Abstract
Objective
To identify patients with hypertension with resistant and controlled blood pressure (BP) using electronic health records (EHRs) in order to elucidate practices in the real-world clinical treatment of hypertension and to enable future genetic studies.
Design
Using EHRs, we developed and validated algorithms to identify patients with resistant and controlled hypertension.
Setting
An academic medical centre in Nashville, Tennessee.
Population
European-American (EA) and African-American (AA) patients with hypertension.
Main outcome measures
Demographic characteristics: race, age, gender, body mass index, outpatient BPs and the history of diabetes mellitus, chronic kidney disease stage 3, ischaemic heart disease, transient ischaemic attack, atrial fibrillation and sleep apnoea.
Medication treatment
All antihypertensive medication classes prescribed to a patient at the time of classification and ever prescribed following classification.
Results
The algorithms had performance metrics exceeding 92%. The prevalence of resistant hypertension in the total hypertensive population was 7.3% in EA and 10.5% in AA. At diagnosis, AA were younger, heavier, more often female and had a higher incidence of type 2 diabetes and higher BPs than EA. AA with resistant hypertension were more likely to be treated with vasodilators, dihydropyridine calcium channel blockers and alpha-2 agonists while EA were more likely to be treated with angiotensin receptor blockers, renin inhibitors and beta blockers. Mineralocorticoid receptor antagonists use was increased in patients treated with more than four antihypertensive medications compared with patients treated with three (12.4% vs 2.6% in EA, p<0.001; 12.3% vs 2.8% in AA, p<0.001). The number of patients treated with a mineralocorticoid receptor antagonist increased to 37.4% in EA and 41.2% in AA over a mean follow-up period of 7.4 and 8.7 years, respectively.
Conclusions
Clinical treatment of resistant hypertension differs in EA and AA patients. These results demonstrate the feasibility of identifying resistant hypertension using an EHR.
Keywords: resistant hypertension, electronic health record, race, antihypertensive medications, prescribing trends
Strengths and limitations of this study.
A strength of this study was the accuracy of the electronic algorithms for the identification of resistant hypertension.
The use of stringent definitions to phenotype resistant and controlled hypertension in the electronic health record will permit future genetic studies using large-scale electronic health records with linked genetic data.
The inclusion of a large number of European (n=13 541) and African (n=3541) Americans with resistant hypertension allowed us to compare the treatment of hypertension in these two groups.
The limitations of the study include the inability to confirm medication compliance or track use of over-the-counter medications that affect blood pressure (BP) response, and the lack of ambulatory BP measures in the electronic medical records reviewed.
Introduction
Patients with resistant hypertension, defined as persistently elevated blood pressure (BP) despite concurrent treatment with three or more different antihypertensive medications including a thiazide diuretic, are at an elevated risk of chronic kidney disease (CKD) and a 47% higher risk of cardiovascular events compared with patients with controlled hypertension.1 2 The estimated prevalence of resistant hypertension ranges from 8.4% to 50% of all treated patients with hypertension in clinical trials and epidemiological studies due to varying definitions of resistant hypertension and methods of BP assessment.3–11 A meta-analysis by Achelrod et al estimated a prevalence of resistant hypertension of 13.72% in 20 observational studies and 16.32% in 4 randomised control trials.12 These rates are consistent with a separate meta-analysis of North American and European studies that reported a resistant hypertensive prevalence rate of 14.8% in treated patients with hypertension.9 These rates may be inflated because the studies did not assess adequacy of drug treatment or medication non-adherence.
The rates of inadequate drug treatment or medication non-adherence are not known precisely for the population. In a study by Egan et al, half of the patients with uncontrolled BP on greater than three antihypertensive medications were prescribed optimal treatment and patients with greater cardiovascular risk were more often prescribed optimal treatment.13 Medication non-adherence, in particular, may lead to overestimation of the prevalence of resistant hypertension. Studies assessing medication adherence using blood and urine levels to measure antihypertensive medication intake estimate the rate of non-adherence, including partial and complete non-adherence, to be approximately 50% in resistant patients with hypertension and 25% in patients with hypertension with uncontrolled BP.14–16 These conditions may contribute to uncontrolled BP in some but not all patients with resistant hypertension. The molecular mechanisms underlying resistant hypertension in the remaining population remain unknown. Therefore, understanding the genetic underpinning of resistant hypertension versus readily controlled hypertension is of great value.
To date, however, the identification of novel genetic underpinnings of population-wide hypertension has met with limited success. Genome-Wide Association Studies (GWAS) can analyse up to millions of genetic variants from across the human genome in an effort to identify genetic risk factors of disease. GWAS studies of BP and hypertension have identified a number of variants that associate with BP and hypertension.17–21 The contribution of these variants to the overall heritability of hypertension, however, has been relatively small.22–24 Conversely, studies of Mendelian forms of hypertension have identified novel rare variants with large effect size that contribute to hypertension.22 25–28 These findings have provided mechanistic insight into the aetiology of hypertension. Resequencing efforts in the Framingham Heart Study have further supported the role for some of these rare variants in BP regulation.29 Because much is still left to learn about the genetic contributions to hypertension, there is a need to study more severe hypertension in large datasets for which clinical and medication data are available. Electronic health records (EHRs) linked with genetic material provides a robust resource to do just that.30 31
We hypothesised that we could use the EHR to develop algorithms to identify patients within a clinical population who had resistant hypertension or controlled hypertension for use in future genetic studies. Resistant hypertensive cases were defined as patients with BP > 140/90 mm Hg despite concurrent use of three or more antihypertensive medications including a thiazide, thiazide-like diuretic or a dihydropyridine calcium channel blocker (DHP CCB) or those taking four or more antihypertensive medications including a thiazide, thiazide-like diuretic or DHP CCB. Controls with controlled hypertension were defined as patients with BP <135/90 mm Hg on one and only ever one medication. Our case and control definitions were designed to be stringent, excluding secondary causes of hypertension and CKD stages 4 and 5, to select a more homogeneous population for use in genetic studies. The accuracy of the algorithms was validated by chart review. In developing the method, we observed differences in the patterns of prescribing of antihypertensive medications between African-American (AA) and European-American (EA) patients with resistant hypertension. We describe here the characteristics and medication treatment of AA versus EA patients with resistant hypertension at a large academic health centre.
Methods
Electronic health record
We used the Vanderbilt University Medical Center (VUMC) Synthetic Derivative (SD) for this research. The SD is a de-identified copy of the VUMC EHR system with Health Insurance Portability and Accountability Act identifiers removed by established de-identification software as well as custom algorithms.32 The institutional review board reviewed this project and deemed it exempt as non-human subjects research in accordance with 45 code of federal regulations (CFR) 46. To date the SD contains approximately 2.5 million records and approximately one million of these records contain detailed longitudinal data. The SD contains almost all available clinical data including basic demographics, such as race and sex, text from clinical care notes, laboratory values, inpatient and outpatient medication data, International Classification of Disease (ICD) and Current Procedural Terminology (CPT) codes, and other diagnostic reports.
Resistant hypertension algorithm development
To identify patients with resistant hypertension (cases) and patients with controlled hypertension (controls) in the VUMC SD, we developed the following algorithms, updated and modified from a previously published algorithm to define resistant hypertension within the Electronic Medical Records and Genomics network.21 33
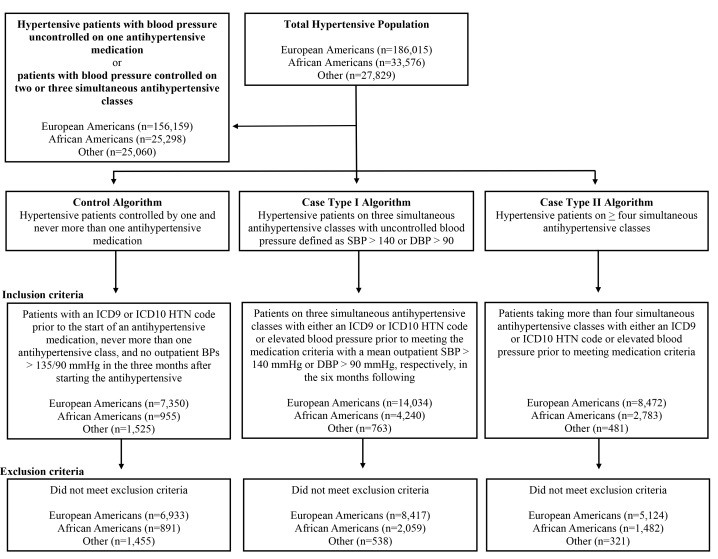
Resistant hypertension was defined by one of two possible case definitions. Case type I identified patients with elevated BP despite simultaneous treatment with at least three different classes of antihypertensive medications, including a thiazide diuretic, amlodipine or other DHP CCB (figure 1). Because thiazide-induced hyponatraemia is a relatively common clinical condition and clinical guidelines suggest thiazide or CCB use, we allowed for the replacement of thiazide diuretics with DHP CCBs.34–37 Antihypertensive medication classes were ACE inhibitors, angiotensin receptor blockers (ARBs), beta blockers (BBs), alpha-2 agonists, CCBs, thiazide and thiazide-like diuretics, aldosterone antagonists, other non-thiazide diuretics, direct-acting vasodilators, alpha antagonists, renin inhibitors and miscellaneous antihypertensives (online supplementary table 1). In the case of combination therapies, each component antihypertensive medication was counted separately. Simultaneous treatment with three different classes of antihypertensive medication was confirmed by documentation in the EHR on two occasions separated by more than 1 month. Uncontrolled hypertension was defined by at least two recorded outpatient measurements of systolic BP (SBP) greater than 140 mm Hg or diastolic BP (DBP) greater than 90 mm Hg at least 1 month after medication criteria were met, as well as by a mean outpatient SBP or DBP calculated as greater than 140/90 mm Hg during the 6 months after the medication criteria were met.
Figure 1.
Diagram of the algorithms for the identification of patients with resistant (cases) and controlled (control) hypertension in Vanderbilt University Medical Center’s Synthetic Derivative of the electronic health record. BPs, blood pressures; DBP, diastolic blood pressure; HTN, hypertension; ICD-9, International Classification of Disease, Ninth Revision; SBP, systolic blood pressure.
bmjopen-2018-021640supp001.pdf (231.5KB, pdf)
The case type II definition of resistant hypertension identified patients who were treated simultaneously with four or more classes of antihypertensive medication, including a thiazide-type diuretic, amlodipine or other DHP CCB, as documented on at least two occasions more than 1 month apart, regardless of BP. Patients who met the criteria for case type I but subsequently met the definition of case type II by virtue of having a new medication class added were considered to meet the definition of case type II resistant hypertension for all analyses.
Controls, patients with controlled hypertension, were defined as patients who had been assigned an ICD-9 or ICD-10 code for hypertension (401.* and I10, respectively) and who were treated with a single antihypertensive medication. The control definition required that at least one BP be recorded in the month following the initiation of the antihypertensive medication and that all recorded SBP and DBP in the month were less than 135 and 90 mm Hg, respectively. In addition, these patients never received concurrent treatment with more than one antihypertensive class at any time in the EHR. Therefore, control patients could never be classified as a case at a different time in the EHR. Because we excluded the majority of treated patients with hypertension, for example, patients who achieve control on two or three antihypertensive medications, our controls do not reflect all patients with controlled hypertension; however, we choose a stringent control definition to facilitate future genetic studies using the identified population.
For all three algorithms, drug exposure to antihypertensive medications was identified in the VUMC SD by electronic-prescribing tools and by using MedEx,38 a natural-language processing tool that recognises medication names and information including drug dose, route, frequency and duration from unstructured clinical documents. We required the presence of at least one of the following identifiers—drug dose, route, frequency or duration—for a MedEx-identified drug to be counted. The utility of these tools for extracting medication data from the EHR has been shown previously.39 40
All patient characteristics—age, gender, body mass index (BMI), BPs and history of type two diabetes mellitus (T2DM), sleep apnoea, ischaemic heart disease (IHD), transient ischaemic attack (TIA), congestive heart failure, atrial fibrillation/flutter and CKD stage 3—were extracted from the VUMC SD using a combination of ICD-9 and ICD-10 codes, CPT codes, laboratory measures, and natural-language processing. For each patient, age and BMI at the earliest time point for which a patient met case or control inclusion criteria, that is the decision date, is reported. All outpatient SBP and DBP collected during the 6 months following the decision date were obtained for each patient and individual means were calculated. History of CKD stage 3 was determined by an estimated glomerular filtration rate >30 mL/min/1.73 m2 and <60 mL/min/1.73 m2 calculated using the Modification of Diet in Renal Disease formula41 at any point before case/control identification or within 6 months of identification. Patient race was administratively assigned in the SD based on either patient or physician report. Previous work has shown that self-identified race is highly correlated with genetic ancestry42 43 and administratively assigned race in the VUMC SD is sufficient for many genetic association analyses44 and correlates tightly with genetic ancestry.43
Online supplementary table 2 lists exclusion criteria for both cases and controls. Exclusion criteria included recorded ICD-9 and ICD-10 codes for conditions known to cause secondary hypertension. For both case types as well as controls, patients were also excluded if they had left ventricular dysfunction defined as an ejection fraction <35%. Patients with CKD stages 4 and 5, defined by an estimated glomerular filtration rate <30 mL/min 1.73 m2, were also excluded.
After the algorithms were iteratively refined, a blinded review of randomly chosen, never overlapping, individual electronic medical records were performed to determine electronic algorithm efficacy. Based on a population size of 24 906, all cases and controls regardless of race, review of 138 records would allow us to estimate a misclassification rate of 10% with a margin of error of 5%. We, therefore, chose to review 150 records to determine algorithm efficacy. Two of three reviewers were not affiliated with the development of the electronic algorithms. Each review drew patient records from the SD that had not been previously reviewed. The algorithms were refined until a negative predictive value (NPV), positive predictive value (PPV), sensitivity and specificity greater than 90% was achieved based on the review of 150 records subdivided equally among the two case types and controls (50 each). The final version of the algorithm is available at Phenotype KnowledgeBase.45
Statistical methods
Data are presented as frequencies for categorical variables and median and IQR for continuous variables. Between-group comparisons were made using Pearson test for categorical variables and the Wilcoxon test for continuous variables. The false discovery rate-adjusted p values are reported for the multiple comparisons in medication use. Analyses were conducted for the total group and also within EA or AA. Multivariable logistic regression models for medication use in resistant hypertensive cases were fit for race, age, gender and history of T2DM, sleep apnoea, IHD, TIA, atrial fibrillation/flutter, congestive heart failure with preserved ejection fraction (HFpEF) and CKD stage 3. All statistical analyses were run using the SPSS software V.24 (SPSS) or R V.3.3.0.46
Patient and public involvement
Because this study involved the use of a de-identified SD of the EHR, patients were not recruited and there was no intervention. Patients were not specifically involved in the development of the research question or design of the study; however, there has been extensive patient and community engagement in the establishment of the Vanderbilt biobank that includes the SD. A community advisory board within the Vanderbilt Institute for Clinical and Translational Research reviews programmes including the SD. Results of the study will be disseminated to patients through local reporting of the publication.
Results
Algorithm validation
NPV, PPV, sensitivity and specificity of the algorithms for resistant hypertension (case type I and type II) and controlled hypertension (control) were determined after a blinded chart review of 150 patients. The algorithm for case type II had the highest PPV and specificity at 96% and 98%, respectively (table 1). The NPV and sensitivity were each 100% for case type I and control.
Table 1.
Algorithm validation metrics following blinded chart review
| Resistant hypertension | Control | ||
| Case type I | Case type II | ||
| Positive predictive value | 94 (83 to 98) | 96 (85 to 99) | 92 (80 to 97) |
| Negative predictive value | 100 (95 to 100) | 99 (94 to 100) | 100 (95 to 100) |
| Specificity | 97 (91 to 99) | 98 (92 to 100) | 96 (90 to 99) |
| Sensitivity | 100 (91 to 100) | 98 (88 to 100) | 100 (90 to 100) |
Data are presented as % (95% CI).
Identification of patients with resistant hypertension and controlled hypertension
Using the occurrence of an ICD-9 or ICD-10 code for hypertension, as well as the presence of a SBP or DBP greater than 140 or 90 mm Hg prior to or at ICD-9 or ICD-10 occurrence, we estimated the total number of patients with hypertension in the VUMC SD to be 247 420 (22.2% of the adult patients in the SD with an available BP measurement), of whom 186 015 were EA (75.2%) and 33 576 were AA (13.6%). A total of 5024 potential EA cases and 2139 potential AA cases were excluded for secondary causes of hypertension. After excluding secondary causes of hypertension, 16.5% of the remaining potential AA cases (n=806) and 11.4% of the potential EA cases (n=1993) were excluded by the algorithm due to the presence of CKD stages 4 and 5. An additional 10 AA cases and 21 EA cases were excluded because of a left ventricular ejection fraction (LVEF) <35% within a year of meeting medication criteria. In total, in the SD after algorithm execution we identified 13 541 EA patients who met one or both of the case definitions for resistant hypertension and 6933 who met the definition for controlled hypertension. We likewise identified 3541 AA cases and 891 AA controls (figure 1). Based on these estimates, we determined the prevalence of resistant hypertension among patients without CKD stages 4 and 5 in the VUMC SD to be 7.3% for EAs and 10.5% for AAs.
Demographics of the algorithm-identified cases and controls
Regardless of race, patients with resistant hypertension were significantly older, heavier and more likely to have T2DM, sleep apnoea, atrial fibrillation, a history of TIA, IHD or CKD stage 3 compared with controls (table 2). There were significantly more female controls than cases among EAs, but not among AAs.
Table 2.
Characteristics of EA and AA patients with resistant hypertension (cases) or controlled hypertension (controls)
| Variable | EA | AA | EA versus AA | |||||
| Cases (n=13 541) | Controls (n=6933) | P values | Cases (n=3541) | Controls (n=891) | P values | Cases P values | Controls P values | |
| SBP, mm Hg | 145.0 (140.0–153.0) | 120.8 (114.0–127.0) | <0.001 | 147.4 (141.0–156.0) | 122.0 (115.0–128.0) | <0.001 | <0.001 | 0.001 |
| DBP, mm Hg | 78.0 (70.0–86.0) | 74.0 (68.0–80.0) | <0.001 | 85.0 (76.4–92.2) | 75.5 (70.0–81.0) | <0.001 | <0.001 | <0.001 |
| Age, years | 66.0 (57.0–73.0) | 53.0 (43.0–64.0) | <0.001 | 56.0 (47.0–65.0) | 46.0 (34.0–55.0) | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 30.8 (26.7–35.8) | 29.3 (25.4–34.2) | <0.001 | 32.9 (28.3–38.9) | 31.0 (26.3–37.0) | <0.001 | <0.001 | <0.001 |
| Female, n (%) | 6615 (48.9%) | 3495 (50.4%) | 0.04 | 2092 (59.1%) | 527 (59.2%) | 0.97 | <0.001 | <0.001 |
| T2DM, n (%) | 2694 (19.9%) | 1026 (14.8%) | <0.001 | 954 (26.9%) | 171 (19.2%) | <0.001 | <0.001 | <0.001 |
| Sleep apnoea, n (%) | 868 (6.4%) | 373 (5.4%) | 0.003 | 252 (7.1%) | 45 (5.1%) | 0.03 | 0.13 | 0.68 |
| Afib, n (%) | 1424 (10.5%) | 272 (3.9%) | <0.001 | 130 (3.7%) | 11 (1.2%) | <0.001 | <0.001 | <0.001 |
| TIA, n (%) | 603 (4.5%) | 110 (1.6%) | <0.001 | 176 (5.0%) | 19 (2.1%) | <0.001 | 0.19 | 0.23 |
| IHD, n (%) | 2493 (18.4%) | 585 (8.4%) | <0.001 | 468 (13.2%) | 32 (3.6%) | <0.001 | <0.001 | <0.001 |
| CKD 3, n (%)* | 4407 (42.4%) | 650 (14.1%) | <0.001 | 870 (29.7%) | 47 (7.2%) | <0.001 | <0.001 | <0.001 |
| HFpEF, n (%) | 1173 (9%) | 102 (1%) | <0.001 | 376 (11%) | 16 (2%) | <0.001 | <0.001 | 0.454 |
The number of subjects with available eGFR data is: 10 405 EA cases, 4602 EA controls, 2927 AA cases, and 653 AA controls.
Data are presented as median (IQR) unless otherwise indicated.
*The percentage for CKD 3 is based on the number of subjects with available eGFR data, not all subjects in the population.
AA, African-American; Afib, atrial fibrillation; BMI, body mass index; CKD 3, chronic kidney disease stage 3; DBP, diastolic blood pressure; EA, European-American; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; IHD, ischaemic heart disease; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack.
Compared with EAs, AA cases and controls were significantly younger, heavier, predominately female and had higher prevalence of T2DM at diagnosis (table 2). The prevalences of atrial fibrillation, IHD and CKD stage 3 were higher in EA compared with AA cases and controls. The prevalence of HFpEF was significantly higher in AA cases compared with EA cases. The prevalences of sleep apnoea and history of TIA were not significantly different between AA and EA patients.
BP and medication use in patients with resistant hypertension and controlled hypertensive controls
By definition, SBP and DBP were significantly higher in patients with resistant hypertension compared with those with controlled hypertension (table 2). Also by definition, all patients with resistant hypertension were prescribed either a thiazide type diuretic or a CCB (amlodipine or other DHP CCB) (table 3). Patients with resistant hypertension were significantly more likely to be prescribed every class of antihypertensive compared with controls except for miscellaneous antihypertensives.
Table 3.
Medication use in EA and AA patients with resistant hypertension (cases) and controlled hypertension (controls)
| Variable | EA | AA | EA versus AA | |||||
| Case (n=13 541) | Control (n=6933) | P values | Case (n=3541) | Control (n=891) | P values | Case P values | Control P values | |
| Thiazide/CCB, n (%) | 13 541 (100%) | 1167 (16.8%) | <0.001 | 3541 (100%) | 292 (32.8%) | <0.001 | 1.0 | <0.001 |
| ACE inhibitor, n (%) | 6999 (51.7%) | 2439 (35.2%) | <0.001 | 1916 (54.1%) | 264 (29.6%) | <0.001 | 0.02 | 0.003 |
| ARB, n (%) | 5178 (38.2%) | 728 (10.5%) | <0.001 | 1161 (32.8%) | 54 (6.1%) | <0.001 | <0.001 | <0.001 |
| BB, n (%) | 8697 (64.2%) | 1803 (26.0%) | <0.001 | 1996 (56.4%) | 152 (17.1%) | <0.001 | <0.001 | <0.001 |
| Alpha-2 agonist, n (%) | 1921 (14.2%) | 140 (2.0%) | <0.001 | 736 (20.8%) | 29 (3.3%) | <0.001 | <0.001 | 0.04 |
| CCB, n (%) | 9272 (68.5%) | 615 (8.9%) | <0.001 | 2625 (74.1%) | 144 (16.2%) | <0.001 | <0.001 | <0.001 |
| Amlodipine, n (%) | 6759 (49.9%) | 318 (4.6%) | <0.001 | 1749 (49.4%) | 90 (10.1%) | <0.001 | 0.74 | <0.001 |
| DHP CCB, n (%) | 1508 (11.1%) | 75 (1.1%) | <0.001 | 585 (16.5%) | 27 (3.0%) | <0.001 | <0.001 | <0.001 |
| Non-DHP CCB, n (%) | 1005 (7.4%) | 222 (3.2%) | <0.001 | 291 (8.2%) | 27 (3.0%) | <0.001 | 0.18 | 0.84 |
| Thiazide Diuretic, n (%) | 8812 (65.1%) | 774 (11.2%) | <0.001 | 2319 (65.5%) | 175 (19.6%) | <0.001 | 0.76 | <0.001 |
| Aldosterone antagonist, n (%) | 854 (6.3%) | 57 (0.8%) | <0.001 | 240 (6.8%) | 8 (0.9%) | <0.001 | 0.43 | 0.85 |
| Non-thiazide Diuretic, n (%) | 4271 (31.5%) | 326 (4.7%) | <0.001 | 1190 (33.6%) | 56 (6.3%) | <0.001 | 0.04 | 0.08 |
| Furosemide, n (%) | 3114 (23.0%) | 254 (3.7%) | <0.001 | 874 (24.7%) | 44 (4.9%) | <0.001 | 0.07 | 0.10 |
| Triamterene, n (%) | 1155 (8.5%) | 45 (0.7%) | <0.001 | 352 (9.9%) | 11 (1.2%) | <0.001 | 0.02 | 0.09 |
| Torsemide, n (%) | 176 (1.3%) | 17 (0.2%) | <0.001 | 23 (0.7%) | 1 (0.1%) | 0.06 | 0.004 | 0.57 |
| Bumetanide, n (%) | 141 (1.0%) | 8 (0.1%) | <0.001 | 28 (0.8%) | 0 (0.0%) | 0.01 | 0.28 | 0.43 |
| Amiloride, n (%) | 32 (0.2%) | 1 (0.0%) | <0.001 | 10 (0.3%) | 0 (0.0%) | 0.12 | 0.75 | 0.79 |
| Ethacrynic acid, n (%) | 11 (0.1%) | 1 (0.0%) | 0.07 | 7 (0.2%) | 0 (0.0%) | 0.19 | 0.10 | 0.79 |
| Vasodilator, n (%) | 903 (6.7%) | 17 (0.3%) | <0.001 | 422 (11.9%) | 2 (0.2%) | <0.001 | <0.001 | 0.93 |
| Minoxidil, n (%) | 161 (1.2%) | 2 (0.0%) | <0.001 | 101 (2.9%) | 0 (0.0%) | <0.001 | <0.001 | 0.75 |
| Hydralazine, n (%) | 742 (5.5%) | 15 (0.2%) | <0.001 | 321 (9.1%) | 2 (0.2%) | <0.001 | <0.001 | 0.96 |
| Alpha antagonist, n (%) | 626 (4.6%) | 33 (0.5%) | <0.001 | 139 (3.9%) | 7 (0.8%) | <0.001 | 0.12 | 0.33 |
| Renin inhibitor, n (%) | 275 (2.0%) | 1 (0.0%) | <0.001 | 43 (1.2%) | 0 (0.0%) | 0.001 | 0.004 | 0.79 |
| Misc antihtn, n (%) | 3 (0.0%) | 0 (0.0%) | 0.21 | 0 (0.0%) | 0 (0.0%) | 1.0 | 0.51 | 1.0 |
P values were adjusted to control for false discovery rate.
AA, African-American; ARB, angiotensin II receptor blocker; BB, beta blocker; CCB, calcium channel blocker; DHP, dihydropyridine; EA, European-American; Misc antihtn, miscellaneous antihypertensive.
SBP and DBP were significantly higher in AA compared with EA whether within the resistant hypertensive group or within the controlled hypertensive group (table 2). Among patients with resistant hypertension, AA were more likely to be prescribed the non-thiazide diuretics, CCBs, alpha-2 agonists, ACE inhibitors and direct-acting vasodilators (minoxidil and hydralazine specifically) than EA patients and less likely to be prescribed ARBs, BBs, torsemide or renin inhibitors (table 3). In multivariable logistic regression models including age, race, gender and history of T2DM, sleep apnoea, atrial fibrillation, TIA, IHD, HFpEF and CKD stage 3, race remained a significant independent predictor of torsemide (p=0.002), ARB (p<0.001), BB (p<0.001), CCB (p<0.001), DHP CCB (p<0.001), alpha-2 agonist (p<0.001), direct-acting vasodilator (p<0.001), minoxidil (p<0.001), hydralazine (p<0.001) and renin inhibitor (p<0.001) use.
To better understand prescribing patterns and patient characteristics, we compared patients with resistant hypertension defined by case definition case type I (uncontrolled hypertension despite treatment with three classes of antihypertensive medications) versus those defined by case type II (patients with hypertension prescribed four or more classes of antihypertensive medications). Patients defined by case type II were heavier, more often male and more likely to have T2DM, IHD, CKD stage 3, HFpEF and sleep apnoea than those defined by the case type I definition (online supplementary table 3). Among EA, case type II patients were more likely to have atrial fibrillation than case type I patients (online supplementary table 3). Among AA, case type II patients were significantly older than case type I patients (online supplementary table 3). DBP and SBP were significantly higher in case type I patients than case type II patients (online supplementary table 3) in both racial groups.
Consistent with the case type I algorithm, all patients were prescribed three simultaneous antihypertensive medication classes. All classes of medications were prescribed more frequently in patients who met the case type II definition versus the case type I definition except for thiazide diuretics and ethacrynic acid (in both EA and AA), and miscellaneous antihypertensives, amlodipine and amiloride (in AA). In particular, spironolactone use was increased from 2.6% to 12.4% in EA and 2.8% to 12.3% in AA. Among AA, direct-acting vasodilator use was increased from 4.7% to 22.0%, and among EA from 2.8% to 13.0% (table 4).
Table 4.
Medication use in EA and AA patients with resistant hypertension based on case type definition
| Variable | EA | AA | EA versus AA | |||||
| Case I (n=8417) | Case II (n=5124) | P values | Case I (n=2059) | Case II (n=1482) | P values | Case I P values | Case II P values | |
| Thiazide/CCB, n (%) | 8417 (100%) | 5124 (100%) | 1.0 | 2059 (100%) | 1482 (100%) | 1.0 | 1.0 | 1.0 |
| ACE inhibitor, n (%) | 4051 (48.1%) | 2948 (57.5%) | <0.001 | 1037 (50.4%) | 879 (59.3%) | <0.001 | 0.14 | 0.32 |
| ARB, n (%) | 2861 (34.0%) | 2317 (45.2%) | <0.001 | 571 (27.7%) | 590 (39.8%) | <0.001 | <0.001 | <0.001 |
| BB, n (%) | 4718 (56.1%) | 3979 (77.7%) | <0.001 | 934 (45.4%) | 1062 (71.7%) | <0.001 | <0.001 | <0.001 |
| Alpha-2 agonist, n (%) | 544 (6.5%) | 1377 (26.9%) | <0.001 | 237 (11.5%) | 499 (33.7%) | <0.001 | <0.001 | <0.001 |
| CCB, n (%) | 5297 (62.9%) | 3975 (77.6%) | <0.001 | 1433 (69.6%) | 1192 (80.4%) | <0.001 | <0.001 | 0.05 |
| Amlodipine, n (%) | 4027 (47.8%) | 2732 (53.3%) | <0.001 | 1000 (48.6%) | 749 (50.5%) | 0.26 | 0.64 | 0.13 |
| DHP CCB, n (%) | 752 (8.9%) | 756 (14.8%) | <0.001 | 293 (14.2%) | 292 (19.7%) | <0.001 | <0.001 | <0.001 |
| Non-DHP CCB, n (%) | 518 (6.2%) | 487 (9.5%) | <0.001 | 140 (6.8%) | 151 (10.2%) | <0.001 | 0.39 | 0.52 |
| Thiazide diuretic, n (%) | 5448 (64.7%) | 3364 (65.7%) | 0.28 | 1363 (66.2%) | 956 (64.5%) | 0.30 | 0.31 | 0.52 |
| Aldosterone antagonist, n (%) | 219 (2.6%) | 635 (12.4%) | <0.001 | 58 (2.8%) | 182 (12.3%) | <0.001 | 0.64 | 0.91 |
| Non-thiazide Diuretic, n (%) | 1643 (19.5%) | 2628 (51.3%) | <0.001 | 397 (19.3%) | 793 (53.5%) | <0.001 | 0.84 | 0.24 |
| Furosemide, n (%) | 1074 (12.8%) | 2040 (39.8%) | <0.001 | 241 (11.7%) | 633 (42.7%) | <0.001 | 0.31 | 0.11 |
| Triamterene, n (%) | 599 (7.1%) | 556 (10.9%) | <0.001 | 171 (8.3%) | 181 (12.2%) | <0.001 | 0.13 | 0.25 |
| Torsemide, n (%) | 43 (0.55) | 133 (2.6%) | <0.001 | 4 (0.2%) | 19 (1.3%) | <0.001 | 0.12 | 0.01 |
| Bumetanide, n (%) | 47 (0.6%) | 94 (1.8%) | <0.001 | 8 (0.4%) | 20 (1.3%) | 0.002 | 0.46 | 0.31 |
| Amiloride, n (%) | 5 (0.15) | 27 (0.5%) | <0.001 | 3 (0.1%) | 7 (0.5%) | 0.08 | 0.31 | 0.84 |
| Ethacrynic acid, n (%) | 4 (0.0%) | 7 (0.1%) | 0.09 | 2 (0.1%) | 5 (0.3%) | 0.12 | 0.51 | 0.21 |
| Vasodilator, n (%) | 236 (2.8%) | 667 (13.0%) | <0.001 | 96 (4.7%) | 326 (22.0%) | <0.001 | <0.001 | <0.001 |
| Minoxidil, n (%) | 27 (0.3%) | 134 (2.6%) | <0.001 | 7 (0.3%) | 94 (6.3%) | <0.001 | 0.91 | <0.001 |
| Hydralazine, n (%) | 209 (2.5%) | 533 (10.4%) | <0.001 | 89 (4.3%) | 232 (15.7%) | <0.001 | <0.001 | <0.001 |
| Alpha antagonist, n (%) | 159 (1.9%) | 467 (9.1%) | <0.001 | 35 (1.7%) | 104 (7.0%) | <0.001 | 0.64 | 0.03 |
| Renin inhibitor, n (%) | 58 (0.7%) | 217 (4.2%) | <0.001 | 11 (0.5%) | 32 (2.2%) | <0.001 | 0.52 | <0.001 |
| Misc antihypertensive, n (%) | 0 (0.0%) | 3 (0.1%) | 0.03 | 0 (0.0%) | 0 (0.0%) | 1.0 | 1.0 | 0.47 |
P values were adjusted to control for false discovery rate.
AA, African-American; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker; DHP, dihydropyridine; EA, European-American; Misc antihtn, miscellaneous antihypertensive.
Among EA case type II patients, 3385 patients (66.1%) were prescribed four different classes of antihypertensive medications, 1373 (26.8%) were prescribed five and 366 (7.1%) were prescribed six. Among AA case type II patients, 935 patients (63.1%) were prescribed four different classes, 416 (28.1%) were prescribed five and 131 (8.8%) were prescribed six different classes of antihypertensive medications.
Online supplementary table 4 lists the number of case type II patients prescribed specific medication classes at initial diagnosis and at any point following their identification. There was an increase in prescription rate for all classes when the time frame was extended to include any point in the SD following initial identification of resistant hypertension. 37.4% of EA and 41.2% of AA case type II patients were eventually prescribed an aldosterone antagonist. While less than half (46.6%) of EA case type II patients were ever prescribed an alpha-2 agonists such as clonidine, more than half (56.4%) of AA case type II patients were ever prescribed an alpha-2 agonist.
Discussion
We developed algorithms to identify patients with resistant hypertension and with controlled hypertension using the EHR. Electronic support has been shown to improve accuracy of clinical data acquisition and to improve control of major cardiovascular risk factors.47 The algorithms exhibited high accuracy with PPVs, NPVs, sensitivity and specificity measures all exceeding 92%. We found that the characteristics of patients with resistant hypertension identified through EHR were similar to those reported previously in observational studies. We identified significant differences in the pharmacological treatment of resistant hypertension in patients of European and African ancestry.
We observed a prevalence of resistant hypertension of 7.3% in EA and 10.6% in AA, at the lower end of prevalence estimates of 8.4%–50% from previous epidemiological studies and clinical trials.3–11 The exclusion of patients with CKD stages 4 and 5,48–50 as well as of patients with secondary causes of hypertension likely accounts for the lower prevalence rates. Consistent with prior studies, the prevalence of resistant hypertension was greater among AA compared with EA, and patients with resistant hypertension were significantly older, heavier, more likely to have CKD stage 3, and had a higher incidence of T2DM than patients with controlled hypertension.1 48–51 While the prevalence of sleep apnoea was higher in patients with resistant hypertension compared with patients with controlled hypertension, the prevalence among resistant hypertensive patients in the current study, in which sleep apnoea was diagnosed by ICD-9 or ICD-10 code, is lower than that reported in previous studies in which sleep apnoea was defined prospectively,52–54 suggesting the need for more rigorous diagnostic approaches to sleep apnoea in the clinical setting. The prevalence of CKD stage 3 was increased in EA compared with AA among both cases and controls. This unexpected finding likely resulted from the exclusion of glomerulonephritis, as a secondary cause of hypertension.55 56 In addition, a higher proportion of AA with resistant hypertension was excluded for CKD stages 4 and 5 compared with EA.55 57 58
Importantly, we found that prescribing trends differed in EA and AA patients with resistant hypertension. AA patients were more likely to be treated with direct-acting vasodilators, hydralazine and minoxidil and less likely to receive an ARB or renin inhibitor compared with EA. The prevalence of salt-sensitive hypertension is increased in AA compared with EA patients with hypertension, and thiazide-type diuretics and vasodilators are most effective in salt-sensitive hypertension.59–61 Hydralazine has also been shown to reduce mortality in AA treated for heart failure62 and awareness of this may account for increased use.63 64 The lower use of ARBs and renin inhibitors in AA may reflect clinician awareness of reduced efficacy of drugs that interrupt the renin–angiotensin–aldosterone system (RAAS) in studies of AA.65–67 Thiazide diuretics or DHP CCBs, used by definition in all patients classified as having resistant hypertension, enhance the response to RAAS interrupting drugs in AA, however.68 Similarly aliskiren may decrease BP in patients with resistant hypertension who do not respond to spironolactone.69 For these reasons, the decreased use of ARBs and renin inhibitors in AA with resistant hypertension prescribed a thiazide diuretic or DHP CCB is surprising. Whether differences in patterns of drug treatment in AA and EA patients with resistant hypertension reflect personalised prescribing or prescribing bias requires further study.
We also evaluated trends in the escalation of antihypertensive treatment in resistant hypertension by comparing medication use between case types I and II. The efficacy of spironolactone as an add-on therapy for BP lowering in patients with resistant hypertension has been supported by many studies and is suggested as a fourth-line treatment by various international guidelines.37 70–75 In the present clinical population aldosterone antagonist use increased with the addition of a fourth med with a prescription rate of approximately 3% in case type I patients compared with 12% in case type II patients, regardless of race. With extended follow-up of patients who met the case type II definition, use of an aldosterone antagonist increased to 37.4% in EA and to 41.2% in AA.
The identification of the resistant hypertensive population using the EHR is not without limitations. First, patients may not adhere to prescribed medication. The true prevalence of medication non-adherence in the resistant hypertensive population is unknown and the estimates from various studies range from as low as 16% up to 53%.15 76–78 While we could confirm that patients with resistant hypertension were prescribed three or more antihypertensive medication classes simultaneously in their EHR, without directly measuring the medication or its metabolites in a patient’s plasma or urine we are unable to confirm adherence. Using a pharmacy fill rate of <80% to exclude patients who were non-adherent with antihypertensive medications, Pimienta and Calhoun reported an incidence of true resistant hypertension of 1.9%.79 We recently reported a rigorous adherence rate of 58.8%, among hypertensive patients in an emergency department prescribed three or more antihypertensive medications based on the detection of drugs in the plasma.14 Second, we used outpatient office BP measurements to define resistant hypertension in the EHR, but ambulatory measurements would be necessary to distinguish between apparent resistant and true resistant hypertension.7 Lastly, it is possible that offsite prescriptions or discontinuations of antihypertensive medications were not captured in the EHR; we overcame this potential limitation by requiring repeated documentation of a medication over more than a month in the study algorithms. Nevertheless, in summary, we demonstrate the feasibility of identifying a large number of patients with resistant hypertension and controlled hypertension using an EHR. Using the methodology, we replicated findings previously reported in population studies,1 9 80 and identified differing patterns of antihypertensive medication use in AA and EA with resistant hypertension. Because our data are from a real-world clinical population, the findings are more generalisable to other clinical populations. Future research using these algorithms has the potential to provide larger patient populations than have been studied previously for the evaluation of outcome studies as well as genetic associations in any system where the EHRs are linked to DNA.
Supplementary Material
Acknowledgments
The authors would like to thank Dan Roden and James (Matt) Luther for their clinical suggestions that were instrumental in refining the resistant hypertension and controlled hypertension algorithms.
Footnotes
Contributors: MMS, JCD and NJB contributed to the design of the study. MMS, JSG, CPC, HN, CY and NJB contributed to the analysis and interpretation of the results. MMS and NJB contributed to the drafting of the manuscript. MMS, JSG, CPC, HN, CY, JCD and NJB contributed to the editing of the manuscript and final approval for submission. Dan Roden and Matt Luther contributed clinical insight that was instrumental to the creation of the algorithms; their contributions are outlined in the acknowledgements.
Funding: This study was supported by the National Institutes of Health (DK108444, K23AR064768, U01HG008672, HL125426 and DK038226) and a Rheumatology Research Foundation K-supplement. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s Synthetic Derivative, which is supported by institutional funding, the 1S10RR025141 instrumentation award and by the CTSA grant UL1TR000445 from National Center for Advancing Translational Sciences/National Institutes of Health.
Disclaimer: MMS affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Competing interests: NJB reports consulting for Shire HGT, Novartis Pharmaceuticals, Viamet Pharmaceuticals and serving on the Advisory Board of Alnylam Pharmaceuticals.
Patient consent: Not required.
Ethics approval: The study was approved by the Vanderbilt University Medical Center Institutional Review Board (#130848).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The full dataset is available on request to the corresponding author.
References
- 1. Calhoun DA, Jones D, Textor S, et al. . Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008;51:1403–19. 10.1161/HYPERTENSIONAHA.108.189141 [DOI] [PubMed] [Google Scholar]
- 2. Daugherty SL, Powers JD, Magid DJ, et al. . Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012;125:1635–42. 10.1161/CIRCULATIONAHA.111.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jamerson K, Weber MA, Bakris GL, et al. . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28. 10.1056/NEJMoa0806182 [DOI] [PubMed] [Google Scholar]
- 4. Hans S, Reilly JP. Resistant hypertension in 2017. Curr Opin Cardiol 2017;32:389–96. 10.1097/HCO.0000000000000412 [DOI] [PubMed] [Google Scholar]
- 5. Cai A, Calhoun DA. Resistant hypertension: an update of experimental and clinical findings. Hypertension 2017;70:5–9. 10.1161/HYPERTENSIONAHA.117.08929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahlöf B, Devereux RB, Kjeldsen SE, et al. . Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003. 10.1016/S0140-6736(02)08089-3 [DOI] [PubMed] [Google Scholar]
- 7. de la Sierra A, Segura J, Banegas JR, et al. . Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011;57:898–902. 10.1161/HYPERTENSIONAHA.110.168948 [DOI] [PubMed] [Google Scholar]
- 8. Falaschetti E, Chaudhury M, Mindell J, et al. . Continued improvement in hypertension management in England: results from the Health Survey for England 2006. Hypertension 2009;53:480–6. 10.1161/HYPERTENSIONAHA.108.125617 [DOI] [PubMed] [Google Scholar]
- 9. Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens 2014;28:463–8. 10.1038/jhh.2013.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. . A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805–16. 10.1001/jama.290.21.2805 [DOI] [PubMed] [Google Scholar]
- 11. Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension 2011;57:1076–80. 10.1161/HYPERTENSIONAHA.111.170308 [DOI] [PubMed] [Google Scholar]
- 12. Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens 2015;28:355–61. 10.1093/ajh/hpu151 [DOI] [PubMed] [Google Scholar]
- 13. Egan BM, Zhao Y, Li J, et al. . Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension 2013;62:691–7. 10.1161/HYPERTENSIONAHA.113.01448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNaughton CD, Brown NJ, Rothman RL, et al. . Systolic blood pressure and biochemical assessment of adherence: a cross-sectional analysis in the emergency department. Hypertension 2017;70:307–14. 10.1161/HYPERTENSIONAHA.117.09659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung O, Gechter JL, Wunder C, et al. . Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013;31:766–74. 10.1097/HJH.0b013e32835e2286 [DOI] [PubMed] [Google Scholar]
- 16. Tomaszewski M, White C, Patel P, et al. . High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 2014;100:855–61. 10.1136/heartjnl-2013-305063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adeyemo A, Gerry N, Chen G, et al. . A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet 2009;5:e1000564 10.1371/journal.pgen.1000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato N, Takeuchi F, Tabara Y, et al. . Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011;43:531–8. 10.1038/ng.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy D, Ehret GB, Rice K, et al. . Genome-wide association study of blood pressure and hypertension. Nat Genet 2009;41:677–87. 10.1038/ng.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newton-Cheh C, Johnson T, Gateva V, et al. . Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009;41:666–76. 10.1038/ng.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dumitrescu L, Ritchie MD, Denny JC, et al. . Genome-wide study of resistant hypertension identified from electronic health records. PLoS One 2017;12:e0171745 10.1371/journal.pone.0171745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doris PA. The genetics of blood pressure and hypertension: the role of rare variation. Cardiovasc Ther 2011;29:37–45. 10.1111/j.1755-5922.2010.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franceschini N, Le TH, Th L. Genetics of hypertension: discoveries from the bench to human populations. Am J Physiol Renal Physiol 2014;306:F1–F11. 10.1152/ajprenal.00334.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehret GB, Munroe PB, Rice KM, et al. . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–9. 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansson JH, Nelson-Williams C, Suzuki H, et al. . Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 1995;11:76–82. 10.1038/ng0995-76 [DOI] [PubMed] [Google Scholar]
- 26. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001;104:545–56. 10.1016/S0092-8674(01)00241-0 [DOI] [PubMed] [Google Scholar]
- 27. Shimkets RA, Warnock DG, Bositis CM, et al. . Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 1994;79:407–14. 10.1016/0092-8674(94)90250-X [DOI] [PubMed] [Google Scholar]
- 28. Luft FC. Mendelian forms of human hypertension and mechanisms of disease. Clin Med Res 2003;1:291–300. 10.3121/cmr.1.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ji W, Foo JN, O’Roak BJ, et al. . Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 2008;40:592–9. 10.1038/ng.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaziano JM, Concato J, Brophy M, et al. . Million veteran program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214–23. 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 31. McCarty CA, Chisholm RL, Chute CG, et al. . The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics 2011;4:13 10.1186/1755-8794-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roden DM, Pulley JM, Basford MA, et al. . Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008;84:362–9. 10.1038/clpt.2008.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newton KM, Peissig PL, Kho AN, et al. . Validation of electronic medical record-based phenotyping algorithms: results and lessons learned from the eMERGE network. J Am Med Inform Assoc 2013;20:e147–e154. 10.1136/amiajnl-2012-000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow KM, Szeto CC, Wong TY, et al. . Risk factors for thiazide-induced hyponatraemia. QJM 2003;96:911–7. 10.1093/qjmed/hcg157 [DOI] [PubMed] [Google Scholar]
- 35. Leung AA, Wright A, Pazo V, et al. . Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med 2011;124:1064–72. 10.1016/j.amjmed.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 36. Rodenburg EM, Hoorn EJ, Ruiter R, et al. . Thiazide-associated hyponatremia: a population-based study. Am J Kidney Dis 2013;62:67–72. 10.1053/j.ajkd.2013.02.365 [DOI] [PubMed] [Google Scholar]
- 37. Weber MA, Schiffrin EL, White WB, et al. . Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014;32:3–15. 10.1097/HJH.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 38. Xu H, Stenner SP, Doan S, et al. . MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc 2010;17:19–24. 10.1197/jamia.M3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu H, Doan S, Birdwell KA, et al. . An automated approach to calculating the daily dose of tacrolimus in electronic health records. AMIA Jt Summits Transl Sci Proc 2010;2010:71–5. [PMC free article] [PubMed] [Google Scholar]
- 40. Xu H, Jiang M, Oetjens M, et al. . Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc 2011;18:387–91. 10.1136/amiajnl-2011-000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levey AS, Bosch JP, Lewis JB, et al. . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 42. Tang H, Quertermous T, Rodriguez B, et al. . Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet 2005;76:268–75. 10.1086/427888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dumitrescu L, Ritchie MD, Brown-Gentry K, et al. . Assessing the accuracy of observer-reported ancestry in a biorepository linked to electronic medical records. Genet Med 2010;12:648–50. 10.1097/GIM.0b013e3181efe2df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birdwell KA, Grady B, Choi L, et al. . The use of a DNA biobank linked to electronic medical records to characterize pharmacogenomic predictors of tacrolimus dose requirement in kidney transplant recipients. Pharmacogenet Genomics 2012;22:32–42. 10.1097/FPC.0b013e32834e1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirby JC, Speltz P, Rasmussen LV, Basford M, et al. . PheKB: a catalog and workflow for creating electronic phenotype algorithms for transportability. J Am Med Inform Assoc 2016;23:1046–52. 10.1093/jamia/ocv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Team RC. R: a language and environment for statistical computing. Vienna, Austria, 2017. [Google Scholar]
- 47. Tocci G, Ferrucci A, Guida P, et al. . Use of Electronic Support for Implementing Global Cardiovascular Risk Management. High Blood Pressure & Cardiovascular Prevention 2010;17:37–47. 10.2165/11311750-000000000-00000 [DOI] [Google Scholar]
- 48. Abdel-Kader K, Dohar S, Shah N, et al. . Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens 2012;30:960–6. 10.1097/HJH.0b013e328351d08a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Nicola L, Borrelli S, Gabbai FB, et al. . Burden of resistant hypertension in hypertensive patients with non-dialysis chronic kidney disease. Kidney Blood Press Res 2011;34:58–67. 10.1159/000322923 [DOI] [PubMed] [Google Scholar]
- 50. Egan BM, Zhao Y, Axon RN, et al. . Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation 2011;124:1046–58. 10.1161/CIRCULATIONAHA.111.030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McAdam-Marx C, Ye X, Sung JC, et al. . Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in an ambulatory care setting. Clin Ther 2009;31:1116–23. 10.1016/j.clinthera.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 52. Logan AG, Perlikowski SM, Mente A, et al. . High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001;19:2271–7. 10.1097/00004872-200112000-00022 [DOI] [PubMed] [Google Scholar]
- 53. Martínez-García MA, Gómez-Aldaraví R, Gil-Martínez T, et al. . [Sleep-disordered breathing in patients with difficult-to-control hypertension]. Arch Bronconeumol 2006;42:14–20. 10.1016/S1579-2129(06)60108-0 [DOI] [PubMed] [Google Scholar]
- 54. Gonçalves SC, Martinez D, Gus M, et al. . Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007;132:1858–62. 10.1378/chest.07-1170 [DOI] [PubMed] [Google Scholar]
- 55. Byrne C, Nedelman J, Luke RG. Race, socioeconomic status, and the development of end-stage renal disease. Am J Kidney Dis 1994;23:16–22. 10.1016/S0272-6386(12)80806-7 [DOI] [PubMed] [Google Scholar]
- 56. Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005;68:914–24. 10.1111/j.1523-1755.2005.00485.x [DOI] [PubMed] [Google Scholar]
- 57. Collins AJ, Foley RN, Chavers B, et al. . ’United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012;59(Suppl 1):e1–420. 10.1053/j.ajkd.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 58. Satko SG, Freedman BI, Moossavi S. Genetic factors in end-stage renal disease. Kidney Int 2005;67:S46–S49. 10.1111/j.1523-1755.2005.09411.x [DOI] [PubMed] [Google Scholar]
- 59. Graves JW, Bloomfield RL, Buckalew VM. Plasma volume in resistant hypertension: guide to pathophysiology and therapy. Am J Med Sci 1989;298:361–5. 10.1097/00000441-198912000-00001 [DOI] [PubMed] [Google Scholar]
- 60. Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension 2002;39:982–8. 10.1161/01.HYP.0000016176.16042.2F [DOI] [PubMed] [Google Scholar]
- 61. Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol 2008;52:1749–57. 10.1016/j.jacc.2008.08.036 [DOI] [PubMed] [Google Scholar]
- 62. Taylor AL, Ziesche S, Yancy C, et al. . Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–57. 10.1056/NEJMoa042934 [DOI] [PubMed] [Google Scholar]
- 63. Dai WS, Kuller LH, Miller G. Arterial blood pressure and urinary electrolytes. J Chronic Dis 1984;37:75–84. 10.1016/0021-9681(84)90128-0 [DOI] [PubMed] [Google Scholar]
- 64. Frisancho AR, Leonard WR, Bollettino LA. Blood pressure in blacks and whites and its relationship to dietary sodium and potassium intake. J Chronic Dis 1984;37:515–9. 10.1016/0021-9681(84)90002-X [DOI] [PubMed] [Google Scholar]
- 65. Mokwe E, Ohmit SE, Nasser SA, et al. . Determinants of blood pressure response to quinapril in black and white hypertensive patients: the Quinapril Titration Interval Management Evaluation trial. Hypertension 2004;43:1202–7. 10.1161/01.HYP.0000127924.67353.86 [DOI] [PubMed] [Google Scholar]
- 66. Saunders E, Weir MR, Kong BW, et al. . A comparison of the efficacy and safety of a beta-blocker, a calcium channel blocker, and a converting enzyme inhibitor in hypertensive blacks. Arch Intern Med 1990;150:1707–13. 10.1001/archinte.1990.00040031707020 [DOI] [PubMed] [Google Scholar]
- 67. Wright JT, Dunn JK, Cutler JA, et al. . Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005;293:1595–608. 10.1001/jama.293.13.1595 [DOI] [PubMed] [Google Scholar]
- 68. Black HR, Weinberger MH, Purkayastha D, et al. . Comparative efficacy and safety of combination aliskiren/amlodipine and amlodipine monotherapy in African Americans with stage 2 hypertension. J Clin Hypertens 2011;13:571–81. 10.1111/j.1751-7176.2011.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ferrario CM. Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: antihypertensive effects and benefits beyond BP control. Life Sci 2010;86:289–99. 10.1016/j.lfs.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Djoumessi RN, Noubiap JJ, Kaze FF, et al. . Effect of low-dose spironolactone on resistant hypertension in type 2 diabetes mellitus: a randomized controlled trial in a sub-Saharan African population. BMC Res Notes 2016;9:187 10.1186/s13104-016-1987-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oxlund CS, Henriksen JE, Tarnow L, et al. . Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens 2013;31:2094–102. 10.1097/HJH.0b013e3283638b1a [DOI] [PubMed] [Google Scholar]
- 72. Oliveras A, Armario P, Clarà A, et al. . Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study - a randomized controlled trial. J Hypertens 2016;34:1863–71. 10.1097/HJH.0000000000001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams B, MacDonald TM, Morant S, et al. . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059–68. 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. NICE. Hypertension: the clinical management of primary hypertension in adults: update of Clinical Guidelines 18 and 34. Updated Nov 2016 http://guidance.nice.org.uk/CB127 (accessed Mar 2018). [PubMed]
- 75. Mancia G, Fagard R, Narkiewicz K, et al. . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 76. Burnier M, Schneider MP, Chioléro A, et al. . Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens 2001;19:335–41. 10.1097/00004872-200102000-00022 [DOI] [PubMed] [Google Scholar]
- 77. Garg JP, Elliott WJ, Folker A, et al. . Resistant hypertension revisited: a comparison of two university-based cohorts. Am J Hypertens 2005;18(5 Pt 1):619–26. 10.1016/j.amjhyper.2004.11.021 [DOI] [PubMed] [Google Scholar]
- 78. Schmieder RE, Ott C, Schmid A, et al. . Adherence to antihypertensive medication in treatment-resistant hypertension undergoing renal denervation. J Am Heart Assoc 2016;5 10.1161/JAHA.115.002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation 2012;125:1594–6. 10.1161/CIRCULATIONAHA.112.097345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sim JJ, Bhandari SK, Shi J, et al. . Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc 2013;88:1099–107. 10.1016/j.mayocp.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-021640supp001.pdf (231.5KB, pdf)