Abstract
Pre-exposure prophylaxis (PrEP), the use of antiretroviral medications by HIV-uninfected individuals to prevent acquisition of HIV, represents a promising prevention option but important public health questions about PrEP remain. This review article updates the current evidence base for PrEP to address questions about effectiveness, safety and risk compensation.
Daily oral FTC-TDF PrEP is highly efficacious in preventing HIV acquisition in individuals at risk from a range of different types of sexual exposure. There is good evidence of efficacy in women and men, and when men who have sex with men use event-based dosing. Studies have been conducted in several country contexts and epidemics. It is clear that adherence differs substantially and as a consequence there are questions about the public health benefit of PrEP. Oral FTC-TDF PrEP has been shown to be extremely safe with minimal impact on kidney, bone or pregnancy outcomes, and to date there is no evidence that the effectiveness of PrEP has been diminished by risk compensation during open-label and programmatic follow-up. It is too early to fully assess the impact of PrEP rollout on STI incidence at a population level.
Many challenges remain. Access to PrEP is limited and disparities exist, including by race and gender. Different pricing and access models for PrEP need to be explored to avoid further widening inequalities. The optimal combination prevention programme needs to be defined, and this will depend on local epidemiology, service provision and cost-effectiveness.
Introduction
Pre-exposure prophylaxis (PrEP), the use of antiretroviral medications by HIV-uninfected individuals to prevent acquisition of HIV, represents a promising prevention option.
HIV prevention strategies that include both targeted and broad ‘test and treat’ interventions have not had the predicted impact on the HIV epidemic (1). Modelling studies suggest that PrEP has the potential to curtail the HIV epidemic when used as part of a combination public health prevention strategy(2–4). The estimated number needed to treat to prevent one new infection might be as low as 13 when PrEP is given to a group at high risk of HIV (e.g. incidence up to 9%)(5). Effectiveness studies and open label extension demonstration projects in both high and low income settings have shown that PrEP can be delivered feasibly within existing health systems. However, uptake, reimbursement packages and provision of PrEP currently vary from country to country, where national PrEP programmes have been established.
Early data from San Francisco suggest that roll-out of PrEP, in combination with other strategies, has been associated with a decline in HIV incidence despite slow initial uptake(6). By the end of 2016, it was estimated that 30% of the city’s gay and bisexual men were using PrEP as part of the ‘Getting to Zero’ initiative that incorporates PrEP, rapid linkage to care and initiation of antiretrovirals, and retention and re-engagement of HIV-positive people in care(7, 8). A decline in HIV diagnoses has also been reported in five sexual health clinics in London, attributed to a combination of early testing, early access to antiretrovirals including as Treatment as Prevention (TasP) and PrEP sourced through private or trial means (9–11).
It has been established that PrEP adherence is probably the strongest determinant of PrEP effectiveness in trial settings. How to optimize adherence outside of a clinical trial is not well understood and is likely to differ by country, culture and population group. Important ethical issues also exist, including how to ensure access to PrEP for everyone who will benefit. This is a particular issue for at-risk, marginalized populations such as Black, Asian and Minority Ethnic (BAME) groups. Finally, PrEP as an intervention sits within a complex social structure, and careful thought about how risk-perception should be conceptualised and presented to ensure those who may benefit from PrEP can access it without contributing to or causing stigma.
This review updates the current evidence base for PrEP to address questions about effectiveness, safety and risk compensation. In doing so, it provides an overview of adherence, adverse events, risk behaviours, breakthrough infections and the cost-effectiveness of PrEP. National and international guidelines are detailed and an overview of alternative drug regimens is provided.
Incidence/prevalence
In 2016, there were an estimated 36.7 million (30.8 million- 42.9 million) adults and children living with HIV in the world, with a prevalence of 0.8 (0.7-0.9) among adults aged 15 to 49. An estimated 1.8 million (1.6-2.1 million) people globally became newly infected with HIV in 2016(12) giving an incidence of 0.43 per 1,000 population (adults 15-49), and the number dying was 1.0 million (0.83-1.2 million).
Data on the uptake of PrEP within PrEP programmes are available through prescription data and health service data. In the United States, an analysis of electronic prescription data from approximately 80% of retail pharmacies between 2012-2016 by Gilead (manufacturer of Truvada) found that 98,732 people started PrEP during this period and estimated that 136,000 people in the US were on PrEP by the end of the second quarter of 2017. These data do not include non-retail pharmacy prescriptions, such as private PrEP or national Medicaid programmes. Uptake of PrEP was characterized by a period of slow initial uptake, followed by an acceleration and then a steady state(13). Data from the cross-sectional National HIV Behavioural Surveillance (NHBS) System found that only 4% of surveyed MSM reported using PrEP, but more than half reported that they would be willing to take it(14). A PrEP programme in New South Wales in Australia has enrolled over 6,500 participants over a 70 week period with steady enrollment(15). In France, 2805 people had started on PrEP in the first year of the programme(16). Data are emerging on uptake from other national PrEP programmes.
Methods
This review extends the comprehensive systematic literature review and meta-analysis conducted by Fonner et al(17) that included all literature on PrEP published in peer-reviewed journals or presented at a scientific conference between 1 January 1990 and 15 April 2015. Key outcomes included HIV infection, adverse events, antiretroviral drug resistance, including effectiveness of hormonal contraception with PrEP and effects on pregnancy, and sexual behaviour. To be included in the Fonner review, studies had to be a randomized controlled trial (RCT), an open label extension (OLE), or a demonstration project evaluating oral PrEP containing TDF to prevent HIV infection and measure one or more key outcomes, comparing those randomized to PrEP vs placebo or no PrEP use.
We replicated the methodology, including the search terms, used by Fonner et al although the search and data extraction were performed by one person instead of two. We included studies published in peer-reviewed journals or presented at a scientific conference between 16 April 2015 and 19 September 2016. Only English language and human studies were included. The following databases were searched: PubMed, Embase, and CINAHL (Cumulative Index to Nursing and Allied Health Literature). For conference abstracts, the Database of Citations Index was searched. Iterative secondary reference searching on all included studies was also conducted.
In addition to the data extracted in the Fonner review, and updated here, this review extracted data on cost-effectiveness and breakthrough infections.
Results of evidence review
The Fonner review screened 3,068 citations. 39 articles and six conference abstracts describing 18 PrEP-related studies were included in the review. 15 of these were RCTs and three were observational OLE or demonstration projects. Seven RCTs were double-blinded, placebo-controlled trials of daily oral PrEP, two studies randomized participants to immediate or delayed PrEP and one study compared daily PrEP with placebo and ‘no pills’.
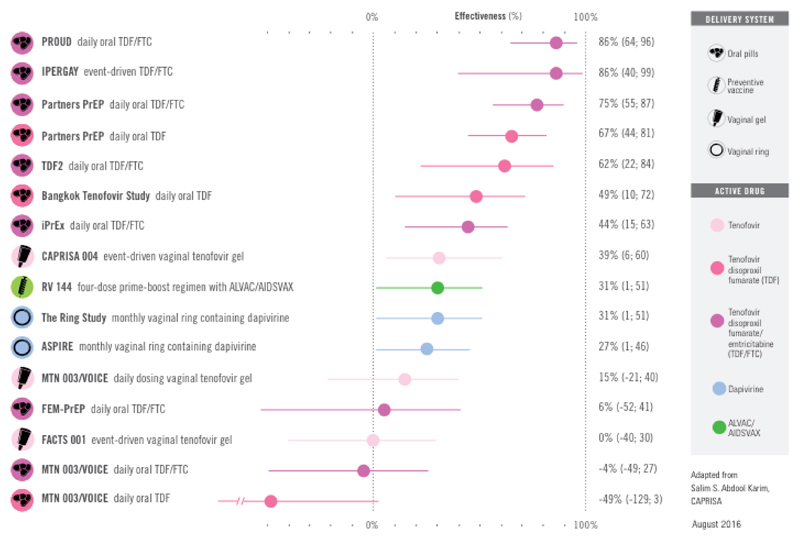
In the extended literature review of articles published between April 2015 and September 2016, a further 1,271 citations were screened. Eight articles and two conference abstracts covering further data on six of the studies identified in the Fonner review were included (fig 1). This extended literature review provides full results of the PROUD(18, 19) and IPERGAY studies(20, 21), additional safety data from the iPrEx(22), Partners PrEP(23, 24) and VOICE(25) studies, and updated sexual behaviour data from the IPERGAY study(21).
Fig 1.
Flow diagram of review
Recent evidence for PrEP
Overview of evidence of efficacy of PrEP
The review by Fonner et al showed that oral PrEP containing TDF-based regimens are highly effective in preventing the transmission of sexually acquired HIV infection across different risk groups (17). The meta-analysis of 11 RCTs that compared PrEP with placebo estimated a 51% reduction in risk of HIV infection comparing PrEP with placebo (risk ratio=0.49, 95% CI 0.33-0.73) in the intention to treat analysis; all randomized participants were included regardless of adherence except those who were acutely HIV infected at baseline. Adherence, indicated by the proportion in the active arms who had any detectable PrEP medications in blood, was strongly associated with PrEP effectiveness between trials. PrEP effectiveness did not depend on gender, route of exposure (penile/vaginal vs anal), or age.
MSM
Evidence from high-quality placebo-controlled RCTs, an open-label RCT comparing PrEP to no-PrEP and open-label extensions (OLE) demonstrate the high level of efficacy and effectiveness of TDF-FTC PrEP for MSM. Meta-analysis suggests that the reduction in HIV transmission comparing PrEP with placebo for rectal exposure is 0.34 (95%CI 0.15-0.80, p=0.01) (17). However, with high adherence this risk reduction is likely greater. Few data exist for trans-populations, though several of the studies described below included trans-men and women. Of note, there was only one small study of PrEP using TDF-alone for MSM which showed no infections after receiving active PrEP, and six emergent infections in the placebo arm and deferred phases of the study(26)
One Phase 3 placebo-controlled RCT has assessed the efficacy of daily PrEP (iPrEx) (27), one Phase 3 open-label RCT has evaluated the effectiveness of daily PrEP compared to no-PrEP (PROUD) (18)and one Phase 3 placebo-controlled RCT has evaluated the efficacy of ‘on demand’ PrEP (IPERGAY) among MSM (28).
The largest study of daily oral PrEP among MSM is the iPrEx study(27). This was a high-quality phase 3, randomized, double-blinded, placebo-controlled multi-centre trial conducted among 2499 MSM and trans-gender male-to-female adults in Peru, Ecuador, Brazil, Thailand, South Africa and the United States. Participants were randomly assigned to either a daily dose of FTC-TDF (1251 participants) or placebo (1248 participants). Participants were followed up every four weeks with interview, HIV testing, risk-reduction counselling, adherence, pill count and dispensing of pills and condoms for a total of 3324 person-years. The primary outcome was HIV infection. 100 participants became infected with HIV over the course of the study; 36 in the FTC-TDF group and 64 in the placebo group, representing a 44% (95% CI 15-63) reduction in HIV incidence using a modified intention to treat (mITT) analysis. After adjustment for age difference between the two groups, the efficacy was estimated to be 43% (95% CI 14-62).
The first study to explore the real-world effectiveness of daily oral PrEP among MSM was the PROUD study(18), full results of which were published after the Fonner review. This was a Phase 3, randomized, open-label waitlisted trial conducted among 544 HIV-negative MSM at 13 sexual health clinics in England. Participants were randomly assigned to either receiving a daily dose of FTC-TDF immediately (275 participants), or after a deferral period of 12 months (269 participants). Participants were followed up every three months with an HIV test and screening for sexually transmitted infections (STIs). Following an interim analysis, the study was halted early and all participants who were still in the deferral period were offered study drug. A total of 243-person years follow-up in the immediate group and 222-person years follow-up in the deferred group had accumulated. 23 participants were infected with HIV during the period of randomized trial observation; three in the FTC-TDF group and 20 in the deferred (no-PrEP) group, representing a rate difference in HIV infection of 7.8 per 100 person years (90% CI 4.3- 11.3) in the modified ITT analysis and a relative risk reduction of 86% (90% CI 64%- 96%). The number needed to treat over one year was 13 (90% CI 9-23). HIV incidence in the no-PrEP group was 9 per 100 person years compared to 1.2 per 100 person years in the PrEP group. HIV incidence in the no-PrEP group was markedly higher than that seen in the MSM population attending sexual health clinics in England, showing that the study had recruited a group at especially high risk of HIV infection(29).
The third trial to show a significant reduction in HIV infections with PrEP was the Phase 3, randomized, placebo-controlled multi-centre IPERGAY trial of PrEP for MSM which evaluated an event-based oral TDF/FTC regimen compared to placebo. Full results were published after the Fonner review. 414 participants were randomly assigned to either receiving an ‘on demand’ regimen of Truvada or placebo(28). The ‘on demand’ regimen involved taking two pills (2 x 300mg TDF/2 x 200 mg FTC) 2-24 hours before sex, and continuing with a daily pill during periods of sexual risk, followed by post-exposure pills 24 hours and 48 hours after the last sexual exposure. The study was unblinded at interim review due to high efficacy of the drug that conferred a relative risk reduction of 86% (95% ci 40%-98%).
There has been one phase 2 safety trial, the CDC MSM Safety Trial(26) that compared TDF to placebo in a randomized, double-blinded, placebo-controlled waitlisted trial among 400 HIV negative MSM. Participants were randomised in a 1:1:1:1 design to receive TDF or placebo immediately or after nine months. Main end points were safety and behavioural effects. No adverse events were associated with tenofovir PrEP and there was no evidence of risk compensation. Participants were followed up with interview, an HIV test and screening for STIs, risk reduction and adherence counselling every three months for 24 months. There were no infections among those taking active drug. Seven participants seroconverted: four in the placebo arm and three among delayed arm participants who were not on study drug. One participant in the placebo group was HIV infected at enrollment.
There have been two smaller studies. The first was a feasibility and acceptability pilot study; Project PrEPare recruited 58 MSM aged 18-22 in the United States. Participants were randomized to receiving a behavioural intervention alone, the behavioural intervention + PrEP or the behavioural intervention + placebo. There were no seroconversions among the 58 participants(30). The study demonstrated the feasibility of enrolling young MSM, in particular young BAME MSM who have a disproportionately high risk of HIV. Retention in the study was 98%. However, self-reported and drug-level based adherence was low, suggesting low acceptability of the drug intervention.
The second was the IAVI Kenya Study, a small safety and adherence study conducted among Kenyan MSM and female commercial sex workers (CSW). 67 MSM and five female CSW were randomized to receive daily FTC-TDF or placebo, or intermittent FTC-TDF or placebo in a 2:1:2:1 ratio. There was one seroconversion in the placebo arm(31).
The iPrEx Open Label Extension (iPrEx-OLE)(32) enrolled 1603 HIV-negative men and transgender women who have sex with men who were previously part of PrEP studies (iPrEx, ATN082/Project PrEPare and US Safety study). Participants were enrolled into the study whether or not they chose to take up PrEP. Participants were followed up for 72 weeks after enrollment into the open-label extension. 76% of those enrolled in the open-label extension initiated PrEP, and this was higher among those reporting condomless receptive anal intercourse and those who were herpes simplex-2 virus seropositive. The majority of participants at risk of HIV and choosing to use PrEP; defined by condomless receptive anal intercourse, more than one anal intercourse partner, recent STI (syphilis, gonorrhea or chlamydia diagnosed at that visit); returned for PrEP at 12 weeks, suggesting use during periods of risk.
As during the randomized phase of the iPrEx study, there were no seroconversions in the open label extension among participants with protective drug levels in dried blood spots (taken quarterly), which was associated with taking 4-7 tablets per week. Retention in the study was not associated with sexual risk behaviour. Overall reduction in HIV incidence compared to the group who did not take up PrEP in the OLE was non-significant at 49% (95% CI -1 to 74%) after adjusting for sexual behaviour, but was significantly lower than the placebo group in iPrEx (51% reduction in HIV incidence, 95% CI 23% to 69%)(32).
The IPERGAY OLE enrolled 333 existing PrEP users and 29 new patients and followed them for 18.4 months (IQR 17.5-19.1). The study demonstrated a marked reduction of 97% in HIV incidence in the PrEP group from the trial and OLE combined (three infections in 734 patient-years of follow up) compared to the placebo arm of the IPERGAY study (14 infections in 212 patient-years of follow up)(20).
Transgender women
iPrEx is the only study to date to report findings in transgender women, albeit in small numbers and without demonstrating evidence of an effect in HIV prevention in this group. Of the 2499 participants in iPrEx, 399 (14%) were classified as trans-gender women. Among transgender women, there were 11 HIV infections in the PrEP group and ten in the placebo group (hazard ratio 1.1, 95% CI 0.5-2.7)(33); the trend toward lower effectiveness among trans women corresponded to lower rates of drug detection, especially among transgender women reporting higher risk behaviour.
During the iPrEx open label extension, 192 transgender women enrolled and were eligible to take PrEP; 151 (79%) chose to take PrEP(33). There were three seroconversions among transgender women with drug levels equivalent to less than two pills per week, below the level of quantification or were off PrEP. No seroconversions were seen among transgender women with drug levels equivalent to 2-3 pills per week or greater. There were lower drug concentrations among transgender women using feminizing hormones compared to other transgender women and could reflect lower PrEP adherence.
Although ex-vivo studies in women have suggested that hormone treatments can have a role in increasing susceptibility to HIV infection by altering intracellular tenofovir concentrations(34), this is a theoretical concern and evidence from studies of antiretroviral interactions with hormonal contraceptives has been reassuring(35). However, there have been no pharmacological interaction studies done in transgender women using both PrEP and hormones. Furthermore, PrEP studies that have included transgender women have not been powered to detect HIV incidence in this group nor a difference between MSM and transgender women.
Heterosexuals
PrEP efficacy among heterosexual populations has been explored in two Phase 3 randomised controlled trials in high-risk heterosexual women (FEM-PrEP, TDF-2, VOICE)(36, 37), one Phase 3 RCT comparing daily PrEP with placebo among sero-discordant couples (Partners PrEP) (38), and one OLE of daily PrEP (Partners Demonstration Project) (39) These studies demonstrated a high level of efficacy of tenofovir-based PrEP in reducing the risk of HIV transmission in heterosexual populations where adherence to drug was high. The meta-analysis by Fonner et al estimated the relative risk for HIV infection comparing PrEP with placebo for penile/vaginal exposure to be 0.54 (95% CI 0.32-0.90; p=0.02) (17).
No studies have evaluated the efficacy of an event-based or intermittent PrEP regimen in heterosexuals and to date there are no heterosexual RCT studies undertaken in high-income countries. While there is no reason to doubt that the biology of transmission is the same worldwide, the trials to date have clearly demonstrated that adherence data cannot be extrapolated from one population to another.
The strongest evidence of effectiveness among serodiscordant heterosexual couples was reported in the large multi-country Partners PrEP study among serodiscordant couples(38). This double-blinded Phase 3 RCT randomized and followed 4747 couples to single (TDF alone) or dual agent (TDF co-formulated with FTC) PrEP or placebo. The study provides evidence of clinical efficacy for daily FTC-TDF (75% (95% CI 55%-87%) or TDF (67% (95% CI 44%-81%) among serodiscordant opposite gender couples in sub-Saharan Africa. No significant difference was observed between FTC-TDF and TDF (p=0.23). Although the placebo arm of this study was stopped earlier than anticipated, the finding of no difference between FTC-TDF and TDF was replicated in the Partners PrEP open-label extension demonstration project.
In the TDF-2 study, 1219 men and women at high risk of HIV in Botswana were randomized to daily oral FTC-TDF or placebo(40). The study provided good evidence for efficacy of PrEP (62%; 95% CI 16%-83%; p=0.03). However, one third of participants did not complete follow up per protocol and 10% were lost of follow-up resulting in risk of bias.
Two studies, FEM-PrEP(36) and VOICE(37), demonstrated no benefit of PrEP among heterosexual women at risk of HIV infection, which is considered due to poor adherence to study medication. FEM-PrEP was a phase 3 double-blinded RCT conducted in sub-Saharan Africa that randomized 2120 heterosexual women at high risk of HIV to daily oral FTC-TDF or placebo. There was no evidence for the clinical efficacy of daily oral FTC-TDF (HR=0.94; 95% CI 0.59-1.52) for heterosexual women. However, there was very low adherence to the study drug in the intervention arm; less than 40% of HIV uninfected women had drug measured in plasma, despite high self-reported adherence. Among the women who seroconverted in the intervention arm, drug levels in blood indicated less than six tablets per week were being used(41).
The VOICE study randomized 5029 women at high risk of HIV infection in sub-Saharan Africa in a Phase 2b double-blinded RCT to oral TDF, oral FTC-TDF, vaginal tenofovir gel or placebo(37). The VOICE study provided no evidence of clinical efficacy for daily FTC-TDF (HR 1.04; 95% CI 0.73-1.49) or TDF (HR 1.49; 95% CI 0.97-2.29) for heterosexual women. Again, despite high self-reported adherence, study drug was only detected in 30% of those randomized to taking oral drug.
People who inject drugs
The only placebo controlled trial of PrEP among PWID randomized 2413 participants in Thailand to receive daily oral TDF or placebo(42). There was a 48.9% reduction in HIV incidence in the PrEP group (95% CI 9.6-72.2; p=0.01) with greater efficacy seen in women compared to men (78.6 per 100py (95% CI 16.8-96.7; p=0.03 in women compared to 37.6 (95% CI -17.8 to 67.9; p=0.15) in men). Efficacy was associated with older age (88.9%, 95% CI 41.1 to 99.4; p=0.01) in those aged >40 compared to 33.6% (95% CI -40.1 to 69.8; p=0.30) in those aged 20-29). Reports of needle sharing was a risk factor of HIV acquisition, and most study participants reported sexual activity that could have exposed them to HIV. The trial sites did not provide access to clean injection equipment which is considered to be the standard of care. PrEP use was directly observed for most of the study(42–44).
Ethnic groups
Black Asian and Minority Ethnic (BAME) populations, particularly MSM, are at increased risk of HIV infection in higher income countries. There are limited data on the efficacy of PrEP in BAME populations and studies are underway to offer PrEP and provide adherence support. In the iPrEx study, a sub-analysis of efficacy of PrEP in Hispanic (N=900 on PrEP, N=901 on placebo) versus non-Hispanic participants (N=351 on PrEP, N=342 on placebo) found no difference in efficacy (HR 0.48 (95%CI 0.14-1.60) in Non-Hispanic versus HR 0.57 (95% CI 0.37-0.89); p=0.79) (27). In the PROUD study, the majority of participants were of white ethnicity (81%) but no data are available on efficacy by ethnicity, similarly for the IPERGAY study.
Adherence
PrEP effectiveness is strongly associated with adherence. Measures of adherence vary in studies, and include self-report, pill count and drug concentrations. Drug concentrations can be measured in red blood cells (RBCs) and peripheral blood mononuclear cells (PMBCs). The median half-life of tenofovir-diphosphate, an active form of the drug, is 17.1 days (interquartile range 15.7-20.2) in red blood cells and 4.2 (3.7-5.2) days in PBMCs. Therefore, estimates of both recent and cumulative drug exposure can be generated. Dried blood spots, which contain millions of RBC can be used as a convenient sampling method to quantify adherence(49).
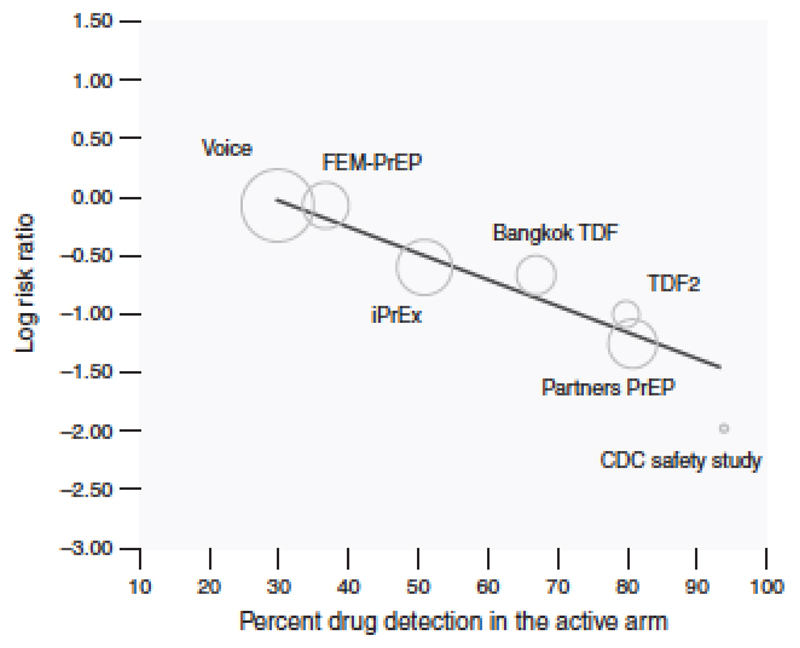
The amount of adherence required for drug detection varies depending on the assays used; in most, use of one or two PrEP tablets per week was sufficient for drug to be detected in blood. Using definitions outlined in the Fonner review ‘high adherence’ was defined as the proportion of those receiving drug with detectable drug levels >70%, ‘moderate adherence’ as 41-70% and ‘low’ as 40% or less. Studies such as FEM-PrEP and VOICE had <30% drug detection overall, an adherence level that conferred no HIV protection. Studies such as Partners PrEP (FTC/TDF arm) where over 80% of blood samples had drug detected demonstrated correspondingly high levels of efficacy. In all studies, in the subset of participants with detectable drug, HIV risk reduction ranged between 70-92%, and risk reduction among MSM was 99% (95% CI 96 to 100%) among MSM whose drug concentrations were commensurate with daily use(50).
Figure 2 demonstrates the close relationship between drug levels and effectiveness.
Fig 2.
Fitted meta-regression line of the relationship between trial-level PrEP adherence and PrEP effectiveness in prevention HIV acquisition (from Fonner et al)(17)
Alternative drug regimens
Reducing the number of tablets taken might lead to more cost-effective use of PrEP if this can be achieved without a loss of HIV prevention activity. Grant et al(51) have described that some MSM have variable periods of higher risk of HIV, and they are inclined to take PrEP more intensively during such periods. Such strategic use periodic use of PrEP may increase impact and lower the overall cost to the health system.
Data extrapolated from RCTs(18, 27, 28) and pharmacokinetic studies(52) suggest that intermittent dosing with at least four tablets per week, or event-based dosing with two tablets 24 hours before and one tablet 24 hours after and 48 hours after sex is protective against HIV acquisition during anal sex. There is no evidence to date for these regimens in insertive or receptive vaginal sex.
Concerns have been raised that less than daily dosing might result in lower adherence to drug. However, this has not been borne out by drug level measurements in the first 113 participants receiving PrEP in the IPERGAY study, where high levels of TDF and FTC were detected (86% and 82% respectively). The efficacy of on-demand PrEP in the IPERGAY study however was in the context of participants using a median number of 15 pills/month and having 10 sexual intercourse per month. Among the subset of 269 participants using ≤15 pills per month systematically or often during sexual intercourse, and contributing to 134 person years of follow-up, there were 6 HIV infections all in the placebo group (incidence 9.3 (95%CI 3.4-20.1) per 100py) with a relative rate reduction of 100% (95% CI 39-100%; 0.013)(53).
PrEP ‘failures’: Breakthrough infections
HIV infections among individuals receiving PrEP during trials were attributed to undiagnosed HIV infection at the time of starting PrEP, or infections during periods of no or low PrEP use as indicated by no or low drug concentrations in blood. Rare breakthrough infections appear to have occurred in clinical practice, possibly due to exposure to a multi-drug resistant HIV or an overwhelming inoculation of virus, although such cases from clinical practice are never as well characterized as in clinical trials.
Two multi-class, drug resistant, apparent breakthrough HIV infections have been described. Importantly, clinical and pharmacokinetic data in these cases suggested good long-term adherence to FTC-TDF by the patients (54, 55), although the drug concentrations were measured several weeks after the patients switched from PrEP to fully suppressive antiretroviral therapy. One case was not tested for HIV in the 2 months prior to starting PrEP (usual standards require HIV testing within 7 days). The other case had missed all of his follow-up appointments after receiving a PrEP prescription. More recently, a PrEP breakthrough infection with wild-type virus was reported from the AmPrEP study in a participant with high self-reported adherence and high drug levels detected on dried blood spot testing(56). This participant’s viral load was undetectable while on PrEP; the rising viral load several weeks after stopping PrEP raised questions about the timing of infection because of viral suppression whilst on PrEP. Also, drug resistance testing performed after therapy is stopped, as was done in this case, can be falsely negative due to overgrowth of drug sensitive strains(57).
A particular problem for HIV infections that are undiagnosed at the time of starting PrEP is that the drug may drive de novo resistance mutations, particularly where drug adherence is poor and drug levels fluctuate. Fonner et al reviewed results from six trials that reported cases of FTC or TDF drug resistance using standardized genotypic laboratory assays(17). The risk of developing an FTC-related mutation among those acutely infected with HIV at enrollment was significantly higher in the group randomized to receive FTC/TDF PrEP compared to placebo (risk ratio=3.72, 95% CI 1.23- 11.23, p=0.02). The risk of a TDF-related mutation was not statistically different between PrEP and placebo regardless of PrEP regimen among those acutely infected at enrollment. Additionally, six (2%) TDF or FTC-resistant infections occurred among 544 postrandomisation HIV infections; five in PrEP groups and one in a placebo group. Numbers were too small to calculate a pooled relative risk.
These cases highlight the importance of systematic monitoring and surveillance of PrEP use and breakthrough infections at a population level.
Adverse events
To date, studies of TDF-FTC PrEP provide robust evidence of safety with short-term use (2-3 years). The metanalysis by Fonner et al(17) demonstrated no difference in the proportion of adverse events comparing PrEP to placebo across 10 placebo-controlled RCTs (OR 1.01, 95% CI 0.99- 1.03, p=0.27), with no differences seen in subgroup analysis that included mode of acquisition, adherence, gender, drug regimen, dosing or age. No differences were seen in grade 3 or 4 adverse events comparing PrEP and placebo groups across 11 placebo-controlled RCTs (Risk ratio= 1.02, 95% CI 0.92-1.13, p=0.76). Results were not presented by sub-group.
The commonest side effects reported in studies were gastrointestinal(28, 40), headache(18), and nausea(18, 40). Depression was commonly reported yet the rates did not differ in the placebo and active arms (27). Of note, gastrointestinal events were more commonly reported in the PrEP group in the IPERGAY study compared to placebo (14% vs 5%; p=0.002), but no such differences were found in the meta-analysis.
Use of TDF-FTC as PrEP has been associated with a mild non-progressive decline in creatinine clearance(24, 27, 28, 37, 40, 58) that is reversible on discontinuation of drug(27, 58).
An association between decline in bone mineral density (BMD) and FTC-TDF PrEP use has also been documented(22, 25, 40, 59), but no evidence has been found of an associated increase in fracture risk. The change in BMD occurred by week 24, did not progress with additional PrEP use, and recovered to levels observed in the placebo arm after stopping PrEP(60, 61).
Risk behaviour
It has been proposed that PrEP might lead to risk compensation, whereby people taking PrEP might have higher risk sexual behavior because the risk of HIV infection is no longer felt to be relevant. Such a behavior change might increase the risk of other STIs, particularly among groups that already have disproportionately high incidence of STIs such as MSM. Studies have investigated the possibility of risk compensation by collecting information about reported condom use and partner numbers and using diagnosed STIs as an objective measurement of risk
However, studies exploring risk compensation are difficult to design as self-reported behavioural data are subject to reporting bias, may vary inter-and intra-individuals and reporting may vary across the study period. STI incidence used as a marker of risk compensation may be affected by increased testing frequency in the study, the types of tests used (e.g. use of dual tests for chlamydia and gonorrhoea may increase detection rates of gonorrhoea), changes in background population-level STI incidence and other simultaneous interventions such as health promotion messages(62). Therefore, monitoring of behavioural and biological markers of risk compensation is important, but inferring associations at population level between use of PrEP and increased risk behaviour is fraught with difficulty in the context of general increases in risk behaviours(63) and increases in STIs(64).
Data are available from RCTs of PrEP, though these were not powered to detect differences in sexual risk or STIs. Furthermore, participants in RCTs do not know whether they are taking active drug and so there is no expectation that they will modify their behaviour in the same way that would happen in the real world. As such, RCTs only provide limited evidence about risk compensation that is generalisable to wider use of PrEP. Fonner et al(17) found no difference in condom use or partner numbers between study arms, and no change or only small increases in condom use and decrease in partners over time among the RCTs of MSM, heterosexuals and PWIDs. However, meta-analysis was not possible due to heterogeneity of the studies. The IAVI Kenya study, which included MSM, was the only trial to report an increase in sexual partners from baseline to follow-up (from 3 at baseline to 4 at month 4), but partners may have been underreported at baseline(31).
Open-label studies and demonstration projects, whose results were published after the Fonner review, provide better evidence regarding risk compensation because patients know whether they are taking drug and the patients will often be aware of the effectiveness of PrEP in preventing HIV infection. Risk compensation may be more likely in this instance. However, the data are conflicting. On the one hand, a number of studies suggest no evidence of risk compensation. The PROUD study showed no difference between the immediate and deferred (no-PrEP) groups in the total number of sexual partners (p=0.57) at 1 year or in the frequency of overall bacterial STIs (p=0.74)(65). However, a greater proportion of the immediate group reported receptive anal sex without a condom with ten or more partners at one year compared to the deferred group (21% vs 12%, p=0.03). The Partners PrEP demonstration follow-up project, reported condomless sex acts in the main partnership decreased compared to the RCT phase, but there was a slight increase in condomless sex acts outside the main partnership. Nevertheless, rates of STIs and pregnancy did not change(66). In iPrEx OLE, self-reported risk behaviours including number of partners and condomless sex declined over the period of the study. Incidence of syphilis was similar among those taking PrEP and those who were not(32). Data from San Francisco’s demonstration project also suggests no increase in risk behaviours or STIs(67, 68). On the other hand, early data from the Australian Victorian PrEP Demonstration Project, although outside of the search period for this review, suggests an increase in risk compensation. Over the first 12 months of the study, self-reported condom use declined concomitantly with a significant increase in incidence of STIs from 43.2 per 100py at months 0-3 to 119.8 per 100py at months 3-12 (incidence rate ratio 2.77 (95%CI 1.52-5.56))(69). However, the study had a small sample size of 114 and data on STI rates prior to study entry were not available. Similar increases in STI rates were reported in the US-based Kaiser cohort of PrEP users (although also outside of the search period for this review); over the first year, rectal chlamydia positivity increased from 0.9% to 2.5% (p=0.012) and urethral gonorrhoea positivity increased from 7.7% to 14.1% (p<0.001), and data from Seattle demonstrate a decline in condom use and increase in STI diagnoses after PrEP initiation(70).(70)
Overall, these studies are difficult to interpret because the observations might be explained by a range of factors, including changing risk behaviour, increased detection of STIs due to more screening, or unrelated changes in STI transmission dynamics in the wider population. However, to date, it remains the case that no large increases in STI incidence have been attributed to PrEP, and PrEP may create opportunities for STI testing and counselling, and for contemplation of sexual goals and risks, which might mitigate risk compensation (71).
Pregnancy, contraception and PrEP
FEM-PrEP and Partners PrEP investigated the effectiveness of oral contraception and PrEP when taken simultaneously to investigate whether one might affect the other. The evidence to date suggests that PrEP does not affect the effectiveness of injectable contraception(72) and injectable contraception does not affect oral PrEP efficacy(73), though numbers are small.
Data indicate that oral PrEP does not have an adverse impact on fertility or pregnancies(74). 431 pregnancies occurred in the Partners PrEP study with no difference in pregnancy incidence, loss, preterm birth, congenital abnormalities or intrauterine? growth between the study arms. However, PrEP was discontinued on detection of pregnancy, and therefore data on the safety of TDF-FTC in HIV positive pregnant women may be more informative for clinical decisions about whether to continue PrEP in pregnancy in light of ongoing risk of HIV infection. A recent review indicated no safety reason to withhold PrEP during pregnancy(75) and data have demonstrated that HIV risk is increased in pregnancy, highlighting the importance of providing effective HIV prevention interventions for pregnant women(76).
Cost-effectiveness
Cost-effectiveness studies for PrEP are mainly based on data from the United States and suggest that PrEP can be cost-effective and have significant budgetary impact, particularly among the highest risk MSM(4, 77–85). Two models from the UK concluded that PrEP is cost-effective when targeting the highest risk MSM if drug prices are reduced(3, 86) and a study using a third model concluded that this would also be the case in the Netherlands(87).
Cost-effectiveness studies are context and epidemic specific and need to consider local factors. Key determinants have been found to include HIV incidence in the target group being offered PrEP, patient adherence to PrEP, levels of condomless sex and numbers of sexual partner. Cost of drug is most likely to fall substantially in the upcoming years due to the availability of generic formulations and, though partly counter-balanced by decreasing treatment costs, this will likely improve the cost-effectiveness of PrEP.
Guidelines
In light of the strong evidence for the efficacy of PrEP, PrEP is increasingly being incorporated into national HIV prevention guidance, such as in the US(88), Europe(89), Kenya, South Africa, and Brazil; Guidelines are being prepared in the UK. The WHO guidelines(90) recommend that oral PrEP containing TDF should be offered to all people at substantial risk of HIV infection; substantial risk is provisionally defined as HIV incidence of approximately 3 per 100 person years or greater in the absence of PrEP, although countries might not have accurate incidence data for relevant populations. MSM and TGW in the placebo arms of diverse PrEP trials routinely had incidence that exceeded 3.0/100PY, as have cohorts of sexually active adolescent girls and young women in Africa.
There are only small differences between these guidelines in terms of drug, regimen and eligibility criteria for PrEP. The Center for Disease Control issued guidelines in 2014(88) in which they recommended use of daily oral PrEP with FTC-TDF for MSM, heterosexual women and men and injecting drug users.
The European AIDS Clinical Society (EACS) guidelines(89) recommends the use of oral FTC-TDF for HIV negative MSM and transgender individuals ‘at risk of HIV’. The guidelines define risk as those who are inconsistent in their use of condoms with casual partners or with HIV-positive partners who are not on treatment. EACS considers use in HIV-negative heterosexual men and women at risk of HIV. The recommended regimen is daily, but an ‘on demand’ regimen as used in the Ipergay trial is also recommended for MSM.
Emerging treatments
Several alternative drugs and delivery modalities for PrEP are being investigated in animal and human studies. Examples include long-acting preparations, vaginal rings and gels, and alternative drug regimens. A search of the AVAC (Global Advocacy for HIV Prevention) HIV Prevention Research & Development Database (http://www.avac.org/pxrd) which is a comprehensive source of information on biomedical HIV prevention clinical trials, found four phase I-III trials of PrEP listed as planned and five ongoing phase I-III trials. These studies include oral, long-acting injectable and topical formulations. There are a large number of planned and ongoing demonstration and open label studies.
Three non-oral products have or will be evaluated for efficacy. Tenofovir formulated as a vaginal gel and administered before and after sex showed modest effectiveness in reducing HIV and herpes simplex virus type 2 in one trial(91), and this result was supported by a secondary analysis from an efficacy trial assessing daily tenofovir gel(92). An intravaginal ring releasing the non-nucleoside reverse transcriptase inhibitor dapivirine showed modest protection in two trials(93, 94) and is now being evaluated in open-label studies. The long-acting integrase inhibitor cabotegravir administered every 2 months as a 3ml intramuscular injection is being evaluated in two efficacy trials. Details of these studies fall outside the scope of this review.
Oral PrEP drug candidates need to have high bioavailablity and/or good penetration to the genital mucosa. A clinical limitation of FTC-TDF PrEP is that high plasma concentrations of tenofovir are required to achieve adequate intracellular penetration, which can lead to worsening renal impairment in those with pre-existing disease. Tenofovir alafenamide (TAF) has greater intracellular bioavailability, and it has been shown that lower doses can be used to achieve the same therapeutic benefit when used for HIV treatment, with less risk of impact on renal function or bone mineral density. The effectiveness of TAF combined with FTC is currently being evaluated in the DISCOVER trial(95).
Another candidate is Maraviroc, a CCR5 inhibitor, which was investigated for safety and acceptability in the NEXT-PrEP/HPTN 069 study(96). Maraviroc has few known side effects and a resistance profile that does not overlap with commonly used treatments for HIV. In the NEXT-PrEP study, maraviroc was given alone or in combination with emtricitabine or with TDF, with FTC-TDF as a control. Explant data from the NEXT-PrEP study demonstrated higher levels of HIV replication in rectal tissue from the Maraviroc group compared to those treated with Maraviroc paired with another antiretroviral(97). There were also two seroconversions in the Maraviroc group in spite of high levels of drug in the tissue. These preliminary data are not encouraging and there are not thought to be current plans to develop maraviroc as a PrEP agent.
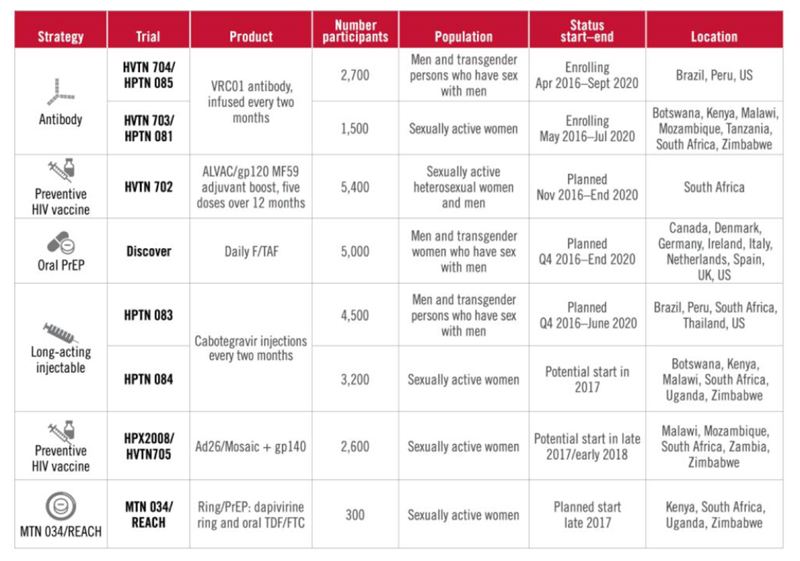
Many other HIV prevention modalities under investigation, including a preventive HIV vaccine and the use of monoclonal antibodies. These are outlined in figure 4, but fall outside the scope of this review.
Fig 4.
Current and ongoing HIV prevention studies (from AVAC http://www.avac.org/infographic/2016-17-percolating-pipeline)
Combination prevention strategies
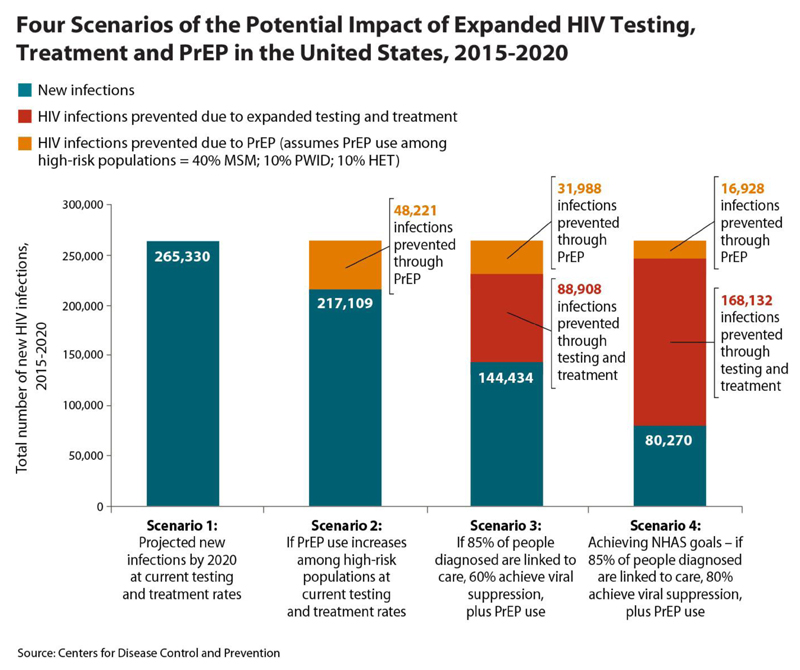
It has long been argued that a successful public health strategy to prevent HIV requires a combination approach because of the complexities involved, and modelling studies support the idea that such an approach is essential to eliminate HIV(98–100). Therefore, PrEP is not being thought of in isolation, and studies are underway to explore what the optimal prevention packages might look like, including behavioural risk reduction strategies and treatment as prevention alongside PrEP. (see figure 3).
Fig 3.
Potential impact of expanded HIV testing, treatment and PrEP in the United States, 2015-2020
Conclusion
This review provides a comprehensive overview of PrEP, including information and discussion on cost-effectiveness, guidelines and breakthrough infections. The PROUD and IPERGAY studies in particular, which were not included in depth in the Fonner review and are discussed in detail in this review, enhance our understanding of the efficacy of PrEP and adherence among high-risk MSM.
It is clear from the evidence available that oral FTC-TDF PrEP is highly effective in reducing the risk of HIV acquisition across different types of sexual exposure, genders, dosing schedules, and different country contexts and epidemics. While only one small trial has assessed TDF monotherapy in MSM, there is high quality evidence for the effectiveness of TDF in reducing heterosexual transmission, contrary to the common misconception that PrEP is not effective among heterosexual women. Only one trial has been conducted in PWID; although it demonstrated effectiveness in HIV prevention, it has not been possible to separate the reduction in the risk of sexual acquisition from the possible effect on reducing intravenous blood borne transmission. An oral event based regimen has only been evaluated in MSM, and pharmacokinetic studies have demonstrated that drug concentrations of TDF are higher in rectal than vaginal tissue. Guidelines do not currently recommend event based regimens for other risk groups.
Oral FTC-TDF PrEP has been shown to be extremely safe with minimal impact on kidney, bone or pregnancy outcomes, and there is no evidence that the effectiveness of PrEP has been diminished by risk compensation during open-label and programmatic follow-up. However, it remains too early to assess the impact of PrEP rollout on STI incidence at a population level, and any changes in STI incidences will be difficult to disentangle from temporal increases in STIs in the wider population.
Lots of challenges remain. Access to PrEP is still limited and there are disparities by race and gender. Different pricing and access models for PrEP need to be explored to avert further widening of these inequalities. Adherence is essential for PrEP to be effective and research is already being conducted to understand how to optimise adherence, including through use of mobile technology. The optimal combination prevention programme needs to be defined, but this will depend on local epidemiology, service provision and cost-effectiveness measures.
Cost-effectiveness studies have highlighted that scale up of PrEP is challenging in countries that have to pay the full price for the branded drug. Roll out is likely to be particularly challenging where there is lack the infrastructure for regular HIV and STI screening. The drugs were licensed for use as PrEP five years ago, and an increasing number of countries are embarking on national programmes, with others having approved reimbursement of PrEP. However, coverage is not universal within many countries and key groups may not be accessing PrEP due to barriers such as cost, knowledge and stigma(101, 102).
San Francisco’s experience has shown that early adoption of PrEP can be slow, but also that subsequent rapid uptake can have a substantial impact on HIV incidence. Although there are residual programmatic questions about eligibility, uptake and duration of use it is clear that in countries where there is access to PrEP, clinicians have a duty of care to inform those at risk of acquiring HIV about the benefits of PrEP. Healthcare providers’ knowledge of PrEP is variable(103). The successes of San Francisco and London may reflect local awareness of, and prioritization and investment in PrEP. Policymakers need to be aware of local PrEP policies and provide training for clinicians who are involved in PrEP care to ensure that access to PrEP is fair and equitable and that structural barriers to PrEP access such as knowledge, stigma and discrimination are challenged.
Glossary.
| Breakthrough infection/PrEP failure | An HIV infection in a person fully adherent to PrEP | |
| Combination prevention | Programmes that use a mix of biomedical, behavioural and structural interventions | |
| FTC | Emtricitabine | An antiretroviral used in PrEP |
| OLE | Open label extension | Typically a follow-on of a placebo-controlled clinical trial, where all participants are given open-label study drug (both they and the investigators know the drug is active and not a placebo) |
| PrEP | Pre-exposure prophylaxis | The use of antiretroviral medications by HIV-uninfected individuals to prevent acquisition of HIV |
| Risk compensation | The adjustment of behaviour in response to perceived changes in risk, in the case of PrEP, protection causing people to increase sexual behaviour that may involve exposure to HIV. | |
| Serodifferent partnership | One partner is infected with HIV and the other is not | |
| Seroconcordant partnership | Both partners are infected with HIV, or both partners are uninfected | |
| TasP | Treatment as Prevention | The public health strategy of treating HIV-infected individuals to reduce HIV incidence in the population |
| TDF | Tenofovir Disoproxil Fumarate | An antiretroviral used in PrEP |
| Waitlisted trial | Trial design where participants receive the intervention immediately or after a deferred period |
Research questions.
What does the inclusion of PrEP in the wider HIV prevention strategy add to the impact on HIV incidence at a population level?
Will there be an impact on STI incidence and antiretroviral resistance at a population level?
How can we target PrEP most appropriately to attain optimal population level impact at a manageable cost?
Are there better alternatives to coformulated tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) PrEP?
What strategies can be used to maximise the adherence to PrEP?
In epidemics where HIV incidence is declining, is there a point at which PrEP is no longer a cost-effective addition to the HIV prevention package?
Fig 1.
Clinical trial evidence for HIV prevention options (http://www.avac.org/infographic/evidence-hiv-prevention-options)
Table 1.
Summary table of included studies for effectiveness of oral HIV preexposure prophylaxis for all populations (adapted from Fonner et al)
| Study | Study design | Location | PrEP regimen | PrEP dosing and comparison | Primary mode of HIV acquisition | Study population | Number of participants | Overall effectiveness of PrEP (95% CI) by MITT* |
|---|---|---|---|---|---|---|---|---|
| iPrEx(27) | RCTa | Peru, Ecquador, S Africa, Brazil, Thailand, USA | FTC/TDFb | Daily PrEP to placebo | Rectal | MSMc and transgender women | 2499 | 44% (15-63%) |
| iPrEx OLEd(32) | Cohort | Peru, Ecquador, S Africa, Brazil, Thailand, USA | FTC/TDF | Daily PrEP to no PrEP use | Rectal | MSM and transgender women | 1603 | 49% (-0.1-74%) |
| PROUD(18) | RCT | England | FTC/TDF | Immediate to delayed PrEP | Rectal | MSM | 545 | 86% (64-96%)e |
| Project PrEPare(30) | RCT | USA | FTC/TDF | Daily PrEP to placebo and to ‘no pill’ | Rectal | Young MSM | 58 | No seroconversions |
| IPERGAY(28) | RCT | France, Canada | FTC/TDF | Intermittent PrEP to placebo | Rectal | MSM | 400 | 86% (40-99%) |
| IPERGAY OLE(20) | Cohort | France, Canada | FTC/TDF | Intermittent PrEP to placebo | Rectal | MSM | 362 | 97% (81-100%) |
| IAVI Kenya(31) | RCT | Kenya | FTC/TDF | Daily/intermittent PrEP to daily/intermittent placebo | Rectal | MSM and FSW | 72 | 1 seroconversion placebo group |
| CDC Safety Study(26) | RCT | USA | TDF | Immediate/delayed PrEP to immediate/delayed placebo | Rectal | MSM | 400 | 7 seroconversions (4 placebo, 3 delayed, 0 TDF) |
| ADAPT HPTN 067(45) | RCT | S Africa, Thailand, USA | FTC/TDF | Daily, time and event driven PrEP | Rectal Vaginal/Penile | MSM Women | 500 | 12 infections, distributed across the arms |
| Bangkok Tenofovir Study(42) | RCT | Thailand | TDF | Daily PrEP to placebo | Vaginal/penile | People who inject drugs | 2413 | 48.9% (9.6-72.2%) |
| Bangkok Tenofovir Study OLE(46) | Cohort | Thailand | TDF | Daily PrEP to placebo | Vaginal/penile | People who inject drugs | 787 | All: 48.9% (9.6-72.2) Females: 78.6 (16.8-96.7) |
| Partners PrEP(38) | RCT | Kenya, Uganda | FTC/TDF and TDF (2 active arms) | Daily PrEP to placebo | Vaginal/penile | Sero-discordant couples | 4747 couples | All: TDF/FTC: 75% (55-87%) TDF: 67% (44-81%) Women: TDF/FTC: 66% (28-84%) TDF: 71% (37-87%) Men: TDF/FTC: 84% (54-94%) TDF: 63% (20-83%) |
| Partners PrEP Demonstration Project (39) | RCT | Kenya, Uganda | FTC/TDF and FTC | Daily FTC/TDF to TDF | Vaginal/penile | Sero-discordant couples | 4410 couples | 33% (-17-41%) (i.e. no difference between TDF/FTC and TDF) |
| TDF-2(40) | RCT | Botswana | FTC/TDF | Daily PrEP to placebo | Vaginal/penile | Heteroseuxal men and women | 1219 | 61.7% (15.9-82.6%) |
| IAVI Uganda(47) | RCT | Uganda | FTC/TDF | Daily/intermittent PrEP to daily/intermittent placebo | Vaginal/penile | Sero-discordant couples | 72 | Not reported |
| FEM-PrEP(36) | RCT | Tanzania, S Africa, Kenya | FTC/TDF | Daily PrEP to placebo | Vaginal/penile | Women | 2056 | 6% (-52-41%) |
| VOICE(37) | RCT | S Africa, Uganda, Zimbabwe | FTC/TDF and TDF (2 active arms) | Daily PrEP to placebo | Vaginal/penile | Women | 4969 | TDF/FTC: -4.4% (-50-27%) TDF: -49% (-130-3%) |
| West African Safety Study(48) | RCT | Nigeria, Cameroon, Ghana | TDF | Daily PrEP to placbeo | Vaginal/penile | Women | 936 | 65% (-93-97%) |
RCT: Randomized controlled trial
FTC: Emtricitabine; TDF: tenofovir disoproxil fumarate
MSM: men who have sex with men
OLE: open label extension
90% confidence interval from the PROUD study
Not adjusted for adherence
Table 3.
Summary of published national and international guidelines for PrEP1
| Indication for PrEP | MSM | Heterosexual men and women | People who inject drugs | |
|---|---|---|---|---|
| CDC (2014) | Detection of substantial risk of acquiring HIV infection | RECOMMEND Any one of: HIV positive sexual partner Recent bacterial STIa High number of sex partners History of inconsistent or no condom use Commercial sex work (Note does not mention trans-populations) |
RECOMMEND Any one of: HIV positive sexual partner Recent bacterial STI High number of sex partners History of inconsistent or no condom use Commercial sex work In a high-prevalence area or network |
RECOMMEND Any one of: HIV-positive injecting partner Sharing injection equipment Recent drug treatment (but currently injecting) |
| WHO (2015) | Offer for people at substantial risk of HIV infection (provisionally defined as >3 per 100py) | HIV incidence >3 per 100 py Includes transgender women |
HIV incidence >3 per 100 person years Includes heterosexual men and women with sexual partners with undiagnosed or untreated HIV where HIV incidence >3 per 100 person years |
No recommendation made |
| EACS (2015) | Use in adults at high risk of acquiring HIV infection | RECOMMEND Inconsistent in use of condoms with casual partners or with HIV-positive partners who are not on treatment. Recent STI or use of PEPb may be markers of increased risk for HIV acquisition (includes transgender individuals) |
CONSIDER Inconsistent in use of condoms and likely to have HIV –positive partners who are not on treatment |
No recommendation made |
|
ANRS (2015) In French* |
Use in adults (aged >18) at high risk of HIV | RECOMMEND For sexually active MSM at high risk of acquiring HIV : Reporting condomless sex with at least 2 partners over a 6 month period Having had multiple episodes of STIs (syphilis, chlamydia, gonococcal infection, primary hepatitis B infection or hepatitis C infection) in the past year Multiple courses of PEP over the past year Use of psychoactive substances during sex in the past year Partners of HIV positive people who are taking part in trials of stopping ARVs NOT RECOMMENDED FOR: Condomless sex that is not associated with high risk of transmission of HIV |
CONSIDER ON A CASE BY CASE BASIS Other people at high risk of acquiring HIV infection: Commercial sex worker having condomless sex Vulnerable individuals having condomless sex at high risk of transmission of HIV (partner from a group with high HIV prevalence-multiple sexual partners, from a high prevalence country (>1%), people who inject drugs, physical factors increasing transmission e.g. genital or anal ulcer, bleeding) Partners of HIV positive people who are taking part in trials of stopping ARVs NOT RECOMMENDED FOR: condomless sex that is not associated with high risk of transmission of HIV Sex with HIV positive partner who is well controlled on antiretroviral therapys (VL<50) PrEP for conception for serodifferent couples |
Injecting drug users sharing needles |
| British Columbia (2016) | Ongoing high risk of acquiring HIV infection | One or more of: One or more HIV positive sexual partner, particularly if HIV positive partner is not receiving stable antiretroviral therapy and/or does not have a consistently undetectable viral load Recent (within last 6 months)STI Multiple sex partners History of inconsistent or no condom use for anal intercourse Repeated courses of PEP |
One or more of: HIV positive sexual partner(s), particularly if HIV positive partner is not receiving stable antiretroviral therapy and/or does not have a consistently undetectable viral load Having sexual partners who are MSM or use injection drugs |
Sharing injection equipment Injecting once or more times per day in an unsafe setting (outside of safe injection sites) Injecting cocaine or methamphetamine Repeated courses of nPEP |
|
Quebec (2013) In French |
For those at very high risk of acquiring HIV | Condomless anal sex with HIV positive or unknown status partners | HIV positive sexual partners with a detectable viral load | Not recommended |
| South Africa (2016) | At significant risk of acquiring HIV | Any sexually active HIV-negative MSM or transgender person who wants PrEP Especially: HIV positive sexual partner who is not confirmed virologically supressed Partner of unknown HIV status Recent STI Multiple sexual partners History of inconsistent or no condom use Commercial sex work Recurrent PrEP users History of sex whilst under the influence of alcohol or recreational drugs Especially vulnerable are young MSM |
Heterosexual men and women who want PrEP, especially: HIV positive sexual partner who is not confirmed virologically supressed Partner of unknown HIV status Recent STI Multiple sexual partners History of inconsistent or no condom use Commercial sex work Serodiscordant couples trying to conceive Recurrent PrEP users History of sex whilst under the influence of alcohol or recreational drugs Especially vulnerable are adolescent girls |
One or more of: HIV positive / unknown status injecting partner Share injecting needles and drug preparation equipment |
Only guidelines published in English and French have been included
STI: sexually transmitted infection
PEP: post-exposure prophlyaxis
Footnotes
Competing interests
We have read and understood the BMJ policy on declaration of interests and declare the following interests:none
Contributorship statement and guarantor
MD conducted the literature review and prepared the first draft of the manuscript. All authors contributed equally and fully in all remaining aspects of this manuscript. SM is the guarantor.
References
- 1.Padian NS, McCoy SI, Karim SS, Hasen N, Kim J, Bartos M, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011;378(9787):269–78. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juusola JL, B ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156(8):541–50. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambiano V, Miners A, Dunn D, McCormack S, Gill N, Nardone A, et al. O1 Is pre-exposure prophylaxis for hiv prevention cost-effective in men who have sex with men who engage in condomless sex in the uk? Sex Transm Infect. 2015 Sep 23;91 [Internet]. Available from: http://sti.bmj.com/content/91/Suppl_1/A1.1.abstract. [Google Scholar]
- 4.Paltiel AD, Freedberg KA, Scott CA, Schackman BR, Losina E, Wang B, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(6):806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchbinder SP, Glidden DV, Liu AY, McMahan V, Guanira JV, Mayer KH, Goicochea P, Grant RM. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014 doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.San Francisco Department of Public Health. HIV Epidemiology Annual Report 2015. 2016 Sep; [Google Scholar]
- 7.Weber S, S B, San Francisco Getting to Zero Consortium . AIDS 2016. Durban, South Africa: 2016. The Getting to Zero San Francisco consortium: early results. [Google Scholar]
- 8.Cohen S. Exapnding PrEP Access and Implementation in San Francisco. San Francisco Health Commission; 2016. [Google Scholar]
- 9.Collins Simon. Four London clinics report dramatic drops in HIV incidence in gay men: PrEP, early testing and early ART likely to be key. HIV treatment Bulletin. 2016 [Google Scholar]
- 10.Delpech V. Towards elimination of HIV amongst gay and bisexual men in the United Kingdom. 22nd Annual Conference of the British HIV Association; Liverpool. 2017. [Google Scholar]
- 11.Brown AE, Mohammed H, Ogaz D, Kirwan PO, Ynug M, Nash SG, Furegato M, Hughes G, Connor N, Delpech VC. Fall in new HIV diagnoses among men who have sex with men (MSM) at selected London sexual health clinics since early 2015: testing or treatment or pre-exposure prophyalxis (PrEP)? Eurosurveillance. 2017;22(25) doi: 10.2807/1560-7917.ES.2017.22.25.30553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS. Fact Sheet 2016. 2016.
- 13.Giller RM, M D, Trevor H, Bush S, Rawlings K, McCallister S. Changes in Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilisation in the United States: (2012-2016). International AIDS Society 2017; Paris, France. 2017. [Google Scholar]
- 14.Hoots BE, Finlayson T, Nerlander L, Paz-Bailey G. Willingness to Take, Use of, and Indications for Pre-exposure Prophylaxis Among Men Who Have Sex With Men-20 US Cities, 2014. Clin Infect Dis. 2016;63(5):672–7. doi: 10.1093/cid/ciw367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New South Wales. NSW HIV Strategy 2016-2020: April-June 2017 Data Report. 2017.
- 16.Molina JM, Pialoux G, Ohayon M, Cotte L, Valin N, Ghosn J, Cua E, Pintado C, Chas J, Petour P, Barriere G, et al. One-year experience with pre-exposure prophylaxis (PrEP) implementation in France with TDF/FTC. International AIDS Conference; Paris, France. 2017. [Google Scholar]
- 17.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O'Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–83. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (London, England) 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. North American Edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolling DI, Desai M, McOwan A, Gilson R, Clarke A, Fisher M, et al. An analysis of baseline data from the PROUD study: an open-label randomised trial of pre-exposure prophylaxis. Trials. 2016;17:1–11. doi: 10.1186/s13063-016-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina JM, Charreau B, Spire L, Cotte J, et al., editors. Efficacy of 'On Demand' PrEP in the ANRS IPERGAY Open-Label Extension Study. International AIDS Conference; 2016 July 18-22; Durban, South Africa. 2016. [Google Scholar]
- 21.Sagaon-Teyssier L, Suzan-Monti M, Demoulin B, Capitant C, Lorente N, Preau M, et al. Uptake of PrEP and condom and sexual risk behavior among MSM during the ANRS IPERGAY trial. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV. 2016;28:48–55. doi: 10.1080/09540121.2016.1146653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, Gonzales P, et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2015;61(4):572–80. doi: 10.1093/cid/civ324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugwanya KK, Wyatt C, Celum C, Donnell D, Kiarie J, Ronald A, et al. Reversibility of Glomerular Renal Function Decline in HIV-Uninfected Men and Women Discontinuing Emtricitabine-Tenofovir Disoproxil Fumarate Pre-Exposure Prophylaxis. Journal of acquired immune deficiency syndromes (1999) 2016;71(4):374–80. doi: 10.1097/QAI.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugwanya KK, Wyatt C, Celum C, Donnell D, Mugo NR, Tappero J, et al. Changes in glomerular kidney function among hiv-1- uninfected men andwomen receiving emtricitabine- tenofovir disoproxil fumarate preexposure prophylaxis a randomized clinical trial. JAMA internal medicine. 2015;175(2):246–54. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirembe BG, Kelly CW, Mgodi N, Greenspan S, Dai JY, Mayo A, et al. Bone Mineral Density Changes Among Young, Healthy African Women Receiving Oral Tenofovir for HIV Preexposure Prophylaxis. Journal of acquired immune deficiency syndromes (1999) 2016;71(3):287–94. doi: 10.1097/QAI.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 27.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. New England Journal of Medicine. 2015;373(23):2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 29.Dolling D, Desai M, Apea V, Mackie N, McOwan A, Youssef E, Bowman C, Lacey C, Schembri G, Gilson R, Sullivan A, et al. Who accesses PrEP? An analysis of baseline data in the PROUD pilot. Third joint conference of BHIVA with BASHH; 4th April 2014; Liverpool. 2014. [Google Scholar]
- 30.Hosek SG, Green KR, Siberry G, Lally M, Balthazar C, Serrano PA, et al. Integrating Behavioral HIV Interventions into Biomedical Prevention Trials with Youth: Lessons from Chicago's Project PrEPare. Journal of HIV/AIDS & social services. 2013;12(3–4) doi: 10.1080/15381501.2013.773575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, Hosek S, Mosquera C, Casapia M, Montoya O, Buchbinder S, et al. Uptake of pre-exposure prophylaxis, sexual practices and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet infectious diseases. 2014 doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2(12):e512–9. doi: 10.1016/S2352-3018(15)00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Kashuba AD, Wira CR. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One. 2014;9(6):e100863. doi: 10.1371/journal.pone.0100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteman MK, Jeng G, Samarina A, Akatova N, Martirosyan M, Kissin DM, et al. Associations of hormonal contraceptive use with measures of HIV disease progression and antiretroviral therapy effectiveness. Contraception. 2016;93(1):17–24. doi: 10.1016/j.contraception.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baeten J, Heffron R, Kidoguchi L, Mugo N, Katabira E, Bukusi E, Asilmwe S, Morton J, Ngure K, Bulya N, Odoyo J, et al. Integrated delivery of PrEP and ART results in sustained near elimination of HIV transmission in African HIV serodiscordant couple: final results from The Partners Demonstration Project. AIDS 2016; Durban, South Africa. 2016. [Google Scholar]
- 40.Thigpen MC, Kebaabetswe PM, Smith DK, Segolodi TM, Soud FA, Chillag K, Chirwa LI, Kasonde M, Mutanhaurwa R, Henderson FL, Pathak S, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study. IAS Rome. 2011 [Google Scholar]
- 41.Grant RM, Sevelius JM, Guanira JV, Aguilar JV, Chariyalertsak S, Deutsch MB. Transgender Women in Clinical Trials of Pre-Exposure Prophylaxis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;72:S226–S9. doi: 10.1097/QAI.0000000000001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 43.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS (London, England) 2015;29(7):819–24. doi: 10.1097/QAD.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 44.Martin MT, Vanichseni S, Suntharasamai P, Sangkum U, Mock P, Leethochawalit M, et al. Preliminary follow-up of injecting drug users receiving preexposure prophylaxis. Topics in antiviral medicine. 2015;23:445–6. [Google Scholar]
- 45.Sivay MV, Li M, Piwowar-Manning E, Zhang Y, Hudelson SE, Marzinke MA, et al. Characterization of HIV Seroconverters in a TDF/FTC PrEP Study: HPTN 067/ADAPT. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017;75(3):271–9. doi: 10.1097/QAI.0000000000001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. The Lancet HIV. 4(2):e59–e66. doi: 10.1016/S2352-3018(16)30207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kibengo FM, Ruzagira E, Katende D, Bwanika AN, Bahemuka U, Haberer JE, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One. 2013;8(9):e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant JM. From subjects to relations: Bioethics and the articulation of postcolonial politics in the Cambodia Pre-Exposure Prophylaxis trial. Social studies of science. 2016;46(2):236–58. doi: 10.1177/0306312716632617. [DOI] [PubMed] [Google Scholar]
- 52.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guillemette A, C T, Charreau I, Cua E, Rojas D, Hall N, Huleux T, Spire B, Capitant C, Cotte L, Meyer L, et al. Is on-demand PrEP with TDF/FTC effective among MSM with infrequent sexual intercourse. A sub-study of the ANRS Ipergay trial. International AIDS Scoiety; Paris, France. 2017. [Google Scholar]
- 54.Knox DC, T D, Harrigna R, Anderson PL. HIV-1 infection with multiclass resistance despite pre-exposure prophylaxis (PrEP). Conference on Retroviruses and Opportunistic Infections; 22-25 February 2016; Boston, USA. 2016. p. 169aLB. [Google Scholar]
- 55.Grossman H. Newly acquired HIV-1 infection with Multi-Drug Resistant (MDR) HIV-1 in a patient on TDF/FTC based PrEP. HIVR4P conference; Chicago. 2016. p. OA03.6LB. [Google Scholar]
- 56.Hoornenborg E, de Bree GL. Acute infection with a wild-type HIV-1 virus in a PrEP user with high TDF levels. Conference on Retroviruses and Opportunistic Infections Seattle; USA. 2017. p. 953. [Google Scholar]
- 57.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344(7):472–80. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 58.Mugwanya KK, Wyatt C, Celum C, Donnell D, Mugo N, Kiarie JN, et al. Reversibility of kidney function decline in HIV-1-uninfected men and women using preexposure prophylaxis. Topics in antiviral medicine. 2015;23:451. [Google Scholar]
- 59.Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688. doi: 10.1371/journal.pone.0023688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant R, Mulligna K, McMahan V, Liu AY, Guanira J, Chariyalertsak S, Bekker L, Schechter M, Veloso VG, Glidden DV, for the iPrEx study team Recovery of bone mineral density after stopping oral HIV preexposure prophylaxis. Conference on retroviruses and opportunisitic infections; March 4-7; Boston. 2016. [Google Scholar]
- 62.Hughes G, Field N. The epidemiology of sexually transmitted infections in the UK: impact of behavior, services and interventions. Future Microbiol. 2015;10(1):35–51. doi: 10.2217/fmb.14.110. [DOI] [PubMed] [Google Scholar]
- 63.Aghaizu A, Wayal S, Nardone A, Parsons V, Copas A, Mercey D, et al. Sexual behaviours, HIV testing, and the proportion of men at risk of transmitting and acquiring HIV in London, UK, 2000-13: a serial cross-sectional study. Lancet HIV. 2016;3(9):e431–40. doi: 10.1016/S2352-3018(16)30037-6. [DOI] [PubMed] [Google Scholar]
- 64.Public Health England, editor. Public Health England. Sexually transmitted infections and chlamydia screening in England, 2015. Health Protection Report; 2016. [Google Scholar]
- 65.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mugwanya K, Donnell D, Celum C, Mugo N, Thomas KK, Ngure K, Ndase P, Katabira E, Baeten JM, for the Partners PreP Study Team Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: post-unblinding analysis of the partners PrEP study. Sex Transm Infect. 2013;89:A46. doi: 10.1016/S1473-3099(13)70226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA internal medicine. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015;61(10):1601–3. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lal L, Audsley J, Murphy DA, Fairley CK, Stoove M, Roth N, et al. Medication adherence, condom use and sexually transmitted infections in Australian preexposure prophylaxis users. AIDS. 2017;31(12):1709–14. doi: 10.1097/QAD.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 70.Montano MA, D J, Barbee LA, Golden MR, Khosropour CM. Changes in sexual behavior and STI diagnoses among MSM using PrEP in Seattle, WA. Conference on Retroviruses and Opportunisitic Infections; Seattle, USA. 2017. p. 979. [Google Scholar]
- 71.Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–6. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murnane PM, Heffron R, Ronald A, Bukusi EA, Donnell D, Mugo NR, et al. Pre-exposure prophylaxis for HIV-1 prevention does not diminish the pregnancy prevention effectiveness of hormonal contraception. AIDS. 2014;28(12):1825–30. doi: 10.1097/QAD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heffron R, Mugo N, Were E, Kiarie J, Bukusi EA, Mujugira A, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014;28(18):2771–6. doi: 10.1097/QAD.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mugo NR, Hong T, Celum C, Donnell D, Bukusi EA, John-Stewart G, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA. 2014;312(4):362–71. doi: 10.1001/jama.2014.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mofenson LM. Tenofovir Pre-exposure Prophylaxis for Pregnant and Breastfeeding Women at Risk of HIV Infection: The Time is Now. PLoS Med. 2016;13(9):e1002133. doi: 10.1371/journal.pmed.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong KJ, Desai S, Desai M, Nardone A, van Hoek AJ, Gill ON. Cost and cost-effectiveness of an HIV pre-exposure prophylaxis (PrEP) programme for high-risk men who have sex with men in England: results of a statis decision analytical model. Lancet. 2015 [Google Scholar]
- 78.Desai K, Sansom SL, Ackers ML, Stewart SR, Hall HI, Hu DJ, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22(14):1829–39. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 79.Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9(10):e108742. doi: 10.1371/journal.pone.0108742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Annals of internal medicine. 2012;156(8):541–50. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernard CL, Brandeau ML, Humphreys K, Bendavid E, Holodniy M, Weyant C, et al. Cost-Effectiveness of HIV Preexposure Prophylaxis for People Who Inject Drugs in the United States. Annals of internal medicine. 2016;165(1):10–9. doi: 10.7326/M15-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kessler J, Myers JE, Nucifora KA, Mensah N, Toohey C, Khademi A, et al. Evaluating the impact of prioritization of antiretroviral pre-exposure prophylaxis in New York. AIDS. 2014;28(18):2683–91. doi: 10.1097/QAD.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Letchumanan M, Coyte PC, Loutfy M. An economic evaluation of conception strategies for heterosexual serodiscordant couples where the male partner is HIV-positive. Antiviral Therapy. 2015;20(6):613–21. doi: 10.3851/IMP2956. [DOI] [PubMed] [Google Scholar]
- 84.Ouellet E, Durand M, Guertin JR, LeLorier J, Tremblay CL. Cost effectiveness of 'on demand' Hiv pre-exposure prophylaxis for non-injection drug-using men who have sex with men in Canada. Canadian Journal of Infectious Diseases and Medical Microbiology. 2015;26(1):23–9. doi: 10.1155/2015/964512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ross EL, Cinti SK, Hutton DW. Implementation and Operational Research Epidemiology and Prevention: A Cost-Effective, Clinically Actionable Strategy for Targeting HIV Preexposure Prophylaxis to High-Risk Men Who Have Sex with Men. Journal of Acquired Immune Deficiency Syndromes. 2016;72(3):e61–e7. doi: 10.1097/QAI.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 86.Ong K, Desai S, Desai M, Nardone A, van Hoek AJ, Gill ON, editors. The cost-effectiveness of Pre-Exposure Prophylaxis (PrEP) to prevent HIV acquisition by high-risk MSM in England – preliminary results of a static decision analytical model. Poster presentation. Public Health England Annual Conference; 2015 15-16 September; Warwick University, UK. [Google Scholar]
- 87.Nichols BE, Boucher CA, van der Valk M, Rijnders BJ, van de Vijver DA. Cost-effectiveness analysis of pre-exposure prophylaxis for HIV-1 prevention in the Netherlands: a mathematical modelling study. Lancet Infect Dis. 2016;16(12):1423–9. doi: 10.1016/S1473-3099(16)30311-5. [DOI] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States-2014. In: Services DoHaH, editor. A clinical practice guideline. 2014. [Google Scholar]
- 89.European AIDS Clinical Society. Guidelines Version 8.0. Oct, 2015. 2015.
- 90.World Health Organisation. Consolidated Guidance on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Second Edition ed. 2016. [PubMed] [Google Scholar]
- 91.Abdool Karim SS, Abdool Karim Q, Baxter C. Antibodies for HIV prevention in young women. Current opinion in HIV and AIDS. 2015;10(3):183–9. doi: 10.1097/COH.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai JY, Hendrix CW, Richardson BA, Kelly C, Marzinke M, Chirenje ZM, et al. Pharmacological Measures of Treatment Adherence and Risk of HIV Infection in the VOICE Study. The Journal of infectious diseases. 2016;213(3):335–42. doi: 10.1093/infdis/jiv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. New England Journal of Medicine. 2016;375(22):2121–32. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nel A, Kapiga S, Bekker L, Devlin B, Borremans M, Rosenberg Z, for the IMP 027/The Ring Study Research Center Teams Safety and efficacy of dapavarine vaginal ring for HIV-1 prevention in African women. Conference on retroviruses and opportunistic infections; March 4-7; Boston, MA. 2016. [Google Scholar]
- 95.ClinicalTrials.gov. Safety and efficacy of Emtricitabine and Tenofovir Alafenamide (F/TAF) fixed dose combination once daily for pre-exposure prophylaxis in men and transgender women who have sex with men and are at risk of HIV-1 infection (DISCOVER) 2016.
- 96.Gulick R, W T, Chen Y, et al. HPTN 069/ACTG 5305: Phase II study of Maraviroc-based regimens for HIV PrEP in MSM. Conference on Retroviruses and Opportunistic Infections; 22-25 February 2016; Boston, USA. 2016. p. 103. [Google Scholar]
- 97.McGowan I, Nikiforov A, Young A, Cranston R, Landovitz RJ, Bakshi R, Andrade A, Mayer K, Wilkin TJ, Gulick R. PrEP Impact on T-cell activation and explant infection: HPTN 069/ACTG 5303 substudy. Conference on retroviruses and opportunistic infections; 4-7 March; Boston. 2016. [Google Scholar]
- 98.Mitchell KM, Lepine A, Terris-Prestholt F, Torpey K, Khamofu H, Folayan MO, et al. Modelling the impact and cost-effectiveness of combination prevention amongst HIV serodiscordant couples in Nigeria. Aids. 2015;29(15):2035–44. doi: 10.1097/QAD.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yaylali E, F PG, Jacobson E, Sansom SL. Impact of Improving HIV Care and Treatment and Initiating PrEP in the U.S., 2015-2020. Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2016. [Google Scholar]
- 100.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bauermeister JA, Meanley S, Pingel E, Soler JH, Harper GW. PrEP awareness and perceived barriers among single young men who have sex with men. Curr HIV Res. 2013;11(7):520–7. doi: 10.2174/1570162x12666140129100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, et al. Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLoS One. 2017;12(2):e0172354. doi: 10.1371/journal.pone.0172354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desai M, Gafos M, Dolling D, McCormack S, Nardone A. Healthcare providers' knowledge of, attitudes to and practice of pre-exposure prophylaxis for HIV infection. HIV Medicine. 2016;17(2):133–42. doi: 10.1111/hiv.12285. [DOI] [PubMed] [Google Scholar]