellent test-retest reliability and strong internal consistency.
Multidimensional Scale of Perceived Social Support (MSPSS) measures caregiver’s cognitive social capital, defined as a subjective measure of what people feel, such as those of trust and reciprocity.69 The MSPSS is a 12-item scale with four items for each source of support, with items rated on a seven-point scale. The measure has good cross-cultural stability, strong internal consistency when tested in a range of samples in a developing country and was significantly associated with two measures of depression and anxiety (the Beck Depression Inventory and the State-Trait Anxiety Inventory).70
Statistical analysis
Study data will be collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted The University of Queensland.71 REDCap is a secure, web-based application designed to support data capture for research studies. All analyses will be undertaken using Stata V.13.1 (Stata, College Station, Texas, USA) with significance set at p<0.05. Professor Robert Ware, lead biostatistician, will provide expert input for the analysis.
Detection substudy
The predictive validity of the GMs and HINE will be tested using receiver operating characteristic curves, and sensitivity and specificity calculated. This will be based on the positive/negative ‘high risk of CP’ status on each screening tool (at 12–40 weeks CA) compared with definite or suspected CP/no CP clinical diagnosis at 18 months CA.
Intervention substudy
Analyses will adhere with the standard principles for an RCT, primarily focusing on differences in outcome between intervention and standard care arms. Intention-to-treat analysis will be undertaken. A generalised linear model will be run to compare the primary outcome (PEDI-CAT mobility domain score) between intervention groups. The model’s main effects will be intervention group and time (baseline/end of intervention/end of study) and a group-by-time interaction will be included. A generalised linear model will also be run to compare the primary caregiver outcome (DASS) between intervention groups. Again, intervention group and time will be included as main effects as well as a group-by-time interaction. First, the full data set will be analysed and then analyses rerun after stratifying using the covariable of neurological severity (binary HINE score <40).72 The distributional family will be Gaussian and the identity link will be used. Secondary analyses will use similar methods to compare outcomes at 18 months, considering differences in goal attainment (COPM), motor development (PDMS-2), cognition/communication (BSID-III Scales), growth (anthropometry), caregiver outcomes (PSCS) and health economics. Skewed continuous data will be transformed, and when indicated, non-parametric tests used.
Validity of results will be determined using baseline and descriptive data, including systematic differences between those completing the intervention and drop-outs. Sensitivity analyses will be conducted using multiply imputed data sets to investigate the effect of possibly differential drop-out, as appropriate.
Patient and public involvement
This study was conceptualised directly from the author’s (KAB) engagement with children with CP and their families living in Bangladesh. Families were predominately rural-based from across all districts in Bangladesh and attending the Paediatric Unit at the Centre for the Rehabilitation of the Paralysed (Savar, Bangladesh). The service delivery model drew on both formal discussions regarding barriers restricting access to therapy for these families as well as informal observations of families attending direct centre-based therapy programmes. It also builds on findings from the distance learning package trial (NZK).
The CDW employed as project staff are all mothers residing in the same communities as the participants. Four of these CDWs are mothers of a child with a disability, and one worker herself, a person with a disability. These women are all actively involved in the everyday recruitment and conduct of the study and represent the user’s voice. An info-graphic summary of the key findings of the study will be developed at the conclusion of the study and distributed to participating families by their CDW.
Outcomes and significance
The proposed study is expected to result in a number of important outcomes spanning several tiers of society, including child, caregiver/family and health systems. Studies in LMIC have shown positive effects of early interventions for the general population and other high risk groups (eg, low birth weight) with significant improvements in children’s cognition, language and social-emotional development. It is anticipated that the proposed intervention package, designed specifically for infants at risk of CP, will result in significant improvements in their language, cognition, mobility and activities of daily living. Consequently, these changes to children’s developmental trajectories are likely to improve their participation in education, employment and community. By reducing the average age of CP detection to the first year of life, high-risk infants are expected to have greater improvements to brain development and motor/cognitive outcomes. The detection programme is also anticipated to better direct limited health resources to those that are at an increased risk of later diagnosis. Early interventions for infants with a disability have also been associated with reductions in maternal anxiety and depression.18 In a country where women are often more socially isolated, which is further compounded by the stigma of having a child with a disability, such improvements in maternal mental health are expected to be significant for families and communities. By empowering mothers as disability resource champions in their local communities, the intervention is also expected to have a lasting and far-reaching benefit, beyond the duration of the study. South Asia has been at the heart of the Community Health Worker model development, which has been subsequently up-scaled around the globe. Building on this model, of up-skilling community members with varied levels of formal/technical education to deliver community-based healthcare, the proposed project is expected to result in a cost-effective and feasible model of care for infants with cerebral palsy that is highly scalable and transposable to other LMICs.
Ethics and dissemination
This study is registered with the Australia and New Zealand Clinical Trials Register (12616000653460 p). A two-stage consent process will be adopted for this study; caregivers will first provide informed consent for the eligibility assessment (detection substudy), and subsequently, those who are eligible for the intervention (‘high risk of CP’) will then provide informed consent for the clinical trial (intervention substudy). Participant data will be stored and managed according to universal privacy and confidentiality standards. There are no known risks associated with the interventions, and both interventions (LEAP-CP and health advice) are anticipated to provide benefit to the infant and their family. Findings from this trial will be disseminated through peer-reviewed publications and at national and international conference presentations.
Strengths and limitations
This study is an adequately powered randomised double-blinded controlled trial of a novel parent-delivered early intervention for infants at risk of CP in LMICs. Eligibility has been determined with gold standard tools (the GMs and HINE), according to an International Clinical Practice Guideline,11 to ensure infants who are likely to have a later diagnosis of CP are targeted. Outcomes are evaluated with standardised measures and evaluate a range of infant and family domains, including functional motor, cognitive, visual and communication developmental outcomes, infant growth and maternal mental health.
As far as possible, this study adheres to guidelines for the conduct of an RCT and is expected provide valid results to test the stated hypotheses. However, this is a pragmatic RCT performed in a vulnerable population and as such certain confounders are present which should be considered when interpreting the findings. All children, regardless of study arm, are able to access care as usual in their community, which may dilute or influence the findings. To control for this, fortnightly recording of the infant’s access to concurrent therapies is completed. Furthermore, as children in LMIC may have limited access to care as usual, it was considered necessary to provide nutrition and health support to all families in the study (with equivalent service delivery model and frequency for both study arms). This additional service provided to the standard care arm beyond ‘standard care’ may reduce the magnitude (or presence) of an effect between groups. Being a novel multidomain study, it is expected that the LEAP-CP intervention may impact on a range of infant and parent outcomes. While the motor domain on the PEDI-CAT was selected as the most meaningful primary infant outcome, gains on the many secondary outcomes will be considered valid in demonstrating the effectiveness of the intervention. At the conclusion of this study, it is expected that the effectiveness of the LEAP-CP programme will be determined, and as such, provide clinicians working in LMIC with the necessary information to inform the optimal management of infants with CP in LMIC.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the invaluable contributions of the implementing partner organisations for the LEAP-CP trial. Specifically, the teams at the Child In Need Institute (Ms Monidipa Ghosh and Ms Shirin Parveen); Asha Bhavan Centre (Mr Johnmary Barui, Ms Mohua Manna); Dr BC Roy Postgraduate Institute of Pediatric Sciences (Mr Pradip Maiti, Mr Debasis Gantait). We would also like to acknowledge the contribution of Apollo Gleneagles Hospital as the host of the Endeavour Scholarship (Mr Jewel Chakraborty) and the Indian Institute of Cerebral Palsy as research partner (Dr Reena Sen, Mr Sayak Chowdhury). Finally, we acknowledge the support of the Queensland Cerebral Palsy and Rehabilitation Research Centre clinical research team (Ms Bernadette Shannon, Ms Kym Morris, Ms Christine Finn, Ms Carly Dickinson, Dr Joanne George) for their clinical inputs and role as advanced General Movements raters.
Footnotes
Contributors: KB conceptualised the study, secured funding for the study, drafted the manuscript and approved the final manuscript as submitted. She is Chief Investigator overseeing all aspects of the project delivery in West Bengal. RNB conceptualised the study, secured funding for the study, provided critical review of the manuscript and approved the final manuscript as submitted. IN, CM and KW conceptualised the study, provided critical review of the manuscript and approved the final manuscript as submitted. RSW advised on statistical design of the study, provided critical review of the manuscript and approved the final manuscript as submitted. AS provided expert advice on the vision components of the programme, including intervention design and measurement. She provided critical review of the manuscript and has approved the final manuscript as submitted. KLB provided expert advice on the nutritional components of the programme, including intervention design and measurement. She provided critical review of the manuscript and approved the final manuscript as submitted. SB provided expert advice on the medical components of the programme, including intervention design and measurement. She provided critical review of the manuscript and approved the final manuscript as submitted. NZK provided expert advice on the cultural aspects of the programme for delivery in South Asia. She provided critical review of the manuscript and approved the final manuscript as submitted. AKG is overseeing the project delivery in West Bengal and provided expert advice on the cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted. AB hosted the Endeavour scholarship, is the study lead at Apollo Gleneagles Hospital and provided expert advice on the cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted. SS is the study lead at Dr BC Roy Hospital which is a referral site and implementing partner. He provided expert advice on the cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted. GM is the study lead at Asha Bhavan Centre which is an implementing partner. He provided expert advice on the cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted. DB is the study lead at Child In Need Institute which is an implementing partner. He provided expert advice on the cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted. ST is the Data Safety Monitoring Representative and provided critical input into the study design and cultural aspects of the programme for delivery in West Bengal. He provided critical review of the manuscript and approved the final manuscript as submitted.
Funding: This work was supported by the Cerebral Palsy Alliance Project Grant (PG6916); Queen Elizabeth II Diamond Jubilee Postdoctoral Scholarship, Endeavour (KB), Australian Commonwealth Government; NHMRC Fellowship (RB); NHMRC Centre for Research Excellence (Australasian Cerebral Palsy Clinical Trials Network).
Competing interests: ST is the Data Safety Monitoring Representative and also a member of the Apollo Gleneagles Hospital Ethics Committee.
Patient consent: Not required.
Ethics approval: Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/16/QRCH/214), The University of Queensland Medical Research Ethics Committee (2016001073), the Apollo Gleneagles Hospital Kolkata Institutional Ethics Committee (IEC/2016/12/35), Dr BC Roy Postgraduate Institute Institutional Ethics Committee (BCH/ME/PR/54).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This is a study protocol only and as such no unpublished data are available.
References
- 1. World Health Organization, The World Bank. World report on disability 2011. 2011. http://whqlibdoc.who.int/publications/2011/9789240685215_eng.pdf [PubMed]
- 2. Kakooza-Mwesige A, Andrews C, Peterson S, et al. Prevalence of cerebral palsy in Uganda: a population-based study. Lancet Glob Health 2017;5:e1275–82. 10.1016/S2214-109X(17)30374-1 [DOI] [PubMed] [Google Scholar]
- 3. Bangladesh Ministry of Health and Family Welfare. Survey of Autism and neurodevelopmental disorders in Bangladesh. Bangladesh: Sudipta Printers & Packagers Ltd, 2013. [Google Scholar]
- 4. Gladstone M. A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr 2010;30:181–96. 10.1179/146532810X12786388978481 [DOI] [PubMed] [Google Scholar]
- 5. Nations U. The millenium development goals report 2015. New York: United Nations, 2015. [Google Scholar]
- 6. Nations U. Transforming our world: the 2030 agenda for sustainable development. New York: United Nations, 2015. [Google Scholar]
- 7. Chomba E, Carlo WA, McClure E, et al. Feasibility of implementing an early intervention program in an urban low-income setting to improve neurodevelopmental outcome in survivors following birth asphyxia. Fiedld Actions Science Reports 2011;5:1–9. [Google Scholar]
- 8. Lehmann U, Sanders D. Community health workers: what do we know about them? Geneva: World Health Organization, 2007. [Google Scholar]
- 9. Association for Social and Health Advancement. Asha development projects: West Bengal. 2008. http://www.ashaindia.in/asha-ngo-development-projects.html
- 10. Morgan C, Crowle C, Goyen TA, et al. Sensitivity and specificity of general movements assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J Paediatr Child Health 2015. [DOI] [PubMed] [Google Scholar]
- 11. Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897–907. 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spittle A, Orton J, Anderson PJ, et al. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev 2015;11:CD005495 10.1002/14651858.CD005495.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan C, Darrah J, Gordon AM, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol 2016;58:900–9. 10.1111/dmcn.13105 [DOI] [PubMed] [Google Scholar]
- 14. Wirz S, Edwards K, Flower J, et al. Field testing of the ACCESS materials: a portfolio of materials to assist health workers to identify children with disabilities and offer simple advice to mothers. Int J Rehabil Res 2005;28:293–302. 10.1097/00004356-200512000-00001 [DOI] [PubMed] [Google Scholar]
- 15. Juneja M, Jain R, Singhal S, et al. Availing services for developmental disabilities: parental experiences from a referral center in developing country. Indian J Pediatr 2012;79:1213–7. 10.1007/s12098-011-0653-0 [DOI] [PubMed] [Google Scholar]
- 16. McConachie H, Huq S, Munir S, et al. Difficulties for mothers in using an early intervention service for children with cerebral palsy in Bangladesh. Child Care Health Dev 2001;27:1–12. 10.1046/j.1365-2214.2001.00207.x [DOI] [PubMed] [Google Scholar]
- 17. Singer GH. Meta-analysis of comparative studies of depression in mothers of children with and without developmental disabilities. Am J Ment Retard 2006;111:155–69.doi:10.1352/0895-8017(2006)111[155:MOCSOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 18. Hadders-Algra M. Early diagnosis and early intervention in cerebral palsy. Front Neurol 2014;5 10.3389/fneur.2014.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosanquet M, Copeland L, Ware R, et al. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013;55:418–26. 10.1111/dmcn.12140 [DOI] [PubMed] [Google Scholar]
- 20. Tomantschger I, Herrero D, Einspieler C, et al. The general movement assessment in non-European low- and middle-income countries. Rev Saude Publica 2018;52:6–10. 10.11606/S1518-8787.2018052000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ministry of Home Affairs GoI. Population finder 2011. 2011. http://www.censusindia.gov.in/
- 22. Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev 2005;11:61–7. 10.1002/mrdd.20051 [DOI] [PubMed] [Google Scholar]
- 23. Einspieler C, Prechtl HF, Ferrari F, et al. The qualitative assessment of general movements in preterm, term and young infants--review of the methodology. Early Hum Dev 1997;50:47–60. 10.1016/S0378-3782(97)00092-3 [DOI] [PubMed] [Google Scholar]
- 24. Romeo DM, Cioni M, Palermo F, et al. Neurological assessment in infants discharged from a neonatal intensive care unit. Eur J Paediatr Neurol 2013;17:192–8. 10.1016/j.ejpn.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 25. Shanker TJ, Anupama B, Abraham J, et al. Psychometric properties of hammersmith infant neurological examination in 12 months old high-risk infants: a cross sectional study. Indian J Physiother Occup Ther 2014;8:169–77. [Google Scholar]
- 26. Romeo DM, Ricci D, Brogna C, et al. Use of the hammersmith infant neurological examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol 2016;58:240–5. 10.1111/dmcn.12876 [DOI] [PubMed] [Google Scholar]
- 27. Dumas HM, Fragala-Pinkham MA, Rosen EL, et al. Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) and Alberta Infant Motor Scale (AIMS): Validity and Responsiveness. Phys Ther 2015;95:1559–68. 10.2522/ptj.20140339 [DOI] [PubMed] [Google Scholar]
- 28. Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in cerebral palsy: systematic review and meta-analysis. Pediatrics 2013;132:e735–e46. 10.1542/peds.2012-3985 [DOI] [PubMed] [Google Scholar]
- 29. Novak I, McIntyre S, Morgan C, et al. State of the evidence: systematic review of interventions for children with cerebral palsy. Dev Med Child Neurol 2013;55:885–910. [DOI] [PubMed] [Google Scholar]
- 30. Morgan C, Novak I, Dale RC, et al. Single blind randomised controlled trial of GAME (Goals - Activity - Motor Enrichment) in infants at high risk of cerebral palsy. Res Dev Disabil 2016;55:256–67. 10.1016/j.ridd.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 31. Whittingham K, Sanders M, McKinlay L, et al. Stepping stones triple P and acceptance and commitment therapy for parents of children with cerebral palsy: trial protocol. Brain Impairment 2013;14:270–80. 10.1017/BrImp.2013.19 [DOI] [Google Scholar]
- 32. Wallander JL, Bann CM, Biasini FJ, et al. Development of children at risk for adverse outcomes participating in early intervention in developing countries: a randomized controlled trial. J Child Psychol Psychiatry 2014;55:1251–9. 10.1111/jcpp.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McConachie H, Huq S, Munir S, et al. A randomized controlled trial of alternative modes of service provision to young children with cerebral palsy in Bangladesh. J Pediatr 2000;137:769–76. 10.1067/mpd.2000.110135 [DOI] [PubMed] [Google Scholar]
- 34. Damiano D. Effects of motor activity on brain and muscle development in cerebral palsy : Shepherd R, Cerebral palsy in infancy: targeted activity to optimize early growth and development. Elsevier: Edinburgh, 2013. [Google Scholar]
- 35. Ramey CT, Sparling JJ, Ramey SL. Abecedarian: the ideas, the approach, and the findings. Los Altos, CA: Sociometrics Corporation, 2012. [Google Scholar]
- 36. Sparling JJ, Lewis IS. Learning games for the first three years: a guide to parent-child play. New York: Walker & Company, 1979. [Google Scholar]
- 37. Morgan C, Novak I, Dale RC, et al. GAME (Goals - Activity - Motor Enrichment): protocol of a single blind randomised controlled trial of motor training, parent education and environmental enrichment for infants at high risk of cerebral palsy. BMC Neurol 2014;14:203–03. 10.1186/s12883-014-0203-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. Integrated management of childhood illness. Geneva: World Health Organization, 2014. [Google Scholar]
- 39. Hill Z, Kirkwood B, Edmond K. Family and community practices that promote child survival, growth and development: a review of the evidence. Geneva: WHO, 2004. [Google Scholar]
- 40. Khan NZ, Ferdous S, Munir S, et al. Mortality of urban and rural young children with cerebral palsy in Bangladesh. Dev Med Child Neurol 1998;40:749–53. 10.1111/j.1469-8749.1998.tb12343.x [DOI] [PubMed] [Google Scholar]
- 41. Beck AK, Baker A, Britton B, et al. Fidelity considerations in translational research: eating as treatment - a stepped wedge, randomised controlled trial of a dietitian delivered behaviour change counselling intervention for head and neck cancer patients undergoing radiotherapy. Trials 2015;16:465 10.1186/s13063-015-0978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powell M, Perkins E. Asian families with a pre-school handicapped child - a study. Journal of the British Institute of Mental Handicap 1984;12:50–2. 10.1111/j.1468-3156.1984.tb00195.x [DOI] [Google Scholar]
- 43. Selvam S, Thomas T, Shetty P, et al. Norms for developmental milestones using VABS-II and association with anthropometric measures among apparently healthy urban Indian preschool children. Psychol Assess 2016;28:1634–45. 10.1037/pas0000295 [DOI] [PubMed] [Google Scholar]
- 44. Haley SM, Coster WJ, Dumas HM, et al. PEDI-CAT Version 1.4.0: development, standardisation and administration manual. Boston: Trustees of Boston Universtiy, 2011. [Google Scholar]
- 45. Haley SM, Coster WJ, Dumas HM, et al. Accuracy and precision of the pediatric evaluation of disability inventory computer-adaptive tests (PEDI-CAT). Dev Med Child Neurol 2011;53:1100–6. 10.1111/j.1469-8749.2011.04107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haley SM, Coster WI, Kao YC, et al. Lessons from use of the pediatric evaluation of disability inventory: where do we go from here? Pediatr Phys Ther 2010;22:69–75. 10.1097/PEP.0b013e3181cbfbf6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Connolly BH, McClune NO, Gatlin R. Concurrent validity of the Bayley-III and the Peabody Developmental Motor Scale-2. Pediatr Phys Ther 2012;24:345–52. 10.1097/PEP.0b013e318267c5cf [DOI] [PubMed] [Google Scholar]
- 48. Kolobe TH, Palisano RJ, Stratford PW. Comparison of two outcome measures for infants with cerebral palsy and infants with motor delays. Phys Ther 1998;78:1062–72. 10.1093/ptj/78.10.1062 [DOI] [PubMed] [Google Scholar]
- 49. Palisano RJ, Kolobe TH, Haley SM, et al. Validity of the Peabody Developmental Gross Motor Scale as an evaluative measure of infants receiving physical therapy. Phys Ther 1995;75:939–48. 10.1093/ptj/75.11.939 [DOI] [PubMed] [Google Scholar]
- 50. Morgan C, Novak I, Dale RC, et al. Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatr 2015;15:1–11. 10.1186/s12887-015-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bayley N. Bayley scales of infant and toddler development. 3rd edn San Antonio TX: Psychological Corporation, 2006. [Google Scholar]
- 52. Sonksen PM, Petrie A, Drew KJ. Promotion of visual development of severely visually impaired babies: evaluation of a developmentally based programme. Dev Med Child Neurol 1991;33:320–35. 10.1111/j.1469-8749.1991.tb14883.x [DOI] [PubMed] [Google Scholar]
- 53. World Health Organization, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: A joint statement by the World Health Organization and the United Nations Children’s Fund. Geneva, Switzerland: WHO Press, 2009. [PubMed] [Google Scholar]
- 54. Boyd RN, Jordan R, Pareezer L, et al. Australian cerebral palsy child study: protocol of a prospective population based study of motor and brain development of preschool aged children with cerebral palsy. BMC Neurol 2013;13:e57–e69. 10.1186/1471-2377-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benfer KA, Jordan R, Bandaranayake S, et al. Motor severity in children with cerebral palsy studied in a high-resource and low-resource country. Pediatrics 2014;134:e1594–e1602. 10.1542/peds.2014-1926 [DOI] [PubMed] [Google Scholar]
- 56. Romeo DM, Cioni M, Scoto M, et al. Neuromotor development in infants with cerebral palsy investigated by the Hammersmith Infant Neurological Examination during the first year of age. Eur J Paediatr Neurol 2008;12:24–31. 10.1016/j.ejpn.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 57. Caldwell BM, Bradley RH. Home observation for measurement of the environment. Little Rock: University of Arkansas at Little Rock, 1984. [Google Scholar]
- 58. Tofail F, Kabir I, Hamadani JD, et al. Supplementation of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J Health Popul Nutr 2006;24:48–56. [PubMed] [Google Scholar]
- 59. Totsika V, Sylva K. The home observation for measurement of the environment revisited. Child Adolesc Ment Health 2004;9:25–35. 10.1046/j.1475-357X.2003.00073.x [DOI] [PubMed] [Google Scholar]
- 60. Hamadani JD, Fuchs GJ, Osendarp SJ, et al. Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: a follow-up study. Lancet 2002;360:290–4. 10.1016/S0140-6736(02)09551-X [DOI] [PubMed] [Google Scholar]
- 61. Hamadani JD, Fuchs GJ, Osendarp SJ, et al. Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. Am J Clin Nutr 2001;74:381–6. 10.1093/ajcn/74.3.381 [DOI] [PubMed] [Google Scholar]
- 62. Johnston C, Mash EJ. A measure of parenting satisfaction and efficacy. J Clin Child Psychol 1989;18:167–75. 10.1207/s15374424jccp1802_8 [DOI] [Google Scholar]
- 63. Badawi N, Watson L, Petterson B, et al. What constitutes cerebral palsy? Dev Med Child Neurol 1998;40:520–7. 10.1111/j.1469-8749.1998.tb15410.x [DOI] [PubMed] [Google Scholar]
- 64. Lovibond S, Lovibond P. Manual for the depression, anxiety and stress scales. 2nd edn Sydney: Psychology Foundation, 1995. [Google Scholar]
- 65. Alim M. Bangla (Bengali) DASS21 questionnaire. Dhaka: Bangladesh Sheikh Mujib Medical University. [Google Scholar]
- 66. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23. 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 67. Cans C. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000;42:816–24. 10.1111/j.1469-8749.2000.tb00695.x [DOI] [PubMed] [Google Scholar]
- 68. Bhuiya A, Mahmood SS, Rana AK, et al. A multidimensional approach to measure poverty in rural Bangladesh. J Health Popul Nutr 2007;25:134–45. [PMC free article] [PubMed] [Google Scholar]
- 69. Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Pers Assess 1988;52:30–41. 10.1207/s15327752jpa5201_2 [DOI] [Google Scholar]
- 70. Eker D, Arkar H. Perceived social support: psychometric properties of the MSPSS in normal and pathological groups in a developing country. Soc Psychiatry Psychiatr Epidemiol 1995;30:121–6. 10.1007/BF00802040 [DOI] [PubMed] [Google Scholar]
- 71. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haataja L, Mercuri E, Guzzetta A, et al. Neurologic examination in infants with hypoxic-ischemic encephalopathy at age 9 to 14 months: use of optimality scores and correlation with magnetic resonance imaging findings. J Pediatr 2001;138:332–7. 10.1067/mpd.2001.111325 [DOI] [PubMed] [Google Scholar]
- 73. Pepper J, Weitzman E. It takes two to talk: a practical guide for parents of children with learning delays. 2nd edn Toronto: The Hanen Centre, 2004. [Google Scholar]
- 74. Law MC, Darrah J, Pollock N, et al. Focus on function: a cluster, randomized controlled trial comparing child- versus context-focused intervention for young children with cerebral palsy. Dev Med Child Neurol 2011;53:621–9. 10.1111/j.1469-8749.2011.03962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fetters L. Perspective on variability in the development of human action. Phys Ther 2010;90:1860–7. 10.2522/ptj.2010090 [DOI] [PubMed] [Google Scholar]
- 76. World Health Organization. Breastfeeding counselling: a training course. Geneva: World Health Organization, 1993. [Google Scholar]
- 77. World Health Organization. Complementary feeding counselling: a training course. Geneva: World Health Organization, 2004. [Google Scholar]
- 78. Whittingham K. Becoming Mum. Brisbane: Australian eBook Publisher, 2015. [Google Scholar]
- 79. Whittingham K, Wee D, Boyd R. Systematic review of the efficacy of parenting interventions for children with cerebral palsy. Child Care Health Dev 2011;37:475–83. 10.1111/j.1365-2214.2011.01212.x [DOI] [PubMed] [Google Scholar]
- 80. Boyd RN, Ziviani J, Sakzewski L, et al. REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open 2017;7:e017204 10.1136/bmjopen-2017-017204 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.