Abstract
In this article, we report a case of a 55-year-old male heart transplant recipient who presented with diarrhoea. An extensive workup for infectious diseases was negative. The patient had a colonoscopy with biopsies showing colitis that mimicked graft-versus-host disease on histopathology. After excluding other potential causes and excluding acute cellular rejection, mycophenolate mofetil was discontinued, and the patient had significant clinical improvement with increased appetite and weight gain.
Keywords: endoscopy, transplant, colitis
Background
We witnessed first-hand how a patient’s quality of life changed when we discontinued his mycophenolate mofetil (MMF) treatment. He went from having diarrhoea multiple times a day to almost back to baseline. He also feared a much worse diagnosis and was relieved when something as simple as discontinuing a medication resolved his disruptive and uncomfortable gastrointestinal (GI) symptoms.
Although MMF-induced colitis has been reported in literature, there are relatively few cases reported in heart transplant recipients, and we strongly believe this group needs to be reminded of this entity, which can have a huge impact on a patient’s quality of life. The diagnosis is one of exclusion and represents a diagnostic challenge since it mimics many other diagnoses including infections, ischaemic colitis, inflammatory bowel disease (IBD) or other medication side effects. This report will emphasise the importance considering this diagnosis when working up immune-suppressed patients with diarrhoea, since discontinuing the medication improves the patient’s symptoms and avoids unnecessary treatments and interventions.
Case presentation
A 55-year-old male patient with a medical history of non-ischaemic cardiomyopathy and an ejection fraction of 15%–20%, atrial fibrillation and ventricular tachycardia underwent an orthotopic heart transplant procedure which was complicated by allograft dysfunction requiring short-term use of inotropes and extracorporeal membrane oxygenation. He was negative for cytomegalovirus (CMV) and the donor was positive. The patient underwent induction immunosuppressive therapy with thymoglobulin, MMF and solumedrol, before he was started on maintenance immunosuppression therapy with tacrolimus 1 mg two times per day, MMF 500 mg two times per day and prednisone 15 mg daily, as well as trimethoprim/sulfamethoxazole and valganciclovir prophylaxis.
He was doing well post-transplantation with close follow-up and monitoring of kidney function which was initially normal but transiently increased thereafter then improved after decreasing tacrolimus until approximately 6 months later when he was admitted to our hospital with symptoms of non-bloody diarrhoea, dysphagia to solid food, nausea and unintentional weight loss of 2 weeks’ duration. The patient denied any associated fever, abdominal pain, vomiting, night sweats, sick contacts or travel history. On examination, vital signs were within the normal range, and the patient was pale and cachectic.
Investigations
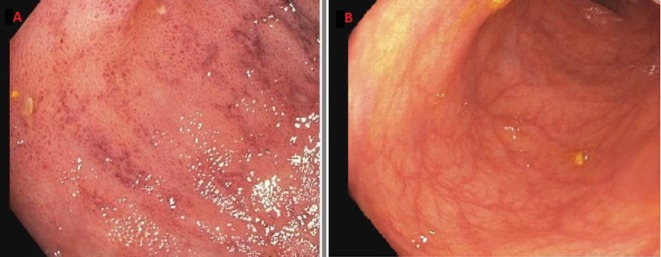
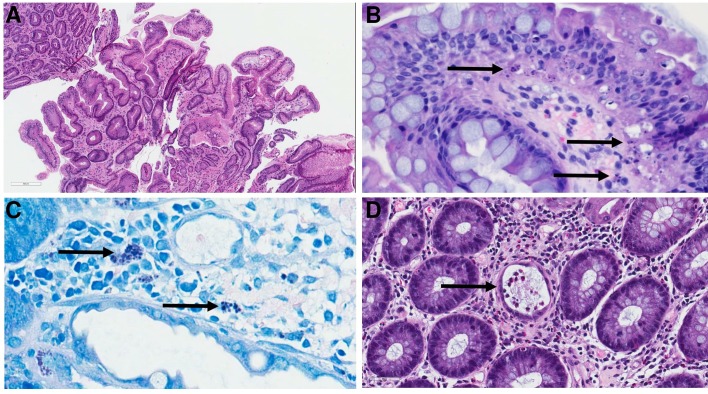
Laboratory workup showed neutropaenia, macrocytic anaemia and mild worsening of kidney function that was attributed to dehydration. Serological tests were negative for CMV (IgM and IgG). Serum levels of mycophenolate acid were not checked. Extensive workup for infectious diseases including CMV, Giardia and Cryptosporidium was negative. CT of the abdomen and pelvis showed bowel wall thickening with surrounding mesenteric inflammatory changes in the region of the cecum concerning for colitis. The patient underwent oesophagogastroduodenoscopy which showed normal oesophagus, multiple small antral ulcers and reactive gastropathy. He also underwent colonoscopy which showed mucosal oedema and erythema with small mucosal haemorrhages and punctate ulcerations in the ascending colon (figure 1A), patchy colitis in the transverse colon (figure 1B) and rectal sparing, hence multiple biopsies were obtained. Gastric biopsies showed reactive gastropathy (figure 2A) and colonic biopsies showed focal crypt abscesses or so called ‘withered crypts’ with occasional apoptosis of epithelial cells (figure 2B,C), frequent tingible body macrophages and eosinophils within the lamina propria (figure 2D). Additional special and immunohistochemical stains (CMV, adenovirus, Gomori Methenamine-Silver Nitrate Stain, Warthin-Starry, Fite and Periodic acid–Schiff) were conducted on the biopsy and no organisms (viruses, bacteria, fungi or parasites) were observed. Testing for parasitic infections, including Strongyloides, was performed due to the presence of abundant eosinophils, and were negative.
Figure 1.
(A) Colonoscopy showing erythema and colitis of the proximal ascending colon. (B) Colonoscopy showing mild colitis of the transverse colon.
Figure 2.
(A) Stomach biopsy showing reactive gastritis with ‘corkscrew’-shaped crypts and minimal inflammation within the lamina propria. (B and C) Biopsies from the colon showing focal areas of crypt apoptosis which were most pronounced within the basal layer of the epithelium (arrows). Tingible-body macrophages (ie, macrophages with engulfed apoptotic fragments present within their cytoplasm) were observed frequently within the lamina propria and these were even more conspicuous in the Fite (C) stain. (D) Colonic biopsy demonstrating crypts with flattened epithelium, intraluminal debris and inflammatory cells (arrow); these have been termed ‘withered crypts’ in the literature (1). Abundant eosinophils were also seen within these crypts and also in the surrounding stroma.
Differential diagnosis
Apoptosis targeting basally located epithelial resembles graft-versus-host disease (GVHD), which is an unlikely diagnosis in a heart transplant recipient, therefore, mimics of GVHD including infections (eg, CMV and adenovirus), medications (eg, MMF, proton pump inhibitors) and bowel preparation were all considered in the differential diagnosis.
Outcome and follow-up
MMF was discontinued and the patient’s GI complaints and appetite slowly improved. Five weeks later, the patient had significant improvement in his symptoms and he underwent an endomyocardial biopsy that was negative for acute T cell-mediated rejection despite modification of his immunosuppression regimen.
Discussion
MMF is a prodrug of the immunosuppressant mycophenolic acid. It is widely used in in solid organ and bone marrow transplantation,1 as well as in treating inflammatory disorders like lupus nephritis, autoimmune hepatitis, myasthenia gravis and autoimmune cytopenias.2 3
MMF is widely used in heart transplant patients. A large randomised controlled trial showed that MMF was associated with significantly lower mortality rates and decreased need for antirejection treatment in patients undergoing their first transplant when compared with azathioprine.4 However, the use of MMF is associated with GI side effects in about 45% of patients, which include diarrhoea (most common adverse effect), nausea, vomiting, GI bleeding, dysphagia and abdominal pain.1 5 6 These side effects commonly occur within the first 6 months of treatment,1 similar to our patient, and can cause adverse events including severe weight loss, dehydration and toxic colitis, which in turn necessitate a decrease in the dose or effects patient medication compliance, leading to subtherapeutic dosing and impaired clinical outcome.7 8 The Gastrointestinal complications in renal transplant recipients study (MITOS study) included 181 heart transplant patients and concluded that the treatment was interrupted in 8.1% of cases and discontinued in 10.9% due to GI complications, all of which were attributed to MMF.9
MMF can affect the entire alimentary tract.1 10 In the upper GI tract, it can cause ulcerative oesophagitis, reactive gastropathy and duodenal ulcers; effects similar to non-steroidal anti-inflammatory drugs.10 Colonic manifestations include diffuse colitis which can have variable histological features, including IBD-like (marked crypt architectural disarray, markedly increased lymphocytes and plasma cells in the lamina propria), ischaemic colitis-like and GVHD-like patterns (mild crypt architectural disarray or villous blunting, oedema and increased epithelial apoptosis).1 10
MMF-induced colitis has been described in literature, and is commonly seen in males and kidney transplant recipients,8 however, data in heart transplant patients are sparse. Calmet et al studied the effect of MMF on 397 patients, 36 (9%) of whom had MMF colitis.1 The majority of these patients had received kidney transplants, with only a few of them being heart transplant recipients. The most common indication for colonoscopy was diarrhoea. Colonoscopy showed mucosal erythema in 12 patients, and erosions and ulcers in 7 patients; our patient had a combination of these findings. The majority of patients had pancolitis, only five patients had right-sided colitis similar to our patient. All patients had rectal sparing. The most common histological findings were acute colitis-like changes, and only three patients had GVHD-like features, similar to our patient. Parfitt et al studied colonic biopsies from 16 patients on MMF.10 Nine of them had GVHD-like changes on biopsy. Among the nine patients, seven had a normal colonoscopy and two had patchy erythema. Papadimitriou et al described 20 cases of MMF colitis mimicking GVHD in renal transplant patients.11 Star et al also described 17 cases of MMF colitis in bone marrow transplant patients with pathological features similar to GVHD.12
The diagnosis of MMF colitis may be challenging given the similarity to other aetiologies, such as infectious colitis, IBD, ischaemic colitis and rarely GVHD. These entities may all have similar clinical presentation, endoscopic features and non-specific histopathology. Although GVHD is extremely rare in heart transplant recipients, it has been reported in the literature,13 and should be considered, since discontinuation of immunosuppression in patients with GVHD may escalate disease severity. In our patient, an exhaustive workup for infectious aetiologies was entirely negative and a recent endomyocardial biopsy showed no evidence of rejection, allowing MMF to be safely discontinued. However, close follow-up was continued since allograft dysfunction, particularly in heart transplant recipients, may have subtle symptoms or clinical manifestations. Fortunately, our patient has had three follow-up biopsies since removing MMF from his immune-suppressive regimen and all have been negative for acute T cell-mediated (International Society for Heart and Lung transplantation (ISHLT) score: 0R) and antibody-mediated (ISHLT score: pAMR0) rejection.14 15
Learning points.
The differential diagnosis for diarrhoea in immune-suppressed transplant patients includes infections and medications, including immune-suppressive drugs and antibiotics.
If the patient is on mycophenolate mofetil (MMF) as part of their immune-suppressive regimen, MMF-induced colitis must be considered.
The time between symptom onset and MMF colitis diagnosis can be unpredictable. Our patient presented with a relatively sudden onset of symptoms.
Once other diagnoses are excluded, discontinuation of MMF may result in significant clinical improvement and when properly diagnosed can prevent unnecessary treatments and interventions.
When immune-suppression regimens are modified, transplant recipients should be closely monitored for allograft rejection.
Footnotes
Contributors: OT and HS wrote the manuscript and reviewed the literature. MAH and HLS critically revised the manuscript for important intellectual content, supervised the process and approved the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Calmet FH, Yarur AJ, Pukazhendhi G, et al. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol 2015;28:366–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin A. Mycophenolate mofetil in the treatment of systemic lupus erythematosus. Eurasian J Med 2009;41:180–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Mok CC. Mycophenolate mofetil for lupus nephritis: an update. Expert Rev Clin Immunol 2015;11:1353–64. 10.1586/1744666X.2015.1087314 [DOI] [PubMed] [Google Scholar]
- 4.Kobashigawa J, Miller L, Renlund D, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 1998;66:507–15. [DOI] [PubMed] [Google Scholar]
- 5.Delacruz V, Weppler D, Island E, et al. Mycophenolate mofetil-related gastrointestinal mucosal injury in multivisceral transplantation. Transplant Proc 2010;42:82–4. 10.1016/j.transproceed.2009.12.027 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen T, Park JY, Scudiere JR, et al. Mycophenolic acid (cellcept and myofortic) induced injury of the upper GI tract. Am J Surg Pathol 2009;33:1355–63. 10.1097/PAS.0b013e3181a755bd [DOI] [PubMed] [Google Scholar]
- 7.Tierce JC, Porterfield-Baxa J, Petrilla AA, et al. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal transplant recipients. Clin Transplant 2005;19:779–84. 10.1111/j.1399-0012.2005.00421.x [DOI] [PubMed] [Google Scholar]
- 8.Dhakal P, Gami R, Giri S, et al. Mycophenolate Mofetil (MMF)-Induced Colitis, 2016. [Google Scholar]
- 9.Díaz B, González Vilchez F, Almenar L, et al. Gastrointestinal complications in heart transplant patients: MITOS study. Transplant Proc 2007;39:2397–400. 10.1016/j.transproceed.2007.07.061 [DOI] [PubMed] [Google Scholar]
- 10.Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol 2008;32:1367–72. 10.1097/PAS.0b013e31816bf3fe [DOI] [PubMed] [Google Scholar]
- 11.Papadimitriou JC, Cangro CB, Lustberg A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol 2003;11:295–302. 10.1177/106689690301100406 [DOI] [PubMed] [Google Scholar]
- 12.Star KV, Ho VT, Wang HH, et al. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol 2013;37:1319–28. 10.1097/PAS.0b013e31829ab1ef [DOI] [PubMed] [Google Scholar]
- 13.Pfitzmann R, Hummel M, Grauhan O, et al. Acute graft-versus-host disease after human heart-lung transplantation: a case report. J Thorac Cardiovasc Surg 1997;114:285–7. 10.1016/S0022-5223(97)70159-4 [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710–20. 10.1016/j.healun.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 15.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013;32:1147–62. 10.1016/j.healun.2013.08.011 [DOI] [PubMed] [Google Scholar]