Abstract
For proteins to cause IgE-mediated allergic reactions, several common characteristics have to be defined, including small molecular size, solubility and stability to changing pH levels and enzymatic degradation. Nevertheless, these features are not unique for potent allergens, but are also observed in non-allergenic proteins. Due to the increasing awareness by regulatory authorities regarding the allergy pandemic, definition of characteristics unique to potent allergens would facilitate allergenicity assessment in the future. Despite major research efforts even to date the features unique for major allergens have not been elucidated so far. The route of allergen entry into the organism determines to a great extent these required characteristics. Especially orally ingested allergens are exposed to the harsh milieu of the gastrointestinal tract but might additionally be influenced by food processing. Depending on molecular properties such as disulphide bonds contributing to protein fold and formation of conformational IgE epitopes, posttranslational protein modification or protein food matrix interactions, enzymatic and thermal stability might differ between allergens. Moreover, also ligand binding influences structural stability. In the current review article, we aim at highlighting specific characteristics and molecular pattern contributing to a stabilized protein structure and overall allergenicity.
Keywords: Allergen, Allergen stability, Protein sequence, Protein structure, Protein digestion, Protein processing, Posttranslational protein modification
1. Introduction
Since many years analyzing common allergen characteristics has been a major research focus worldwide. To answer the question what makes certain proteins to become allergens, the terms allergy and allergens have to be well defined. As proposed by an expert panel of the World Allergy Organization (WAO) in 2003, an allergic response is defined as a hypersensitivity reaction with objectively documented symptoms triggered by immunological mechanisms, which is initiated by an IgE- or non-IgE-mediated response (Johansson et al., 2004). Due to essential mechanistic differences only the impact of protein stability in the context of IgE-mediated response will be discussed in this review. Referring to IgE-mediated allergic reactions, the term allergen describes an antigen triggering an allergic response by IgE binding after initial sensitization (Aalberse, 2000). Based on this definition the most important property of allergens distinguishing them from non-allergenic proteins is the sensitizing capacity in susceptible subjects (Aalberse, 2000). Moreover, also the elicitation of an allergic immune response is an important characteristic of allergens (Masilamani et al., 2012). To trigger an allergic immune response, allergens have to come in contact with immune cells via mucosal body surfaces or the skin. In scientific literature, allergen characteristics such as solubility, stability, as well as molecular properties and molecular size have been repeatedly reviewed and summarized (Aalberse, 2000; Huby et al., 2000). Thus, specific protein properties are essential to reach the organ specific immune induction sites.
2. Implications of allergen stability based on organ specific entry routes
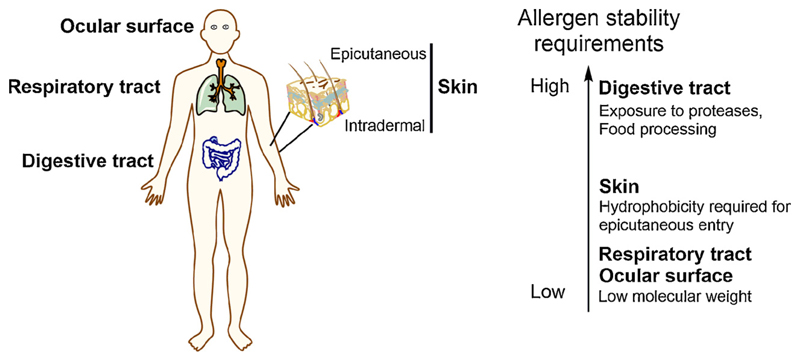
Allergens get access to the human immune system by two different entry pathways, i.e. via the skin or via mucosal surfaces (Dunkin et al., 2011). Depending on the location of allergen uptake, specific molecular properties, which in part are linked with allergen stability, are required to enable transport through epithelial barriers and interaction with organ resident immune cells. In turn, requirements for allergens stability can be allocated to different organ systems based on knowledge regarding specific characteristics of different entry routes (Fig. 1). Allergen stability can be defined as the ability of proteins to preserve their native, three-dimensional pattern after chemical, physical or protease treatment over time (Breiteneder and Mills, 2005; Deller et al., 2016).
Fig. 1.
Allergen uptake via skin and mucosal surfaces. To survive the compartment specific environment, different stability requirements enable allergens to penetrate epithelial barriers and to interact with immune cells for induction of an allergic response.
Allergens entering the organism via the skin have to be able to cross the epidermal barrier. Between the outer layer of corneocytes, lines of intercellular lipids form hydrophobic and hydrophilic structures modulating skin permeability (De Benedetto et al., 2012). Thus, allergens penetrating outer barrier of intact skin have to show hydrophobic properties or need binding to a lipophilic carrier. Due to lack of protein degrading enzymes no specific enzyme stability is required in this compartment. Furthermore, the need for specific stability properties of intradermally injected allergens such as insect venoms is even lower as after injection no further barrier has to be crossed (Fig. 1).
Inhalant allergens, such as pollen, house dust mite proteins or spores, do not require specific stability to low pH levels or to enzymes. Upon inhalation allergens need to exhibit extensive solubility in the aqueous milieu of the mucosal surfaces and the correct particular size to enter and escape mucus binding properties, as both mucus and tissue of the respiratory tract might act as an allergen reservoir (Rimmer et al., 2015). Tear film mucins show similar functional properties, due to their genuine role to prevent antigen and pathogen interaction with the ocular surface (Hodges and Dartt, 2013). Of interest, the proteolytic activity of allergens themselves seems to confer enhanced sensitizing potential by disruption of epithelial barrier layers as it has been shown e.g. for house dust mite allergens (Wan et al., 1999).
Allergen sensitizing via the gastrointestinal tract require high protein stability to ensure proteolytic and hydrolytic resistance (Breiteneder and Mills, 2005). If food processing precedes ingestion, major allergens triggering an allergic response via the oral route even have to show heat resistance. Due to the high stability requirements for proteins triggering an allergic response after oral ingestion, this article will primarily focus on this route of allergen exposure to highlight the relevance of protein stability in allergic disease.
3. Characteristics of food allergens associated with protein stability
When it comes to allergens triggering an allergic response via the oral route, several specific characteristics have been described in the scientific literature. Only few proteins account for most food allergies and many food allergens share some common characteristics, which nevertheless are not unique to them (Bannon, 2004; Breiteneder and Mills, 2005; Vickery et al., 2011). Food allergens are usually relatively abundant in the food source, which seems to enhance their chance to interact with the immune system (Bannon, 2004). Allergenic food proteins are generally relatively small with a molecular size below 70 kDa (Vickery et al., 2011) However, a molecular size of at least 3.5 kDa is necessary to induce an antibody response, as smaller fragments of e.g. digested β-lactoglobulin (BLG) were no longer immunoreactive (Bogh et al., 2013). For a long time food allergens were typically classified into two groups. Class I allergens or complete allergens were described to sensitise via the oral route (Bannon, 2004). This group included allergens found in peanut, milk or wheat, which were accepted to elicit severe, generalized symptoms. Class II allergens, also known as non-sensitising elicitors or incomplete allergens, were revealed to share homologies with aeroallergens (Aalberse, 1997; Vieths et al., 2002). Sensitisation was defined to occur indirectly towards cross-reactive aeroallergens via the respiratory route. Class II allergens were considered to be associated with milder, local symptoms such as the oral allergy syndrome (Webber and England, 2010).
In line with this classification, frequently found linear IgE binding epitopes seem to play an important role in food allergy as conformational epitopes are lost during the passage through the gastrointestinal tract (Bannon, 2004). Especially in milk and egg allergy, the presence of IgE recognizing linear epitopes was described to be associated with reactions to heated allergens and persistence of allergic symptoms (Chatchatee et al., 2001; Jarvinen et al., 2007). However, the ratio of recognised linear versus conformational epitopes was shown to vary between different milk allergens α-lactalbumin (ALA), β-casein and BLG (Madsen et al., 2014). Thus, the sensitising capacity after gastrointestinal digestion may depend on the one hand on allergen stability to proteolysis during passage through the digestive tract and on the other hand on the abundance of linear epitopes with immunostimulating capacity, as conformational epitopes may get destroyed.
4. Gastrointestinal physiology or food associated factors influencing allergen stability
4.1. Stability to gastrointestinal protein digestion
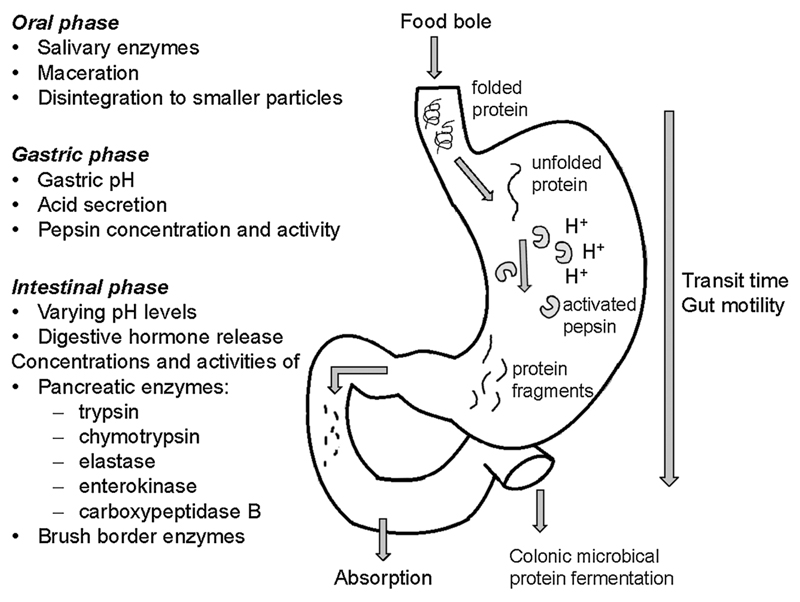
As outlined above, one of the main characteristic of proteins triggering an allergic response via the gastrointestinal tract seems to be resistance to gastrointestinal digestion. Protein digestion is influenced by several factors and consequently varies among different individuals. Digestion of ingested proteins starts with mastication and maceration in the oral cavity. Breaking food into smaller particles facilitates the subsequent gastrointestinal enzymatic digestion. In the oral cavity the enzymes α-amylase, secreted by the salivary glands, and lingual lipase start enzymatic degradation. The cleavage of 1,4-glycosidic bonds of carbohydrates by amylase affects allergens, as most are glycoproteins (Fig. 2) (Boehlke et al., 2015). After this initial oral digestion, ingested proteins are swallowed. Following a quick esophageal passage, proteins enter the stomach and denature due to the acidic gastric fluid. Besides denaturation, a low gastric pH is essential for pepsin activation. At low pH levels, pepsinogen, the inactive zymogen secreted by the parietal cells, gets activated. Moreover, unfolded proteins are more susceptible to enzymatic hydrolysis due to exposed cleavage sites. With an enzymatic optimum between pH 1.8–3.2, pepsin, an aspartic protease, hydrolyses proteins preferentially, but not exclusively, between hydrophobic and aromatic amino acid residues phenylalanine, tyrosine, tryptophan and leucine (Oka and Morihara, 1970; Tang, 1963; Trout and Fruton, 1969). In fasted state, gastric fluids of healthy adults are acidic with a pH between 1.5 and 3 and with a pepsin concentration between 0.11–0.22 mg/ml increasing up to 0.58 mg/mL after eating (Fig. 2) (Kalantzi et al., 2006; Lu et al., 2010). Gastric fluid of newborns is less acidic since the acid secretion increases with parietal cell mass (Gan et al., 2018). This implies a reduced activity of pepsin, which together with lower concentrations of other digestive enzymes may cause overall less proteolysis in the gastrointestinal tract of infants than in adults (Gan et al., 2018). Also in advanced age a reduced gastric acid production was reported, mainly in association with Helicobacter pylori infection and atrophic gastritis, and age additionally seems to impact on pepsin output (Feldman et al., 1996). Though a certain pH is needed for optimal enzyme activity, the gastric pH is far from being constant. Fluctuations of pH levels depend on the amount and composition of ingested food. While a protein rich meal has a high buffer capacity, a high fat content delays gastric emptying and leads to acidification (Charman et al., 1997). The gastric pH can increase from pH 2 in the fasted state up to pH 6.5 depending on the buffer capacity of the ingested food and pH levels decrease again with time (Gan et al., 2018; Kalantzi et al., 2006).
Fig. 2.
Protein digestion during the gastrointestinal transit. Gastrointestinal enzymes and varying pH levels influence digestion of orally ingested proteins.
Despite its clear physiological function, the role of gastric acidity is still controversially discussed in scientific literature. Due to increasing knowledge regarding pH fluctuations depending on the composition of the ingested meals, the importance of an acidic gastric environment has been questioned (Keller, 2012). Other studies concluded that enzymatic digestion in the stomach is not essential for overall protein digestion, as patients after gastric bypass surgery, which excludes main parts of the stomach from digestion, did not show signs of protein malabsorption (Bojsen-Moller et al., 2015). In contrast, the important protein digestive function of the stomach in allergies was underlined by the fact that gastric bypass patients were revealed to have an increased risk for developing allergies evidenced by increasing numbers of sensitizations after Roux-en-Y gastric bypass surgery (Shakeri-Leidenmühler et al., 2015). Moreover, pharmacologically induced gastric hypoacidity was found to be associated with food sensitization and food allergy induction in both experimental mouse studies and human observational studies in patients being treated with anti-ulcer medication due to dyspeptic disorders (DeMuth et al., 2013; Pali-Schöll et al., 2010; Schöll et al., 2005; Trikha et al., 2013; Untersmayr et al., 2005; Untersmayr et al., 2003).
As the food bole enters the intestine, the low pH of the gastric content induces secretin release, which in turn leads to secretion of bicarbonate from the pancreas to increase the pH up to 7 (Keller and Layer, 2005). The presence of amino acids in the duodenum leads to secretion of cholecystokinin which triggers release of pancreatic enzymes trypsin, chymotrypsin, carboxypeptidase A and B, enteropeptidases and elastase (Fig. 2). They are secreted as inactive zymogens and have a pH optimum at 7–8 (Keller and Layer, 2005). Activated trypsin and chymotrypsin cleave peptide bonds at the carboxyl side of basic and at the carbonyl group of aromatic amino acids, respectively (Erickson and Kim, 1990). Also the duodenal pH shows fluctuations. With a pH at 6.2 in the fasted state, the duodenal pH increases after administration of a complete nutrient drink, but slowly decreases with gastric emptying (Kalantzi et al., 2006).
4.2. Matrix effects influencing protein stability
In addition to the physiological conditions in the gut, the protein source itself influences stability to gastrointestinal protein digestion. Beside the intrinsic susceptibility of an allergen to enzymatic degradation, other components of the food can alter digestion patterns. The lipid content of food prolongs transit time and β-casein and BLG proteolysis has been shown to differ between emulsions and solutions (Macierzanka et al., 2009; Mackie et al., 2012). Therefore, also the activity of lingual, gastric and pancreatic lipase may play a significant role in allergen digestion and absorption. Phosphatidylcholine, a lipid contained in milk and gastric secrets, has been shown to hinder proteolysis of BLG (Mandalari et al., 2009). Different digestion products were found in a simulated gastrointestinal digestion with and without addition of phosphatidylcholine (Mandalari et al., 2009).
Other properties of the food matrix influence the susceptibility of proteins to digestive enzymes (Di Stasio et al., 2017; Schulten et al., 2011). A food matrix rich in protein, carbohydrates and fat was shown to influence the digestion of three different allergens, Mal d 1, Bos d 5 and Cor a 8. Food composition slowed digestion, but simultaneously also reduced the allergen uptake by Caco-2 cells, a small intestinal epithelial cell model (Schulten et al., 2011). Ber e 1, the major allergen from Brazil nut, revealed its allergenic potential only when interacting with the specific lipid fraction C of the nut. Thus, it seems to be essential to consider interactions of food components when investigating allergenic properties of proteins (Mirotti et al., 2013).
4.3. The role of protein stability in the context of food processing
Food preservation and improvement of food quality is substantially influenced by different forms of food processing (Sathe and Sharma, 2009). There is growing evidence that food processing also substantially impacts on protein structure and stability and subsequently also allergenicity (Verhoeckx et al., 2015). Especially, heat treatments such as baking, cooking, roasting, pasteurization and others change allergen stability and activity by chemical modification, unfolding protein structure and/or aggregation with the food matrix (Besler et al., 2001). Prominent examples are members of the PR-10 Bet v 1 protein family losing their allergenic activity after heating due to protein unfolding (Bohle et al., 2006). Heat treatment might also be associated with enhanced allergenic potential due to conformational changes. Pasteurization was revealed to induce aggregation of the milk proteins BLG and ALA with direct impact on allergic sensitization due to enhanced uptake via M-cells overlying intestinal Peyer’s patches (Roth-Walter et al., 2008). Nevertheless, denaturing effects of heating were also shown to reduce IgE binding capacity of BLG (Ehn et al., 2005). With regards to thermal processing the presence of food matrix such as lipids and carbohydrates is of paramount importance. A prominent example is protein glycation during the Maillard reaction (Teodorowicz et al., 2017). After roasting, peanut allergenicity was found to be enhanced by formation of aggregates influencing also protein digestibility (Gruber et al., 2005; Maleki et al., 2000). Also for the shellfish allergen tropomyosin, Maillard modification in dried fish products was described to have a higher IgE binding capacity due to creation of new epitopes being associated with primary sensitization to the glycated tropomyosin (Nakamura et al., 2005). However, glycation of fruit allergens seems to have a varying effect on allergencity. While the Maillard reaction decreased the IgE binding capacity of the PR-10 protein Pru av 1, the major cherry allergen (Gruber et al., 2004), it protected the lipid transfer protein (LTP) apple allergen Mal d 3 from denaturing thermal processing effects (Sancho et al., 2005).
In addition, there are also numerous non-thermal processing methods such as e.g. hydrolysis, fermentation UV-exposure and radiation influencing protein stability. In most cases, hydrolysis of food allergens by digestive enzymes is associated with a reduced allergenicity, which is also used for e.g. the preparation of hypoallergenic hydrolysed milk formula (Bahna, 2008). Additionally, fermentation of milk proteins by Lactobacilli was found to reduce IgE binding capacity and allergenicity (Yao et al., 2015). Lactic acid bacteria found in sourdough were furthermore able to hydrolyze wheat allergens and degrade IgE epitopes (De Angelis et al., 2007; Rizzello et al., 2006).
Nevertheless, stability to both enzymatic digestion along the gastrointestinal tract as well as resistance to different methods of food processing depends on protein characteristics such as folding and ligand binding.
5. Protein intrinsic characteristics affecting allergen stability
5.1. Protein sequence and three-dimensional protein structure
The molecular protein structure is directly linked to allergen stability. The knowledge regarding allergen structure has substantially increased during the past years and more than one hundred non-redundant allergen structures have been published so far (Dall’Antonia et al., 2014). Nevertheless, the major allergens can be grouped in a limited number of protein families (Masilamani et al., 2012).
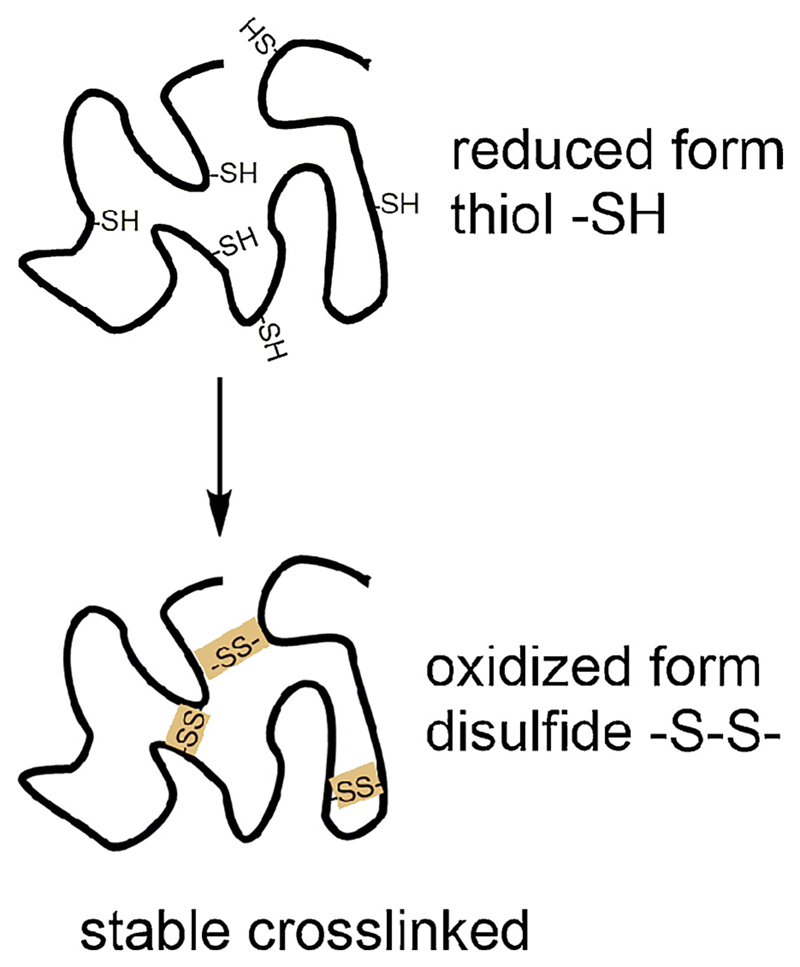
Disulphide bonds may contribute to the digestion resistance of allergens by stabilising the three-dimensional structure in the harsh environment of the gastrointestinal tract or by hindering the dislocation of digestion fragments and stabilizing IgE binding epitopes (Fig. 3) (Sen et al., 2002). The 17 kDa protein Ara h 2 has eight cysteine residues with the potential to form four intramolecular disulphide bonds. Digestion with pepsin, chymotrypsin and trypsin leads to a 10 kDa digestion resistant fragment, which contains multiple IgE binding epitopes. The chemical reduction of the disulphide bonds decreased overall allergenicity, changing the secondary and tertiary structure of the molecule and making it susceptible to proteases (Sen et al., 2002). The importance of disulphide bonds for the allergenicity of Ara h 2 as well as Ara h 6 was confirmed by another study (Apostolovic et al., 2013). Chemical modification, reduction and alkylation, changed the tertiary structure and decreased IgE binding. After the digestion of peach and barley LTP the intramolecular disulphide bonds prevent the dissociation of digestion fragments (Wijesinha-Bettoni et al., 2010). As a consequence, allergen epitopes remain intact and allergenic. The importance of disulphide bonds for the stability to digestion was confirmed for Pru p 3, in another study. While the unfolded protein has a reduced sensitising capacity, it was still able to activate T-cells (Toda et al., 2011).
Fig. 3.
Disulphide bonds contribute to protein stability. Due to the presence of intramolecular disulphide bonds, the three-dimensional allergen structure and IgE binding epitopes are preserved by enhanced protein resistance to proteolytic enzymes.
The importance of the structural integrity of allergens has been shown in a study comparing the reactions against native and denatured allergens. All three proteins, BLG, ALA and β-casein had a reduced antibody binding capacity when denatured, due to the loss of conformational epitopes during denaturation. When given orally, the sensitizing capacity of the three allergens correlated with their susceptibility to digestion (Madsen et al., 2014). The three-dimensional structure determines whether potential enzymatic cleavage sites are accessible to proteases. Cleavage sites of wheat lipid transfer protein (LTP) are found in loop regions on the surface of the protein (Abdullah et al., 2016). In a study evaluating Gly m 5, a major soybean allergen, three stable fragments remained after simulated gastric and intestinal digestion and were modelled on the three-dimensional molecule structure (Zheng et al., 2017). From several cleavage sites only those found solvent exposed on the protein surface were cleaved. This suggests that the compact trimeric complex of the protein with its β-barrel structures protects from enzymatic degradation.
The molecular structure also determines the stability under varying pH levels, which in turn has impact on the enzymatic degradation during gastric and intestinal digestion. To give an example, the secondary structure of peanut conglutins does not change when lowering the pH (Apostolovic et al., 2016). In line also LTP from peach and barley remain folded at pH 1.8 (Wijesinha-Bettoni et al., 2010). These proteins were described to be completely resistant to gastric digestion at pH 2.5 and retain the structure even in the acidic gastric environment with a very rigid structure preventing degradation by pepsin. Under duodenal conditions only two out of 14 possible cleavage sites were cut. Molecular dynamics simulations revealed proteolytic cleavage to preferentially take place at sites with relatively mobile amino acid side chains, which also explained the higher digestion rate of peach LTP compared to barley LTP. This indicates that the flexibility of the protein structure plays an important role in its digestibility, by granting access to peptide bonds and, thus, facilitating enzymatic cleavage (Wijesinha-Bettoni et al., 2010). In contrast, the Bet v 1 homologues Api g 1, Mal d 1, Pru p 1 and Cor a 1 unfold, at least partially, at a pH of 2.5. Pepsin digestion rapidly degraded their IgE binding sites and eliminated the histamine releasing ability. These results suggest that, apart from slight differences, all proteins of the PR-10 family are highly susceptible to digestion, due to their shared structural scaffold (Sancho et al., 2011). However, the allergens Mal d 1 and Cor a 1.04 retain their T cell-activating capacity after digestion, and can therefore induce T-cell mediated immune reactions (Schimek et al., 2005).
Protein structure also seem to be involved in stability to heat treatment as higher abundance of β-structures were found in proteins being resistant to thermal processing (Chakravarty and Varadarajan, 2000).
5.2. Posttranslational protein modification and allergen stability
Posttranslational protein modification such as glycosylation, nitration, oxidation or reduction substantially influence protein fold and structure and were shown to impact on protein stability. It is well accepted that many allergens undergo posttranslational glycosylation in the endoplasmic reticulum and have surface exposed carbohydrate structures (Verhoeckx et al., 2015). These glycosylation sites contribute to the interaction of allergens with specific immune cell receptors facilitating type 2 polarization (Chieppa et al., 2003; Shreffler et al., 2006). Furthermore, the protein structure stabilizing effect of glycosylation was demonstrated and discussed in the context of glycosylated seed storage proteins (Pedrosa et al., 2000; Wormald and Dwek, 1999).
Succinylation of the soy proteins β-conglycinin and glycinin was associated with structural changes. Depending on the number of succinyl groups added to lysine residues, the secondary and tertiary structure especially of glycinin was substantially changed in association with a reduced thermal stability and suppression of thermal aggregation of soy proteins (Wan et al., 2018).
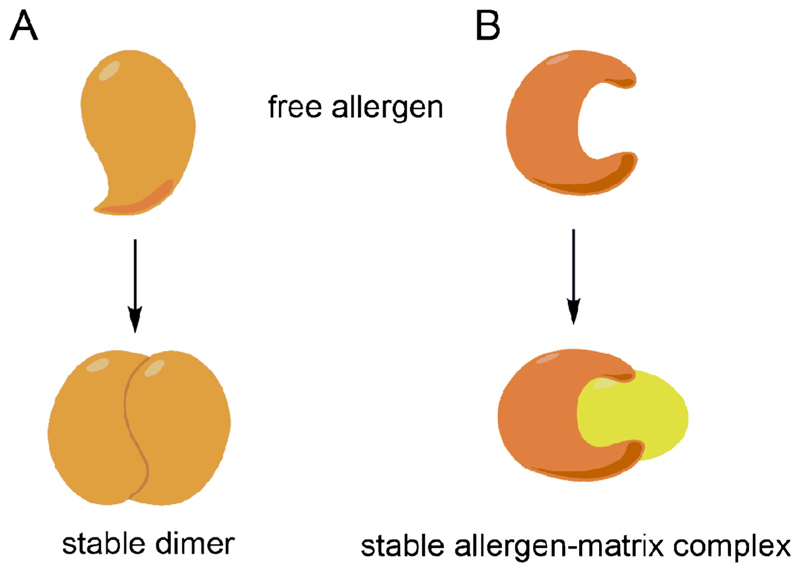
Also for nitration of tyrosine residues (Bottari, 2015), a paramount impact of this chemical protein modification occurring due to environmental pollution or during inflammation in the human body on protein stability was observed. For the egg-white allergen ovalbumin an enhanced susceptibility to gastric digestion associated with a suppressed de novo sensitizing capacity via the oral route was observed in an experimental model of food allergy (Untersmayr et al., 2010). However, in situations with preformed IgE antibodies enhanced anaphylactic capacity of the nitrated food protein BLG was observed, which could be attributed to structural changes associated with dimerization (Fig. 4A) (Diesner et al., 2015).
Fig. 4.
Different forms of protein interactions account for protein stability. Either by formation of oligomers or by interaction with matrix components, food allergens are modified, which contribute to conserved three-dimensional structure and overall stability.
5.3. The influence of ligand binding on protein stability
Protein structure is also influencing the availability of ligand binding sites, while ligand binding is essential for protein fold. Binding of ligands such as metal ions, lipids or vitamins is associated with changes in stability of allergens to thermal and proteolytic treatment (Fig. 4B) (Breiteneder and Mills, 2005). Some allergens bind ligands on the surface, others have binding domains buried within the molecule in cavities or tunnel like structures. Parvalbumins and caseins are both able to bind calcium, but in different ways (Breiteneder and Mills, 2005). Parvalbumins have an EF-hand calcium-binding domain and calcium binding influences protein conformation and conformational IgE-epitopes (Declercq et al., 1991; Untersmayr et al., 2006). For caseins chelating metal ions such as calcium was described to be associated with nanocluster and micelles formation (Holt et al., 1998; Tuinier and de Kruif, 2002). In both cases, however, calcium-binding was described to substantially influence structural behavior, stability and IgE binding potential (Bugajska-Schretter et al., 2000; Tuinier and de Kruif, 2002).
Due to exposed hydrophobic structures on allergens, lipids frequently interact with allergens (Bublin et al., 2014). Lipid binding is common for LTPs or Bet v 1 homologues, as well as BLG. The stabilizing effect of lipid ligands has also been shown for the digestion-labile allergen Ara h 8, a Bet v 1 homologous allergen in peanuts (Petersen et al., 2014). The recombinant Ara h 8 molecule without lipid ligands degraded more rapidly during in vitro digestion than the native molecule (Petersen et al., 2014). In contrast, in vitro gastroduodenal digestion of wheat LTP is enhanced after lipid ligand binding (Abdullah et al., 2016). After 120 min of wheat LTP digestion only 30% of the protein with a lipid ligand remained intact compared to 80% of the protein without ligand bound. While the cleavage pattern remains the same, the rate of proteolysis is accelerated. The lipid ligand increases the flexibility of the tyrosine residue 79 by displacing it from the hydrophobic cavity. As a result the cleavage site is more accessible to enzymes. In contrast lipid binding to peach LTP did not affect digestion (Abdullah et al., 2016).
Very recently binding of retinoic acid to the hydrophobic intramolecular pocket of BLG was shown to enhance stability to lysosomal degradation, which was associated with suppressed immunogenicity (Hufnagl et al., 2018). Of interest, stability to degradation in this specific case was associated with a lower allergenic potential, which has to be taken into consideration in future research studies.
6. Future directions and clinical outlook
Allergen stability directly contributes to allergenicity. Even though in scientific literature the correlation between digestion stability and allergenicity is not fully clear (Bogh and Madsen, 2016), it is increasingly accepted that gastrointestinal digestion clearly has an influence in food allergies (Untersmayr and Jensen-Jarolim, 2008). Despite common properties shared by digestion stable allergens, such as three-dimensional structure under changing pH and hindering enzymatic cleavage, disulphide bonds and binding of ligands stabilizing protein structure, the allergenic potential of food proteins may always be considered in the context of encountered gastrointestinal conditions, including pH and enzyme levels, food matrix or the immune status of the individual. To date there is no test available sufficiently predicting allergenicity of novel food proteins (Naegeli et al., 2017). Therefore, an EFSA expert panel recently recommended a stepwise approach to assess allergenic potential of novel food proteins (Naegeli et al., 2017). Still, further knowledge about the complex interaction of food processing and enzymatic degradation in the gastrointestinal tract and subsequent interaction with the intestinal immune system is needed to be able to reduce the disease burden of allergies and develop effective therapies. Additionally, new insights shall help allergic patients to minimise the risk for severe allergic reactions and anaphylaxis. Thus, it is extremely important to generate more protein specific knowledge and data regarding protein-matrix interactions to define in detail the role of allergen stability in the context of allergenicity.
Acknowledgements
The research work of the authors is supported by the Austrian Science Fund (FWF) project KLI284-B00, by the BMWFW-AWS PRIZE project P1621673 as well as by a research grant of Nordmark GmbH (all to EU).
References
- Aalberse RC. Food allergens. Environ Toxicol Pharm. 1997;4:55–60. doi: 10.1016/s1382-6689(97)10042-4. [DOI] [PubMed] [Google Scholar]
- Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–238. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- Abdullah SU, Alexeev Y, Johnson PE, Rigby NM, Mackie AR, Dhaliwal B, Mills EN. Ligand binding to an allergenic lipid transfer protein enhances conformational flexibility resulting in an increase in susceptibility to gastroduodenal proteolysis. Sci Rep. 2016;6:30279. doi: 10.1038/srep30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolovic D, Luykx D, Warmenhoven H, Verbart D, Stanic-Vucinic D, de Jong GA, Velickovic TC, Koppelman SJ. Reduction and alkylation of peanut allergen isoforms Ara h 2 and Ara h 6; characterization of intermediate- and end products. Biochim Biophys Acta. 2013;1834:2832–2842. doi: 10.1016/j.bbapap.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Apostolovic D, Stanic-Vucinic D, de Jongh HH, de Jong GA, Mihailovic J, Radosavljevic J, Radibratovic M, Nordlee JA, Baumert JL, Milcic M, Taylor SL, et al. Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci Rep. 2016;6:29249. doi: 10.1038/srep29249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahna SL. Hypoallergenic formulas: optimal choices for treatment versus prevention. Ann Allergy Asthma Immunol. 2008;101:453–459. doi: 10.1016/S1081-1206(10)60281-5. quiz 459-61, 481. [DOI] [PubMed] [Google Scholar]
- Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4:43–46. doi: 10.1007/s11882-004-0042-0. [DOI] [PubMed] [Google Scholar]
- Besler M, Steinhart H, Paschke A. Stability of food allergens and allergenicity of processed foods. J Chromatogr B Biomed Sci Appl. 2001;756:207–228. doi: 10.1016/s0378-4347(01)00110-4. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Zierau O, Hannig C. Salivary amylase - the enzyme of unspecialized euryphagous animals. Arch Oral Biol. 2015;60:1162–1176. doi: 10.1016/j.archoralbio.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Bogh KL, Barkholt V, Madsen CB. The sensitising capacity of intact beta-lactoglobulin is reduced by co-administration with digested beta-lactoglobulin. Int Arch Allergy Immunol. 2013;161:21–36. doi: 10.1159/000343042. [DOI] [PubMed] [Google Scholar]
- Bogh KL, Madsen CB. Food Allergens: is There a correlation between stability to digestion and allergenicity? Crit Rev Food Sci Nutr. 2016;56:1545–1567. doi: 10.1080/10408398.2013.779569. [DOI] [PubMed] [Google Scholar]
- Bohle B, Zwolfer B, Heratizadeh A, Jahn-Schmid B, Antonia YD, Alter M, Keller W, Zuidmeer L, van Ree R, Werfel T, Ebner C. Cooking birch pollen-related food: divergent consequences for IgE- and T cell-mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. 2006;118:242–249. doi: 10.1016/j.jaci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller KN, Jacobsen SH, Dirksen C, Jorgensen NB, Reitelseder S, Jensen JE, Kristiansen VB, Holst JJ, van Hall G, Madsbad S. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr. 2015;102:600–607. doi: 10.3945/ajcn.115.109298. [DOI] [PubMed] [Google Scholar]
- Bottari SP. Protein tyrosine nitration: a signaling mechanism conserved from yeast to man. Proteomics. 2015;15:185–187. doi: 10.1002/pmic.201400592. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Mills EN. Molecular properties of food allergens. J Allergy Clin Immunol. 2005;115:14–23. doi: 10.1016/j.jaci.2004.10.022. quiz 24. [DOI] [PubMed] [Google Scholar]
- Bublin M, Eiwegger T, Breiteneder H. Do lipids influence the allergic sensitization process? J Allergy Clin Immunol. 2014;134:521–529. doi: 10.1016/j.jaci.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajska-Schretter A, Grote M, Vangelista L, Valent P, Sperr WR, Rumpold H, Pastore A, Reichelt R, Valenta R, Spitzauer S. Purification, biochemical, and immunological characterisation of a major food allergen: different immunoglobulin E recognition of the apo- and calcium-bound forms of carp parvalbumin. Gut. 2000;46:661–669. doi: 10.1136/gut.46.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Varadarajan R. Elucidation of determinants of protein stability through genome sequence analysis. FEBS Lett. 2000;470:65–69. doi: 10.1016/s0014-5793(00)01267-9. [DOI] [PubMed] [Google Scholar]
- Charman WN, Porter CJ, Mithani S, Dressman JB. Physiochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86:269–282. doi: 10.1021/js960085v. [DOI] [PubMed] [Google Scholar]
- Chatchatee P, Jarvinen KM, Bardina L, Beyer K, Sampson HA. Identification of IgE- and IgG-binding epitopes on alpha(s1)-casein: differences in patients with persistent and transient cow's milk allergy. J Allergy Clin Immunol. 2001;107:379–383. doi: 10.1067/mai.2001.112372. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- Dall’Antonia F, Pavkov-Keller T, Zangger K, Keller W. Structure of allergens and structure based epitope predictions. Methods. 2014;66:3–21. doi: 10.1016/j.ymeth.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M, Rizzello CG, Scala E, De Simone C, Farris GA, Turrini F, Gobbetti M. Probiotic preparation has the capacity to hydrolyze proteins responsible for wheat allergy. J Food Prot. 2007;70:135–144. doi: 10.4315/0362-028x-70.1.135. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq JP, Tinant B, Parello J, Rambaud J. Ionic interactions with parvalbumins. Crystal structure determination of pike 4.10 parvalbumin in four different ionic environments. J Mol Biol. 1991;220:1017–1039. doi: 10.1016/0022-2836(91)90369-h. [DOI] [PubMed] [Google Scholar]
- Deller MC, Kong L, Rupp B. Protein stability: a crystallographer's perspective. Acta Crystallogr F Struct Biol Commun. 2016;72:72–95. doi: 10.1107/S2053230X15024619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMuth K, Stecenko A, Sullivan K, Fitzpatrick A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013;34:227–232. doi: 10.2500/aap.2013.34.3657. [DOI] [PubMed] [Google Scholar]
- Di Stasio L, Picariello G, Mongiello M, Nocerino R, Berni Canani R, Bavaro S, Monaci L, Ferranti P, Mamone G. Peanut digestome: identification of digestion resistant IgE binding peptides. Food Chem Toxicol. 2017;107:88–98. doi: 10.1016/j.fct.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Diesner SC, Schultz C, Ackaert C, Oostingh GJ, Ondracek A, Stremnitzer C, Singer J, Heiden D, Roth-Walter F, Fazekas J, Assmann VE, et al. Nitration of beta-lactoglobulin but not of ovomucoid enhances anaphylactic responses in food allergic mice. PLoS One. 2015;10:e0126279. doi: 10.1371/journal.pone.0126279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011;128:1251–1258 e2. doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehn BM, Allmere T, Telemo E, Bengtsson U, Ekstrand B. Modification of IgE binding to beta-lactoglobulin by fermentation and proteolysis of cow’s milk. J Agric Food Chem. 2005;53:3743–3748. doi: 10.1021/jf048121w. [DOI] [PubMed] [Google Scholar]
- Erickson RH, Kim YS. Digestion and absorption of dietary protein. Annu Rev Med. 1990;41:133–139. doi: 10.1146/annurev.me.41.020190.001025. [DOI] [PubMed] [Google Scholar]
- Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–1052. doi: 10.1053/gast.1996.v110.pm8612992. [DOI] [PubMed] [Google Scholar]
- Gan J, Bornhorst GM, Henrick BM, German JB. Protein digestion of baby foods: study approaches and implications for infant health. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber P, Becker WM, Hofmann T. Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J Agric Food Chem. 2005;53:2289–2296. doi: 10.1021/jf048398w. [DOI] [PubMed] [Google Scholar]
- Gruber P, Vieths S, Wangorsch A, Nerkamp J, Hofmann T. Maillard reaction and enzymatic browning affect the allergenicity of Pru av 1, the major allergen from cherry (Prunus avium) J Agric Food Chem. 2004;52:4002–4007. doi: 10.1021/jf035458+. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Dartt DA. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013;117:62–78. doi: 10.1016/j.exer.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C, Timmins PA, Errington N, Leaver J. A core-shell model of calcium phosphate nanoclusters stabilized by beta-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements. Eur J Biochem. 1998;252:73–78. doi: 10.1046/j.1432-1327.1998.2520073.x. [DOI] [PubMed] [Google Scholar]
- Huby RD, Dearman RJ, Kimber I. Why are some proteins allergens? Toxicol Sci. 2000;55:235–246. doi: 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- Hufnagl K, Ghosh D, Wagner S, Fiocchi A, Dahdah L, Bianchini R, Braun N, Steinborn R, Hofer M, Blaschitz M, Roth GA, et al. Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T-cell epitope. Sci Rep. 2018;8 doi: 10.1038/s41598-018-19883-0. 1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62:758–765. doi: 10.1111/j.1398-9995.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, october 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23:165–176. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- Keller J. Do we need gastric acid? Dtsch Med Wochenschr. 2012;137:1818–1821. doi: 10.1055/s-0032-1305051. [DOI] [PubMed] [Google Scholar]
- Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(Suppl. 6):vi1–28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PJ, Hsu PI, Chen CH, Hsiao M, Chang WC, Tseng HH, Lin KH, Chuah SK, Chen HC. Gastric juice acidity in upper gastrointestinal diseases. World J Gastroenterol. 2010;16:5496–5501. doi: 10.3748/wjg.v16.i43.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macierzanka A, Sancho AI, Mills EN, Rigby NM, Mackie AR. Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin. Soft Matter. 2009;5:538–550. [Google Scholar]
- Mackie A, Knulst A, Le TM, Bures P, Salt L, Mills EN, Malcolm P, Andreou A, Ballmer-Weber BK. High fat food increases gastric residence and thus thresholds for objective symptoms in allergic patients. Mol Nutr Food Res. 2012;56:1708–1714. doi: 10.1002/mnfr.201200330. [DOI] [PubMed] [Google Scholar]
- Madsen JL, Kroghsbo S, Madsen CB, Pozdnyakova I, Barkholt V, Bogh KL. The impact of structural integrity and route of administration on the antibody specificity against three cow’s milk allergens - a study in brown Norway rats. Clin Transl Allergy. 2014;4:25. doi: 10.1186/2045-7022-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000;106:763–768. doi: 10.1067/mai.2000.109620. [DOI] [PubMed] [Google Scholar]
- Mandalari G, Mackie AM, Rigby NM, Wickham MS, Mills EN. Physiological phosphatidylcholine protects bovine beta-lactoglobulin from simulated gastrointestinal proteolysis. Mol Nutr Food Res. 2009;53(Suppl. 1):S131–S139. doi: 10.1002/mnfr.200800321. [DOI] [PubMed] [Google Scholar]
- Masilamani M, Commins S, Shreffler W. Determinants of food allergy. Immunol Allergy Clin North Am. 2012;32:11–33. doi: 10.1016/j.iac.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirotti L, Florsheim E, Rundqvist L, Larsson G, Spinozzi F, Leite-de-Moraes M, Russo M, Alcocer M. Lipids are required for the development of Brazil nut allergy: the role of mouse and human iNKT cells. Allergy. 2013;68:74–83. doi: 10.1111/all.12057. [DOI] [PubMed] [Google Scholar]
- Naegeli H, Birch AN, Casacuberta J, De Schrijver A, Gralak MA, Guerche P, Jones H, Manachini B, Messéan A, Nielsen EE, Nogué F, et al. Guidance on allergenicity assessment of genetically modified plants. EFSA J. 2017;15:4862–4911. doi: 10.2903/j.efsa.2017.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Watanabe K, Ojima T, Ahn DH, Saeki H. Effect of maillard reaction on allergenicity of scallop tropomyosin. J Agric Food Chem. 2005;53:7559–7564. doi: 10.1021/jf0502045. [DOI] [PubMed] [Google Scholar]
- Oka T, Morihara K. Specificity of pepsin: size and property of the active site. FEBS Lett. 1970;10:222–224. doi: 10.1016/0014-5793(70)80633-0. [DOI] [PubMed] [Google Scholar]
- Pali-Schöll I, Herzog R, Wallmann J, Szalai K, Brunner R, Lukschal A, Karagiannis P, Diesner SC, Jensen-Jarolim E. Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin Exp Allergy. 2010;40:1091–1098. doi: 10.1111/j.1365-2222.2010.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa C, De Felice FG, Trisciuzzi C, Ferreira ST. Selective neoglycosylation increases the structural stability of vicilin, the 7S storage globulin from pea seeds. Arch Biochem Biophys. 2000;382:203–210. doi: 10.1006/abbi.2000.2024. [DOI] [PubMed] [Google Scholar]
- Petersen A, Rennert S, Kull S, Becker WM, Notbohm H, Goldmann T, Jappe U. Roasting and lipid binding provide allergenic and proteolytic stability to the peanut allergen Ara h 8. Biol Chem. 2014;395:239–250. doi: 10.1515/hsz-2013-0206. [DOI] [PubMed] [Google Scholar]
- Rimmer J, Santos C, Yli-Panula E, Noronha V, Viander M. Clinical and laboratory studies of the fate of intranasal allergen. PLoS One. 2015;10:e0127477. doi: 10.1371/journal.pone.0127477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzello CG, De Angelis M, Coda R, Gobbetti M. Use of selected sourdough lactic acid bacteria to hydrolyze wheat and rye proteins responsible for cereal allergy. Eur Food Res Technol. 2006;223:405–411. [Google Scholar]
- Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, Jensen-Jarolim E, Mayer L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- Sancho AI, Rigby NM, Zuidmeer L, Asero R, Mistrello G, Amato S, Gonzalez-Mancebo E, Fernandez-Rivas M, van Ree R, Mills EN. The effect of thermal processing on the IgE reactivity of the non-specific lipid transfer protein from apple, Mal d 3. Allergy. 2005;60:1262–1268. doi: 10.1111/j.1398-9995.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Sancho AI, Wangorsch A, Jensen BM, Watson A, Alexeev Y, Johnson PE, Mackie AR, Neubauer A, Reese G, Ballmer-Weber B, Hoffmann-Sommergruber K, et al. Responsiveness of the major birch allergen Bet v 1 scaffold to the gastric environment: impact on structure and allergenic activity. Mol Nutr Food Res. 2011;55:1690–1699. doi: 10.1002/mnfr.201100025. [DOI] [PubMed] [Google Scholar]
- Sathe SK, Sharma GM. Effects of food processing on food allergens. Mol Nutr Food Res. 2009;53:970–978. doi: 10.1002/mnfr.200800194. [DOI] [PubMed] [Google Scholar]
- Schimek EM, Zwolfer B, Briza P, Jahn-Schmid B, Vogel L, Vieths S, Ebner C, Bohle B. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J Allergy Clin Immunol. 2005;116:1327–1333. doi: 10.1016/j.jaci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, Scheiner O, Jensen-Jarolim E. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–160. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- Schulten V, Lauer I, Scheurer S, Thalhammer T, Bohle B. A food matrix reduces digestion and absorption of food allergens in vivo. Mol Nutr Food Res. 2011;55:1484–1491. doi: 10.1002/mnfr.201100234. [DOI] [PubMed] [Google Scholar]
- Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol. 2002;169:882–887. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- Shakeri-Leidenmühler S, Lukschal A, Schultz C, Bohdjalian A, Langer F, Birsan T, Diesner SC, Greisenegger EK, Scheiner O, Kopp T, Jensen-Jarolim E, et al. Surgical elimination of the gastric digestion by Roux-en-Y gastric bypass impacts on food sensitisation-a pilot study. Obes Surg. 2015;25:2268–2275. doi: 10.1007/s11695-015-1689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- Tang J. Specificity of pepsin and its dependence on a possible ‘hydrophobicbinding site’. Nature. 1963;199:1094–1095. doi: 10.1038/1991094a0. [DOI] [PubMed] [Google Scholar]
- Teodorowicz M, van Neerven J, Savelkoul H. food processing: the influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients. 2017;9 doi: 10.3390/nu9080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Reese G, Gadermaier G, Schulten V, Lauer I, Egger M, Briza P, Randow S, Wolfheimer S, Kigongo V, Del Mar San Miguel Moncin M, et al. Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen. J Allergy Clin Immunol. 2011;128:1022–1030 e1-e7. doi: 10.1016/j.jaci.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Trikha A, Baillargeon JG, Kuo YF, Tan A, Pierson K, Sharma G, Wilkinson G, Bonds RS. Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications. Pediatr Allergy Immunol. 2013;24:582–588. doi: 10.1111/pai.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout GE, Fruton JS. The side-chain specificity of pepsin. Biochemistry. 1969;8:4183–4190. doi: 10.1021/bi00838a041. [DOI] [PubMed] [Google Scholar]
- Tuinier R, de Kruif CG. Stability of casein micelles in milk. J Chem Phys. 2002;117:1290–1295. [Google Scholar]
- Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, Riemer AB, Ankersmit HJ, Scheiner O, Boltz-Nitulescu G, Jensen-Jarolim E. Antiulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–658. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Diesner SC, Oostingh GJ, Selzle K, Pfaller T, Schultz C, Zhang Y, Krishnamurthy D, Starkl P, Knittelfelder R, Förster-Waldl E, et al. Nitration of the egg-allergen ovalbumin enhances protein allergenicity but reduces the risk for oral sensitization in a murine model of food allergy. PLoS One. 2010;5:e14210. doi: 10.1371/journal.pone.0014210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008;121:1301–1308. doi: 10.1016/j.jaci.2008.04.025. quiz 1309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, Riemer A, Kraml G, Kinaciyan T, Spitzauer S, Boltz-Nitulescu G, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–623. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Szalai K, Riemer AB, Hemmer W, Swoboda I, Hantusch B, Schöll I, Spitzauer S, Scheiner O, Jarisch R, Boltz-Nitulescu G, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–1461. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, Herouet-Guicheney C, Holzhauser T, Shimojo R, van der Bolt N, Wichers H, et al. Food processing and allergenicity. Food Chem Toxicol. 2015;80:223–240. doi: 10.1016/j.fct.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Vickery BP, Chin S, Burks AW. Pathophysiology of food allergy. Pediatr Clin North Am. 2011;58:363–376. doi: 10.1016/j.pcl.2011.02.012. ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu J, Guo S. Effects of succinylation on the structure and thermal aggregation of soy protein isolate. Food Chem. 2018;245:542–550. doi: 10.1016/j.foodchem.2017.10.137. [DOI] [PubMed] [Google Scholar]
- Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–108. doi: 10.1016/j.anai.2009.11.007. quiz 109-10, 117. [DOI] [PubMed] [Google Scholar]
- Wijesinha-Bettoni R, Alexeev Y, Johnson P, Marsh J, Sancho AI, Abdullah SU, Mackie AR, Shewry PR, Smith LJ, Mills EN. The structural characteristics of nonspecific lipid transfer proteins explain their resistance to gastroduodenal proteolysis. Biochemistry. 2010;49:2130–2139. doi: 10.1021/bi901939z. [DOI] [PubMed] [Google Scholar]
- Wormald MR, Dwek RA. Glycoproteins: glycan presentation and protein-fold stability. Structure. 1999;7:R155–R160. doi: 10.1016/s0969-2126(99)80095-1. [DOI] [PubMed] [Google Scholar]
- Yao M, Xu Q, Luo Y, Shi J, Li Z. Study on reducing antigenic response and IgE-binding inhibitions of four milk proteins of Lactobacillus casei 1134. J Sci Food Agric. 2015;95:1303–1312. doi: 10.1002/jsfa.6823. [DOI] [PubMed] [Google Scholar]
- Zheng S, Qin G, Tian H, Zhang F. Three-dimensional structure of Gly m 5 (beta-conglycinin) plays an important role in its stability and overall allergenicity. Food Chem. 2017;234:381–388. doi: 10.1016/j.foodchem.2017.05.020. [DOI] [PubMed] [Google Scholar]