Abstract
The UP element stimulates transcription from the rrnB P1 promoter through a direct interaction with the C-terminal domain of the RNA polymerase α subunit (αCTD). We investigated the effect on transcription from rrnB P1 of varying both the location of the UP element and the length of the α subunit interdomain linker, separately and in combination. Displacement of the UP element by a single turn of the DNA helix resulted in a large decrease in transcription from rrnB P1, while displacement by half a turn or two turns totally abolished UP element-dependent transcription. Deletions of six or more amino acids from within the α subunit linker resulted in a decrease in UP element-dependent stimulation, which correlated with decreased binding of αCTD to the UP element. Increasing the α linker length was less deleterious to RNA polymerase function at rrnB P1 but did not compensate for the decrease in activation that resulted from displacing the UP element. Our results suggest that the location of the UP element at rrnB P1 is crucial to its function and that the natural length of the α subunit linker is optimal for utilisation of the UP element at this promoter.
INTRODUCTION
Escherichia coli RNA polymerase holoenzyme (RNAP) is a multi-subunit complex consisting of an α subunit homodimer, single β, β′ and ω subunits, and one of several σ subunit species (1,2). During transcription initiation at many promoters, RNAP containing the major σ factor, σ70, recognises two hexameric promoter elements located ∼10 and 35 bp upstream of the transcription start point (+1) (3). Recognition of the –10 and –35 promoter elements requires regions 2.3–2.5 and 4.2 of σ70, respectively (4,5). However, a third promoter element, the UP element, is frequently required for full promoter activity (6,7). UP elements interact directly with the C-terminal domain of the α subunit of RNAP (αCTD) (8,9) in holoenzymes containing σ70 or alternative σ factors (10–12).
UP elements consist of A+T-rich sequences located upstream of the –35 region at many promoters (6,8,13). At the most well-studied UP element-dependent promoter, rrnB P1, the UP element comprises DNA sequences from approximately positions –40 to –60 and is composed of two subsites centred at about –42 and –52 (termed the proximal and distal subsites, respectively), each of which can bind an αCTD (6,14–17). The interaction between αCTD and the UP element results in an increase in KB, the initial equilibrium constant for RNAP binding to the promoter, but may also affect later steps in the pathway to open complex formation (16,18). The αCTD–UP element interaction is responsible for a 30–70-fold increase in in vivo promoter activity at rrnB P1 (8,16,17). The optimised UP element sequence (‘consensus’ UP element) contains alternating A- and T-tracts and is ∼5-fold more effective than the wild type rrnB P1 UP element (17). UP elements can also be transposed as a separate module from one promoter to another where they retain their ability to stimulate transcription in a factor-independent manner (8,13,16).
Each α subunit consists of 329 amino acids organised in two independently folding domains (9,19). The N-terminal domain (αNTD; residues 8–231) contains determinants for dimerisation and assembly into RNAP (20,21) and plays a role in transcription activation at some promoters (22,23). αCTD (residues 249–329) (24) plays roles in transcription initiation, elongation and termination (25–27). During transcription initiation, αCTD provides a contact site for many transcription activators (25,28,29) and is directly involved in UP element recognition (8,30). αCTD folds into a (HhH)2 domain (31) in which key residues interacting with DNA reside within the loop and second helix of HhH1 and HhH2, respectively (32). Based on genetic, biochemical and NMR studies of the αCTD–DNA interaction, and on the X-ray structure of the RuvA–DNA complex, the α (HhH)2 domain is thought to interact in a sequence-specific way with bases in the minor groove and with the DNA backbone across the minor groove (30,32–35).
Several lines of evidence indicate that a flexible linker connects αCTD to αNTD. First, limited proteolysis studies pointed to an accessible region between amino acids 234 and 249 of E.coli α (9,19). Secondly, NMR analysis of an isolated C-terminal fragment of α (amino acids 233–329) showed that a region of at least 13 amino acids, extending from D233 to E248, exhibits a high degree of flexibility (36). This is consistent with the crystallographic data for αNTD, where the C-terminal limit of helix 3 is assigned to F231 (21). Thirdly, amino acid sequence alignments of α subunits from eubacteria and chloroplasts reveal a non-conserved sequence of variable length corresponding to amino acids V237–P251 of the E.coli α subunit (37). Consistent with its proposed role as a linker, the introduction of substantial deletions or insertions into this region does not affect the efficiency of assembly of α into RNAP, nor do they affect transcription from activator-independent and UP element-independent promoters (37,38). However, deletion of three amino acids from within the linker greatly reduces transcription from a promoter where the activator protein CRP binds to a site centred at –61.5 (Class I), whereas transcription is enhanced from a promoter where CRP binds at –41.5 (Class II). This result, together with the observation that αCTD tethered by a shorter linker is still recruited to DNA sequences upstream of the CRP binding site at the Class II promoter, implies that the α linker deletion compromises the motional freedom of αCTD rather than its inherent ability to bind DNA (37,38).
Although potential UP elements or UP element subsites have been identified in the –40 to –60 region at many promoters, in most cases such searches have not been extended to locations further upstream (6,8,13,17). The recent demonstration that αCTD is able to contact DNA near positions –43, –53, –63, –73, –83 and –93 at the lac promoter, unassisted by CRP (39), suggests that the length of the α linker is unlikely to restrict access of αCTD to UP elements located upstream of –60. However, the question of whether UP elements are able to stimulate transcription efficiently from distant locations has not been satisfactorily addressed.
Gourse and colleagues studied effects of UP element location before the UP element subsites were fully defined (15). They observed that upstream displacement of sequences between positions –47 and –87 by a single turn of the helix did not affect transcription from the rrnB P1 promoter, whereas displacement by two or more turns exerted an inhibitory effect on transcription. However, as the proximal UP element subsite was retained in its normal location in these early experiments, conclusions concerning displacement of the full UP element must be re-evaluated. In cases where UP elements have been identified upstream of the –40 to –60 region, DNA bending by accessory DNA binding proteins such as IHF is also required for full activity (e.g. 12,40,41), presumably to bring α binding sequences closer to the core promoter. Therefore, in this work, we have examined the effect of upstream displacement of the full UP element on transcription from the rrnB P1 promoter in the absence of accessory factors. We have also investigated the role of α linker length in UP element function at the rrnB P1 promoter and the possibility that lengthening the α linker might alleviate the negative effect of UP element displacement. Our results demonstrate that the position of the full UP element is crucial to its function at the rrnB P1 promoter and that the length of the α subunit linker is optimised for utilisation of UP elements in their normal position at rrn P1 promoters.
MATERIALS AND METHODS
rrnB P1 promoter fragments
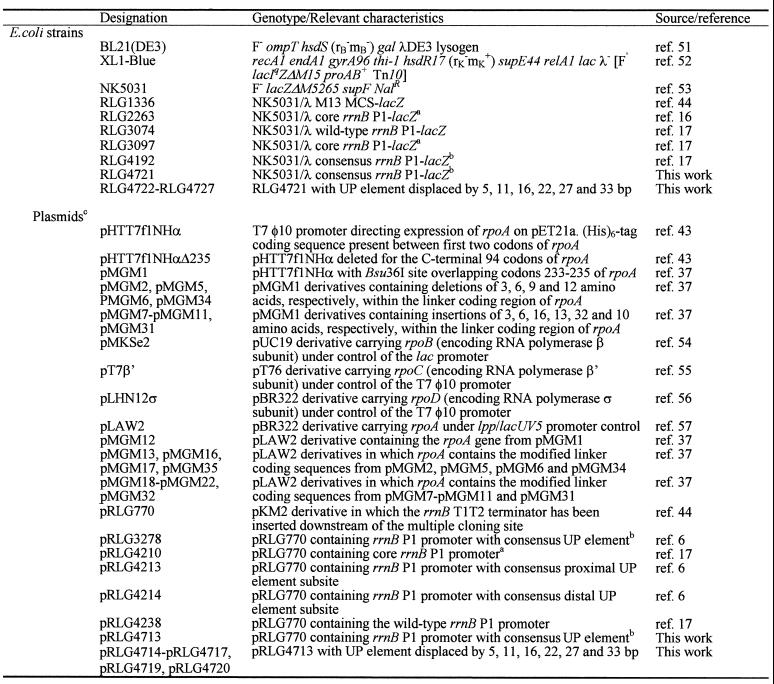
The E.coli strains and plasmids used in this work are listed in Table 1. Standard molecular biology techniques for plasmid isolation and DNA manipulation were used throughout (42). All rrnB P1 promoter derivatives used for transcription assays or single copy lacZ fusion analysis were cloned as EcoRI–HindIII fragments into the transcription vector pRLG770 or λ phage system I (16), respectively, and possess a downstream end point at position +52 with respect to the rrnB P1 transcription initiation site. Upstream end points are at position –66 for promoter fragments present in pRLG4238, pRLG3278, pRLG4213, pRLG4214 and pRLG4210, and in the lacZ fusions present in RLG3074, RLG4192, RLG3097 and RLG2263, but contain different sequences from –38 to –59 (6,17). Promoter fragments present in plasmids pRLG4713–pRLG4717, pRLG4719 and pRLG4720 (and the corresponding lacZ fusions present in RLG4721–RLG4727) possess wild type rrnB P1 sequences up to position –37, upstream of which is a consensus UP element located at various positions. These promoter fragments were constructed by amplifying the rrnB P1 core promoter region from pRLG4210 using primer RLG1620 (5′-GCGCTACGGCGTTTCACTTC-3′) (13), which anneals downstream of the pRLG770 HindIII site, as the reverse primer in each case and one of a series of upstream primers of the type 5′-GCGCGAATTCGGAAAATTTTTTTTAAAAAAGAX-3′ (EcoRI site italicised and UP element emboldened) where ‘X’ corresponds to sequences inserted between the displaced UP element and position –37 of the rrnB P1 promoter (see Fig. 1) plus, in some cases, additional rrnB P1 core promoter sequences (–37 to –16) to allow primer annealing (Table 2). The consensus UP element present in these derivatives is a combination of the individual SELEX selected consensus proximal and distal UP element subsites, and differs from the version constructed by Estrem et al. (6) only by the substitution of a thymine residue for an adenine at position –46 (underlined).
Table 1. Bacterial strains and plasmids.
aThe core rrnB P1 promoter fragment in RLG3097 and pRLG4210 differs from that present in RLG2263 due to the presence of the SUB sequence (GACTGCAGTGGTACCTAGGAAT) from –38 to –59 and rrnB P1 sequences from –60 to –66 in the former. RLG2263 retains rrnB P1 sequences up to position –41 and does not contain the SUB sequence.
bThe consensus UP element present in RLG4192 and pRLG3278 has the sequence GGAAAATTTTTTTTCAAAAGTA (–59 to –38) and retains rrnB P1 promoter sequences from –60 to –66. The consensus UP element in RLG4721 and pRLG4713 has the sequence GGAAAATTTTTTTTAAAAAAGA (–59 to –38) and does not retain additional upstream rrnB P1 sequences.
cAll plasmids encode resistance to ampicillin.
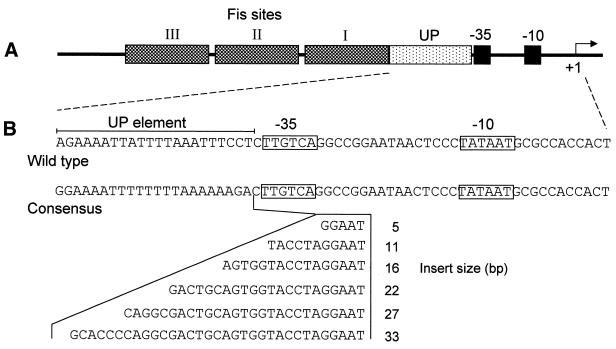
Figure 1.
Displacement of the consensus UP element upstream of the rrnB P1 core region. (A) Organisation of the rrnB P1 promoter region. The rrnB P1 core region constitutes the DNA sequence from –37 to +1 with respect to the transcription initiation site (indicated with a bent arrow). Located immediately upstream of this region is the UP element (–38 to –59) and three tandem Fis sites. (B) DNA sequences from –59 to +3 of the wild type rrnB P1 promoter and of an rrnB P1 derivative containing the consensus UP element present in pRLG4713 and RLG4721. The –35 and –10 regions are boxed and the extent of the UP element sequence is indicated by a horizontal bar. DNA sequences inserted between positions –37 and –38 to displace the consensus UP element are indicated. In promoter derivatives where the UP element has been displaced by 5–22 bp these sequences comprise part or all of the SUB sequence (17), whereas derivatives in which this element has been displaced by 27 and 33 bp contain, in addition, sequences from –37 to –47 of the lac P1 promoter, which do not possess inherent UP element-like activity (13).
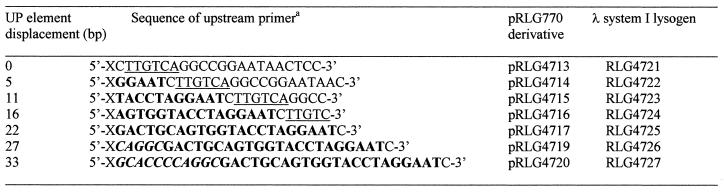
Table 2. Upstream primers used to construct rrnB P1 promoters with displaced consensus UP elements.
aThe 5′ end of each primer has the sequence 5′-GCGCGAATTCGGAAAATTTTTTTTAAAAAAGA-3′, denoted by an ‘X’ in the table, containing an EcoRI site (underlined) and a consensus UP element (emboldened). The rrnB P1 –35 region and SUB sequences are underlined and emboldened, respectively, in the table. rrnB P1 derivatives in which the consensus UP element has been displaced by 5–22 bp contain part or all of the SUB sequence (17) whereas derivatives in which this element has been displaced by 27 and 33 bp contain, in addition, sequences from –37 to –47 of the lac P1 promoter (italicised and emboldened), which do not possess inherent UP element-like activity (13). Zero base pair displacement corresponds to the wild type location for the UP element, i.e. positions –38 to –59.
Subunit purification and reconstitution of RNAP
His-tagged RNAP α subunits containing wild type and modified linkers were overproduced in strain BL21(DE3) harbouring pHTT7f1NHα and the pHTT7f1NHα derivatives pMGM2, pMGM5–pMGM11, pMGM31 and pMGM34, which encoded α subunits with shorter or longer interdomain linkers (37). Following 3 h of induction of the plasmid-borne phage T7 promoter, α subunits were purified by Ni2+-affinity chromatography as described previously (30,43). Preparation of inclusion bodies of RNAP β, β′ and σ subunits from strains XL1-Blue (pMKSe2), BL21(DE3) (pT7β′) and BL21(DE3) (pLHN12σ), respectively, and reconstitution of RNAP were performed as described previously (43).
In vitro transcription
Multiple round transcription reactions were performed at 25°C for 20 min in a volume of 25 µl containing 150 mM NaCl, 40 mM Tris–acetate pH 7.9, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 100 µg/ml bovine serum albumin (BSA), 500 µM ATP, 200 µM CTP, 200 µM GTP, 10 µM UTP and 3–5 µCi [α-32P]UTP (10 mCi/ml, 800 Ci/mmol). Each reaction also contained 0.6 nM supercoiled plasmid DNA, prepared using the Qiagen plasmid midi kit and purified further by phenol–chloroform extraction. All plasmid templates for transcription assays were derivatives of the transcription vector pRLG770 (44) and, therefore, also result in the production of the RNA-I transcript (108 nucleotides). Transcription originating from rrnB P1 promoter fragments terminate at the rrnB T1T2 terminator present on the vector and give rise to transcripts of ∼220 nucleotides in length. Reactions were initiated by addition of RNAP and terminated with 25 µl of stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol). Samples were fractionated on a 5.5% acrylamide gel containing 7 M urea and transcript abundance was quantified using a PhosphorImager. Native RNAP was a generous gift of R. Landick and was 60 ± 10% active in binding to the λPR promoter (45). RNAP reconstituted with the wild type α subunit was 55% as active as native RNAP. Reconstituted RNAPs were used at concentrations that resulted in equivalent transcription from the core rrnB P1 promoter present on pRLG4210 (4.4 nM native RNAP and 8–28 nM reconstituted RNAP) (see legend to Fig. 3).
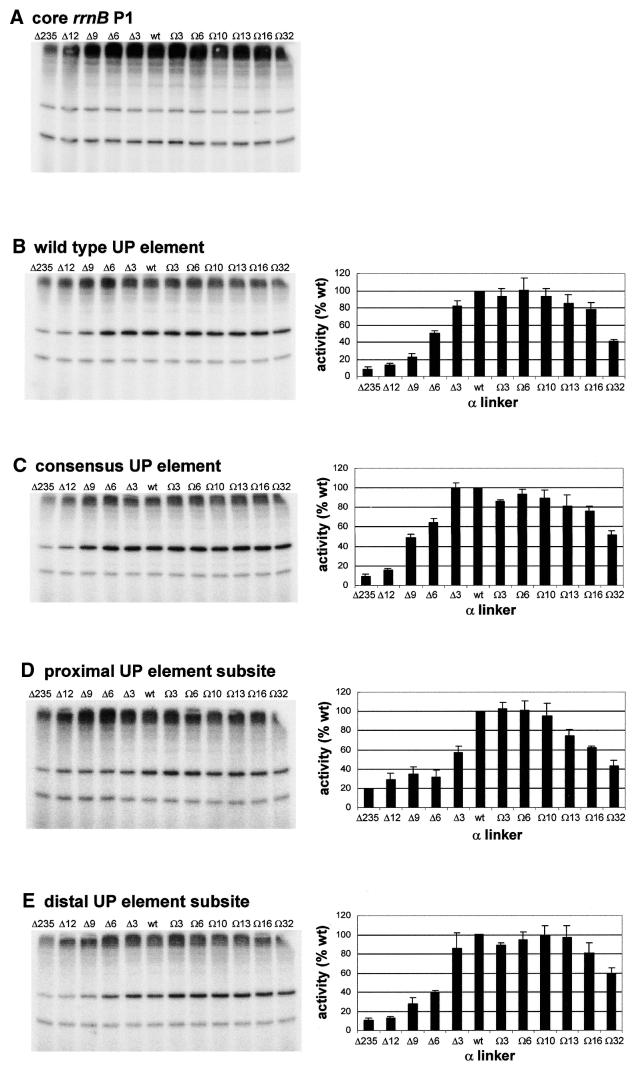
Figure 3.
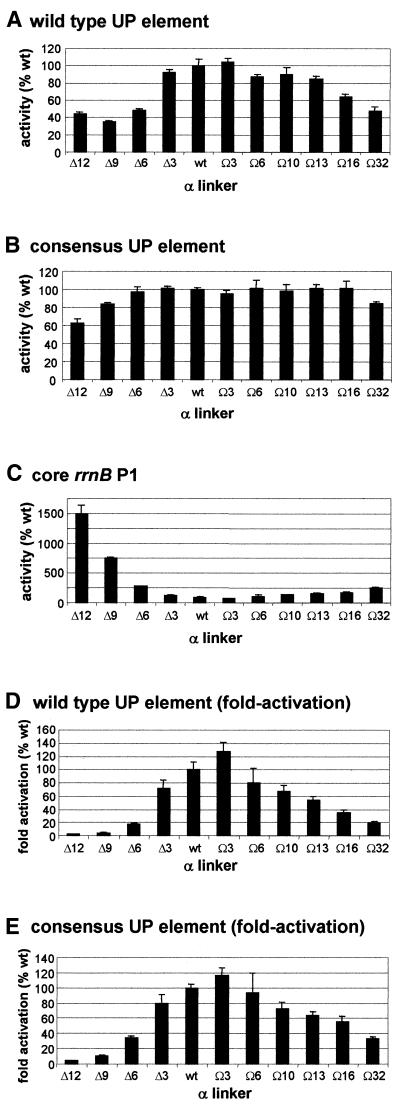
Effect of α subunit linker length on transcription in vitro from rrnB P1 promoter derivatives. The left hand side of each panel shows the result of a typical transcription experiment assaying the activity of RNAPs reconstituted with wild type α or one of the mutant α subunits containing longer or shorter linkers, as indicated, at the core rrnB P1 promoter (A), and at rrnB P1 containing the wild type UP element (B), the consensus full UP element (C), the consensus proximal UP element subsite (D) and the consensus distal UP element subsite (E). RNAPs were used at a concentration which gave equivalent transcription from the rrnB P1 core promoter and were: Δ235α RNAP, 27.8 nM; Δ12α RNAP, 15.2 nM; Δ9α RNAP, 18.2 nM; Δ6α RNAP, 16.8 nM; Δ3α RNAP, 10.8 nM; wild type α RNAP, 8 nM; Ω3α RNAP, 12.6 nM; Ω6α RNAP, 12.2 nM; Ω10α RNAP, 19.6 nM; Ω13α RNAP, 14.6 nM; Ω16α RNAP, 19.8 nM; Ω32α RNAP, 27.0 nM. Transcripts arising from the rrnB P1 and RNA-I promoters are indicated by arrows. On the right of each transcription gel (B–E only) the relative abundance of the transcript originating from the corresponding rrnB P1 promoter derivative in the presence of each RNAP is shown (by definition, the relative abundance of transcripts originating from the rrnB P1 core promoter in the presence of each RNAP would be 100%). The values were calculated from at least three independent experiments and are presented as a percentage (with standard deviations) of transcript obtained with wild type RNAP.
Hydroxyl radical footprinting
Fragments containing the wild type rrnB P1 promoter were prepared for footprinting by first linearising CsCl-purified pRLG4238 DNA with HindIII, then removing the 5′ terminal phosphates with calf intestinal alkaline phosphatase and subsequently making a second cut with AatII. The smaller, 205 bp, DNA fragment was purified from a preparative polyacrylamide gel and was labelled on the 5′ hydroxyl at the HindIII terminus with 10 µCi [γ-32P]ATP (3000 Ci/mmol) using 0.5–1.0 U T4 polynucleotide kinase for 30 min at 37°C. Unincorporated nucleotides were removed by a pass through a Sephadex G-50 spin column and the purified labelled DNA used for footprinting. Binding reactions contained 10 mM Tris–HCl pH 8.0, 30 mM KCl, 10 mM MgCl2, 1 mM DTT, 100 µg/ml BSA, 500 µM ATP, 50 µM CTP, 1.0–4.0 nM labelled DNA fragment and RNAP reconstituted with wild type α (21 nM), Δ6 α (45 nM), Δ9 α (65 nM), Δ12 α (73 nM) or Δ235 α (72 nM), and were incubated at 37°C for 30 min. Prior to hydroxyl radical treatment, heparin was added to a final concentration of 10 µg/ml for 30 s, whereupon hydroxyl radical treatment was performed as previously described (46). The products of the footprinting reaction were fractionated on 8% polyacrylamide sequencing gels that were calibrated with Maxam–Gilbert DNA sequence ladders and resultant footprinting patterns were analysed using a phosphorimager.
Determination of in vivo promoter activity
Analysis of rrnB P1 promoter activity in vivo was performed using strain NK5031 lysogenic for λ system I derivatives (16) in which rrnB P1 promoter variants were transcriptionally fused to trpB′A-lacZ. Construction of recombinant phages harbouring fusions of lacZ to rrnB P1 derivatives in which the consensus UP element had been displaced was carried out as described previously (47–49) and monolysogens of NK5031 (i.e. RLG4721–RLG4727) were identified by a combination of a PCR-based assay (17) and β-galactosidase measurement. Overnight cultures of the lacZ fusion strains were diluted 1:50 into pre-warmed Luria–Bertani broth [containing ampicillin (100 µg/ml) and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when harbouring pLAW2 derivatives expressing mutant rpoA alleles] and grown at 30°C with vigorous shaking for three to four generations (OD600 ∼0.4–0.6). β-Galactosidase activity was determined by the method of Miller (50) using the chloroform–SDS procedure and values were corrected for background β-galactosidase activity by assaying a lysogen containing a promoter-less lacZ fusion (RLG1336). Results are presented as percentages, with the strain harbouring the consensus rrnB P1–lacZ fusion assigned a value of 100% in experiments utilising untransformed strains, and strains harbouring pLAW2 or pMGM12 assigned a value of 100% when measurements were performed on transformants harbouring pLAW2 derivatives.
RESULTS
Effect of UP element displacement on transcription from the rrnB P1 promoter in vitro and in vivo
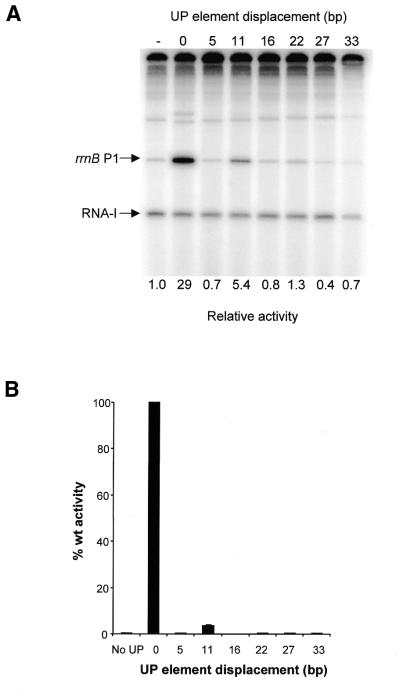
To investigate the relationship between UP element location and UP element-dependent stimulation of transcription from the rrnB P1 promoter, a series of rrnB P1 promoter derivatives were constructed in which the full consensus UP element was displaced upstream from the rrnB P1 core promoter by distances corresponding to 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 turns of the DNA helix, i.e. 5, 11, 16, 22, 27 and 33 bp, respectively (Fig. 1). These promoters were cloned into the transcription vector pRLG770 and their activities in vitro were compared with that of an rrnB P1 promoter derivative containing the consensus UP element in the wild type position, and to the core rrnB P1 promoter, in which the UP element is replaced by a sequence devoid of UP element-like activity (17). The results showed that whereas the presence of the consensus UP element in the wild type location resulted in a 29-fold increase in promoter activity relative to the core promoter, displacement of the UP element by one helical turn (11 bp) resulted in only ∼5-fold activation (Fig. 2A, compare lanes 2 and 4 with lane 1). Upstream displacement of the consensus UP element by 0.5, 1.5, 2, 2.5 or 3 turns of the DNA helix essentially abolished UP element-dependent stimulation (Fig. 2A). These results suggest that the location of the UP element at the wild type rrnB P1 promoter is optimal for UP element-dependent stimulation. The results also confirm the previous observation that activation of rrnB P1 by upstream sequences is face of the helix dependent (15).
Figure 2.
Effect of UP element displacement on transcription from rrnB P1 in vitro and in vivo. (A) Transcription gel showing the results of multiple round in vitro transcription reactions carried out with native RNAP on templates containing the rrnB P1 core promoter (pRLG4210) (denoted by a ‘–’ sign), and the rrnB P1 promoter in which the consensus UP element is located at the normal position (pRLG4713) (indicated by a ‘0’), or displaced upstream by the indicated number of base pairs (pRLG4714–pRLG4720). The position of the transcripts derived from the vector-derived RNA-I promoter and the rrnB P1 promoter are indicated. Promoter activities, as determined from the transcript abundance in at least four transcription assays of the type shown, are presented below each gel lane as fold change in promoter activity relative to the core rrnB P1 promoter, where core promoter activity is assigned a value of 1.0. Values of less than unity arise through inhibition of core promoter activity. Standard deviations are within 18% of the mean and are omitted for clarity. (B) Effect of UP element displacement on transcription from rrnB P1 in vivo. β-Galactosidase activities were measured in lysogens of NK5031 harbouring a single copy lacZ fusion to the rrnB P1 core promoter (RLG3097) (denoted ‘No UP’) or the rrnB P1 promoter containing a consensus UP element located at the normal position (RLG4721) (indicated by a ‘0’), or displaced upstream of the normal location by the indicated number of base pairs (RLG4722–RLG4727). Values are expressed as a percentage of the activity in RLG4721 (100%) and are the means (with standard deviation) of three or more independent assays. 100% activity = 5780 Miller units.
To examine the effect of UP element displacement on transcription from rrnB P1 in vivo, the rrnB P1 promoter derivatives possessing displaced UP elements were cloned upstream of the lacZ gene on a recombinant λ phage and monolysogens were constructed (see Table 1). The results from β-galactosidase assays showed that UP element-dependent stimulation of transcription is maximal when the UP element is present in the wild type location (Fig. 2B). Displacement of the consensus UP element by 11 bp upstream from the wild type location gave rise to >20-fold decrease in transcriptional activity, whereas displacement of the UP element by any other distance, including displacement by only 5 bp, abolished UP element-dependent transcription (Fig. 2B). These results are fully consistent with the data obtained in vitro and emphasise the crucial importance of the position of the UP element to its function at rrnB P1.
Effect of α linker length on UP element-dependent transcription in vitro
To test the requirements for the RNAP α subunit interdomain linker at the rrnB P1 promoter in vitro, RNAPs were reconstituted using a series of 10 mutant α subunits possessing interdomain linkers of different lengths. These mutant derivatives have been described previously (37) and contain deletions of 3, 6, 9 and 12 amino acids (termed Δ3, Δ6, Δ9 and Δ12), and insertions of 3, 6, 10, 13, 16 and 32 amino acids (termed Ω3, Ω6, Ω10, Ω13, Ω16 and Ω32). Multiple round transcription assays were performed with template DNA containing the wild type rrnB P1 promoter, including its natural UP element, or rrnB P1derivatives possessing the consensus full UP element or either the consensus proximal or distal UP element subsites, instead of the rrnB P1 UP element (6,17). As transcription from the rrnB P1 core promoter in comparison to a control promoter, the RNA-I promoter, encoded on the same plasmid, was relatively unaffected by alterations to the length of the α interdomain linker, i.e. the ratio of transcripts originating from the core rrnB P1 and RNA-I promoters remained constant (Fig. 3A), transcription from the rrnB P1 core promoter was used to normalise the activities of the RNAP preparations containing interdomain linkers of different lengths.
At the wild type rrnB P1 promoter, a progressive decrease in RNAP activity was observed as the size of the deletion introduced into the α subunit linker was increased (Fig. 3B). The effect of linker deletion was similar for the rrnB P1 promoter containing the consensus UP element (Fig. 3C), although the extent of the decrease was less marked. Removal of 12 amino acids from the α linker resulted in an 80–90% decrease in transcriptional efficiency from the promoters with the wild type and consensus UP elements, almost as large a decrease as was observed following complete removal of the αCTD. Transcription from these promoters was much less sensitive to increases in the length of the α linker. Thus, insertion of 16 amino acids resulted in only a 20–25% decrease in transcription from both promoters, and RNAP containing a 32 amino acid insertion in the α linker retained approximately half of wild type RNAP activity at these promoters.
The effect of altering α linker length on transcription from the rrnB P1 promoter containing only the consensus distal subsite or only the consensus proximal UP subsite was broadly similar to the overall pattern observed for the wild type rrnB P1 promoter, although the effect of deletion of three amino acids from the α linker was greater on transcription from rrnB P1 containing the proximal UP element subsite than on the other rrnB P1 derivatives (Fig. 3D and E).
Effect of α linker length on UP element-dependent transcription in vivo
To investigate the effects of altering the length of the α interdomain linker on UP element-dependent transcription in vivo, plasmids expressing each of the mutant rpoA alleles were introduced into NK5031 harbouring single copy transcriptional fusions of the lacZ gene to various rrnB P1 promoter derivatives. β-Galactosidase activities were measured following induction of mutant α synthesis for three to four generations. In general agreement with our observations in vitro, decreasing the number of amino acids in the α linker caused a general decrease in the efficiency of transcription from the wild type rrnB P1 promoter, whereas only longer insertions within the linker (Ω16 and Ω32) decreased expression substantially (Fig. 4A). The overall pattern was similar at the consensus rrnB P1 promoter, although this promoter was less sensitive to changes in linker length than the wild type promoter (Fig. 4B).
Figure 4.
Effect of α subunit linker length on transcription in vivo from rrnB P1 promoter derivatives. β-Galactosidase activities were measured in lysogens of NK5031 harbouring a single copy lacZ fusion to (A) the rrnB P1 promoter with its natural UP element (RLG3074), (B) the rrnB P1 promoter with the consensus UP element (RLG4192) or (C) the rrnB P1 core promoter (RLG2263), in each case transformed with pMGM12, expressing wild type rpoA, or one of the pMGM12 derivatives expressing mutant rpoA alleles (pMGM13, pMGM16–pMGM22, pMGM32 or pMGM35), as indicated. For each rrnB P1 derivative, values are expressed as a percentage of the promoter activity in the presence of RNAP containing only wild type α, and are the means (with standard deviation) of three or more independent assays. 100% activity = 11.4, 1865 and 4983 Miller units, respectively, for the pMGM12 transformants of RLG2263 (core rrnB P1 promoter), RLG3074 (wild type rrnB P1 promoter) and RLG4192 (consensus rrnB P1 promoter). Fold activation due to the wild type (D) and consensus (E) UP elements in the presence of each mutant RNAP derivative is presented as a percentage of the fold activation in the presence of wild type RNAP (100%). Values were calculated by expressing the ratios of the wild type (or consensus) rrnB P1 promoter activity to the core rrnB P1 promoter activity for each RNAP derivative as a percentage of the ratio obtained in the presence of only wild type RNAP.
Unlike the results in vitro, deletions of more than three amino acids from within the α linker strongly stimulated transcription from the core rrnB P1 promoter in vivo (Fig. 4C). Thus, cells containing RNAP in which the Δ12 α subunit was incorporated into RNAP gave rise to an ∼15-fold increase in the β-galactosidase activity relative to cells expressing only wild type rpoA. Increasing the length of the α linker caused a gradual but much less profound increase in transcription from the core promoter. The increase in rrnB P1 core promoter activity in vivo can be interpreted in the context of our understanding of rRNA transcription regulation (58,59); i.e. decreased rRNA transcription (from decreased UP element function) leads to compensating derepression of rrnB P1 core promoter activity to keep total rRNA synthesis constant (see ref. 8 and Discussion). Figure 4D and E illustrates the effects of the altered linkers on UP element function in vivo, adjusted for these feedback effects. The fold increases in transcription resulting from the wild type rrnB P1 UP element (Fig. 4D) or from the consensus UP element (Fig. 4E) in the presence of each of the α linker mutants are presented as a percentage of the fold increases in transcription observed in the presence of wild type α. In this representation, it is obvious that the α linker deletions exert a strong negative effect on UP element function in vivo, consistent with their effects on UP element utilisation in vitro. Likewise, it is apparent that UP element-dependent function at rrnB P1 is also impaired by insertions within the α linker, although to a lesser degree.
Effect of shortening the α linker on interactions between αCTD and the UP element at the rrnB P1 promoter
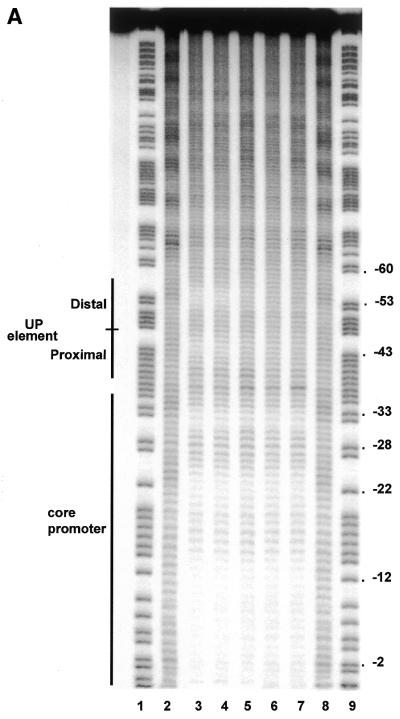
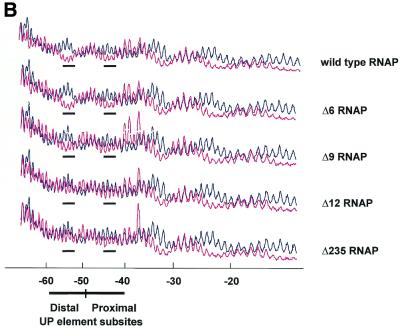
To determine whether there is a correlation between the effect of shortening the α interdomain linker on the activity of RNAP at the rrnB P1 promoter, and the ability of αCTD to bind the UP element, we carried out hydroxyl radical footprinting experiments on RNAP:rrnB P1 binary complexes. As previously observed, wild type RNAP protected a continuous region extending from approximately position –10 to +16 (not shown) and short regions around positions –20 and –30 (14) (Fig. 5A, lane 3, and B). In addition, short protected regions were observed around positions –43 and –53, corresponding to the sites of αCTD interaction within the proximal and distal UP element subsites (14,17). RNAP reconstituted with the Δ6 α derivative affords a similar degree of protection of the proximal and distal UP element subsites as wild type RNAP (Fig. 5A, lane 4, and B). However, protection of both UP element subsites was greatly diminished following deletion of a further three amino acids from the α linker (Δ9 RNAP) and was essentially abolished following deletion of 12 amino acids (Δ12 RNAP), without a concomitant reduction in the protection downstream of –40 (Fig. 5A, lanes 5 and 6, and B). RNAP reconstituted with the Δ235 α subunit failed to protect the UP element subsites from hydroxyl radical attack, as shown previously (8), confirming that the protections observed around –43 and –53 are due to αCTD (Fig. 5A, lane 7, and B). The pattern of protection produced in the core promoter region by each of the linker modified RNAPs and the Δ235 RNAP derivative was essentially identical to that afforded by wild type RNAP. These results indicate that as many as six amino acids can be deleted from the α interdomain linker without significantly compromising the ability of αCTD to engage the UP element subsites in vitro. The strong correlation between the transcriptional activity of the different RNAP deletion derivatives at rrnB P1 and their ability to protect the UP element subsites suggests that that the observed decrease in in vitro and in vivo transcriptional activity at the wild type rrnB P1 promoter upon shortening the α interdomain linker is due to a decreased ability to interact with the UP element.
Figure 5.
Hydroxyl radical cleavage of the wild type rrnB P1 promoter with wild type and mutant RNAPs. (A) Autoradiogram of a typical footprinting gel showing sites within the template (bottom) strand of the wild type rrnB P1 promoter which are protected from hydroxyl radicals by bound RNAP reconstituted with wild type or mutant α subunits. Lanes 1 and 9, Maxam–Gilbert G+A reaction; lanes 2 and 8, no RNAP; lane 3, wild type RNAP; lane 4, Δ6 RNAP; lane 5, Δ9 RNAP; lane 6, Δ12 RNAP; lane 7, Δ235 RNAP. Sequences comprising the core promoter element (as far downstream as +2) and the UP element subsites are indicated by black bars. (B) Scan of the footprinting pattern for each RNAP, reconstituted with the indicated α subunit (magenta trace), aligned in each case with the scan obtained in the absence of RNAP (black trace). The scans are averaged from three independent footprinting experiments. The regions in each scan corresponding to positions around –43 and –53 are underscored by a black bar.
Effect of increasing the length of the α interdomain linker on stimulation of transcription by displaced UP elements in vitro and in vivo
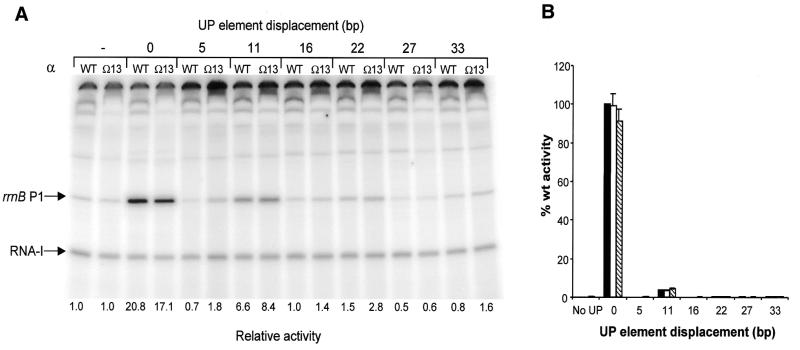
The footprinting experiments suggest that the ‘reach’ of αCTD is a function of linker length. Therefore, we used in vitro transcription assays to investigate whether tethering αCTD by a longer linker could improve the utilisation of displaced UP elements at rrnB P1. We observed no significant difference between the activity of RNAP reconstituted with the Ω13 α derivative and RNAP reconstituted with wild type α at the rrnB P1 promoter containing the UP element either in the normal position (as also shown in Fig. 3B) or when the UP element was displaced by one or two turns of the helix (Fig. 6A). A similar pattern was observed when RNAPs reconstituted with α mutants possessing 10 or 16 additional amino acids were compared with wild type RNAP (results not shown). We conclude that extending the α linker does not significantly enhance the response of RNAP to displaced UP elements at the rrnB P1 promoter in vitro.
Figure 6.
Effect of extended α subunit linker length on in vitro and in vivo RNAP activity at rrnB P1 promoters containing a displaced UP element. (A) Transcription gel showing the results of multiple round in vitro transcription reactions performed with RNAP reconstituted with wild type (WT) or Ω13 α subunits on templates containing the rrnB P1 core promoter (pRLG4210) (indicated by a ‘–’ sign), and the rrnB P1 promoter in which the consensus UP element is located at the normal position (pRLG4713) (denoted by a ‘0’), or displaced by the indicated number of base pairs (pRLG4714–pRLG4720). The position of the transcripts derived from the RNA-I and rrnB P1 promoters are indicated. Promoter activities, as determined from the transcript abundance in at least four in vitro transcription reactions of the type shown, are presented below each gel lane as fold change in promoter activity relative to the rrnB P1 core promoter, where the core promoter activity is assigned a value of 1.0 for each RNAP tested. Values of less than unity arise through inhibition of core promoter activity. Standard deviations were within 22% of the mean and are omitted for clarity. (B) Effect of extended α subunit linker length on in vivo RNAP activity at rrnB P1 promoters containing a displaced UP element. β-Galactosidase activities were measured in lysogens of NK5031 harbouring a single copy lacZ fusion to the rrnB P1 core promoter (RLG3097) (indicated with a ‘–’ sign) or the rrnB P1 promoter containing a consensus UP element located at the normal position (RLG4721) (denoted by a ‘0’), or displaced upstream of the normal location by the indicated number of base pairs (RLG4722–RLG4727). Each lysogen contained pLAW2, expressing wild type rpoA (black bars), or a pLAW2 derivative encoding the Ω6 α subunit (pMGM19; white bars) or the Ω13 α subunit (pMGM21; hatched bars). Values are expressed as a percentage of the activity in the RLG4721/pLAW2 transformant (100%) and are the means (with standard deviation) of three or more independent assays. 100% activity = 4897 Miller units.
To explore the effect of lengthening the α interdomain linker on the response of RNAP to displaced UP elements in vivo, the strains harbouring single copy fusions of lacZ to rrnB P1 promoters possessing displaced consensus UP elements were transformed with plasmids expressing wild type rpoA, or the Ω6 and Ω13 rpoA mutants. Consistent with our in vitro observations, β-galactosidase assays indicated that possession of an extended linker does not significantly increase the activity of RNAP at any of the promoters containing displaced UP elements (Fig. 6B).
Previous studies have demonstrated that displacement of the distal UP element subsite by one helical turn, while retaining the proximal subsite in its normal location, does not impair UP element-dependent transcription, whereas displacement of the distal subsite by two or three turns abolishes its contribution (15). To examine whether extending the α interdomain linker compensates for the negative effect of displacing the distal UP element subsite alone, we performed β-galactosidase measurements on a series of strains harbouring single copy fusions of the lacZ gene to rrnB P1 promoter derivatives in which the distal UP element subsite had been displaced by a half, one, two and three turns of the helix (15). Our results showed that the activity of all the promoter variants was unaffected in cells containing Ω6 or Ω13 α subunits (results not shown). These results suggest that extending the α subunit linker also does not compensate for the negative effect on transcription of displacement of the distal UP element subsite.
DISCUSSION
The experiments described here provide important information regarding the requirements for productive interactions between αCTD and UP elements. We have shown that upstream displacement of the full UP element by 5 bp or more greatly reduces UP element-dependent stimulation of transcription from rrnB P1. Our results are consistent with previous observations that αCTD can sometimes bind to the opposite face of the DNA helix to that occupied by the rest of RNAP, but that such interactions do not stimulate transcription and can in fact induce an inhibitory effect (60,61). As there appears to be a requirement for UP element:αCTD interactions to be located on the same face of the DNA helix at rrnB P1 as the rest of RNAP, adjacent to σ bound at the –35 region, it is possible that optimal UP element function requires contact between αCTD and σ at some promoters. Recent evidence supports the importance of such an interaction at the rrnB P1 promoter containing the proximal UP element subsite (W.E.Ross and R.L.Gourse, unpublished results; 7,32) and at Class I CRP-dependent promoters (23; N.J.Savery, G.Lloyd, S.J.W.Busby, M.S.Thomas, R.H.Ebright and R.L.Gourse, unpublished results). Alternatively, it is possible that insertions between the –35 hexamer and the UP element result in perturbations in DNA structure that interfere with αCTD function.
Displacement of the full UP element by two (or more) turns upstream of rrnB P1 (i.e. moving the centres of the proximal and distal subsites to –64 and –75, respectively) essentially abolishes UP element-dependent stimulation, and displacement of the distal UP element subsite was shown previously to decrease the efficiency of αCTD binding (15). Conversely, experiments with promoters containing displaced CRP sites have revealed that αCTD can bind adjacent to CRP at positions as far as 85 bp upstream of the transcription start site (60,62). αCTD is also able to access naturally occurring UP elements located ∼80 bp upstream of the Pseudomonas putida Pu promoter (12) or ∼90 bp upstream of the λ PL promoter (40,41). However, in these cases, the interaction between αCTD and more distant DNA binding sites is facilitated by the binding of the transcription factors CRP and IHF, respectively, to upstream sequences. Although αCTD can be crosslinked to DNA sites as far as 63, 73, 83 and 93 bp upstream of the lac(ICAP)UV5 promoter in the absence of CRP (39), these presumably transient interactions do not facilitate transcription initiation.
Figures 3–5 indicate that there is a good correlation between α linker length, the ability of αCTD to access the UP element and the degree of UP element-dependent stimulation at the rrnB P1 promoter. Our observations on the effect of α linker deletion on transcription from the rrnB P1 promoter are also broadly similar to the results obtained by Fujita and colleagues (38), although they reported a more pronounced effect of the shorter deletion derivatives on transcription in vitro. In contrast, shortening the α subunit interdomain linker exerted a strong stimulatory effect on the core rrnB P1 promoter in vivo (Fig. 4C). This phenomenon was not observed for the UP element-independent lacUV5 promoter (W.Meng, S.J.W.Busby and M.S.Thomas, unpublished results). The decreased efficiency of UP element utilisation by RNAPs containing deletions within the α subunit linker would be expected to result in a transient decrease in rRNA synthesis. As reported previously (8), when UP element function is compromised by mutations in rpoA, the activity of an rrnB P1 core promoter–lacZ fusion increases. For reasons that remain unclear, the magnitude of the ‘derepression’ observed in the presence of overexpressed linker modified α subunits was greater than the ∼3-fold increase in core promoter activity measured in the presence of α subunits devoid of the C-terminal domain (8). The increase in UP element-independent promoter activity in the seven rrn operons responsible for rRNA biosynthesis in E.coli compensates for the decrease in UP element function and the resulting transient reduction in ribosome synthesis, a phenomenon referred to as ‘rRNA feedback’ (8,58,63). However, the effects of the α linker mutants on UP element function were apparent in vivo when the effects of rRNA feedback were taken into consideration (Fig. 4D and E).
Increasing the length of the α interdomain linker also impairs transcription from the rrnB P1 promoter, although RNAP appears less sensitive to linker insertions than deletions. This result indicates that the length of the wild type α interdomain linker is optimal for efficient utilisation of the UP element at rrnB P1. It is probable that the linker length adopted by bacterial RNAP results, at least in part, from selective pressure for UP element utilisation at rRNA promoters. In this context, it is interesting that chloroplasts contain α subunits possessing considerably longer linkers (37), suggesting that chloroplast RNAP is not subject to the same constraints as the bacterial enzyme.
Increasing the length of the linker by 16 amino acids (essentially doubling its length) would be expected to result in extending the ‘reach’ of αCTD by ∼58 Å, enough to allow utilisation of UP elements positioned one or two turns upstream of the normal location (the pitch of B-form DNA is 34 Å per turn). As RNAP containing α subunits with linkers lengthened by up to 32 amino acids did not increase utilisation of displaced UP elements, it is possible that the α linkers containing insertions are not fully extended. Studies by Fujita and colleagues suggest, in fact, that segments of the α linker may adopt an ordered (helical) structure under certain conditions (38). In conjunction with the possible requirement for α–σ interactions and/or DNA structural requirements at the junction of the –35 region and UP element, the possibility that the linker has structure might explain why lengthening the linker does not extend the operational distance of αCTD with respect to UP element utilisation.
Acknowledgments
ACKNOWLEDGEMENTS
M.S.T. would like to thank members of the Gourse and Landick laboratories for their help and advice. This work was supported by project grants 050794 (to M.S.T. and S.J.W.B.) and 052849 (to R.L.G. and S.J.W.B.) from the Wellcome Trust, and by grant RO1 GM37048 from the National Institutes of Health to R.L.G.
References
- 1.Zhang G., Campbell,E.A., Minakhin,L., Richter,C., Severinov,K. and Darst,S.A. (1999) Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell, 98, 811–824. [DOI] [PubMed] [Google Scholar]
- 2.Ebright R.H. (2000) RNA polymerase: structural similarities between bacterial RNA polymerase and eucaryotic RNA polymerase II. J. Mol. Biol., 304, 687–698. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan M.E., Hawley,D.K., Entriken,R. and McClure,W.R. (1984) Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res., 12, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darst S.A., Roberts,J.W., Malhotra,A., Mark,M., Severinov,K. and Severinova,E. (1997) Pribnow box recognition and melting by Escherichia coli RNA polymerase. In Eckstein,F. and Lilley,D.M.J. (eds), Nucleic Acids and Molecular Biology, Vol. 11. Springer-Verlag, Berlin, pp. 27–40.
- 5.Barne K.A., Bown,J.A., Busby,S.J.W. and Minchin,S.D. (1997) Region 2.5 of the Escherichia coli RNA polymerase σ70 subunit is responsible for the recognition of the ‘extended –10’ motif at promoters. EMBO J., 16, 4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrem S.T., Ross,W., Gaal,T., Chen,Z.W.S., Niu,W., Ebright,R.H. and Gourse,R.L. (1999) Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase α subunit. Genes Dev., 13, 2134–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourse R.L., Ross,W. and Gaal,T. (2000) UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol., 37, 687–695. [DOI] [PubMed] [Google Scholar]
- 8.Ross W., Gosink,K.K., Salomon,J., Igarishi,K., Zou,C., Ishihama,A., Severinov,K. and Gourse,R.L. (1993) A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science, 262, 1407–1413. [DOI] [PubMed] [Google Scholar]
- 9.Blatter E.E., Ross,W., Tang,H., Gourse,R.L. and Ebright,R.H. (1994) Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell, 78, 889–896. [DOI] [PubMed] [Google Scholar]
- 10.Newlands J.T., Gaal,T., Mecsas,J. and Gourse,R.L. (1993) Transcription of the Escherichia coli rrnB P1 promoter by the heat shock RNA polymerase (E sigma 32) in vitro. J. Bacteriol., 175, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredrick K., Caramori,T., Chen,Y.F., Galizzi,A. and Helmann,J.D. (1995) Promoter architecture in the flagellar regulon of Bacillus subtilis: high-level expression of flagellin by the sigma D RNA polymerase requires an upstream promoter element. Proc. Natl Acad. Sci. USA, 92, 2582–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertoni G., Fujita,N., Ishihama,A. and de Lorenzo,V. (1998) Active recruitment of σ54-RNA polymerase to the Pu promoter of Pseudomonas putida: role of IHF and αCTD. EMBO J., 17, 5120–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross W., Aiyar,S.E., Salomon,J. and Gourse,R.L. (1998) Escherichia coli promoters with UP elements of different strength: modular structure of bacterial promoters. J. Bacteriol., 180, 5375–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newlands J.T., Ross,W., Gosink,K.K. and Gourse,R.L. (1991) Factor-independent activation of Escherichia coli rRNA transcription. II. Characterization of complexes of rrnB P1 promoters containing or lacking the upstream activator region with Escherichia coli RNA polymerase. J. Mol. Biol., 220, 560–583. [DOI] [PubMed] [Google Scholar]
- 15.Newlands J.T., Josaitis,C., Ross,W. and Gourse,R.L. (1992) Both FIS-dependent and factor-independent upstream activation of the rrnB P1 promoter are face of the helix dependent. Nucleic Acids Res., 20, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao L., Ross,W., Appleman,J.A., Gaal,T., Leirmo,S., Schlax,P.J., Record,M.T.,Jr and Gourse,R.L. (1994) Factor-independent activation of rrnB P1; an ‘extended’ promoter with an upstream element that dramatically increases promoter strength. J. Mol. Biol., 235, 1421–1435. [DOI] [PubMed] [Google Scholar]
- 17.Estrem S.T., Gaal,T., Ross,W. and Gourse,R.L. (1998) Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl Acad. Sci. USA, 95, 9761–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strainic M.G., Sullivan,J.J., Velvis,A. and deHaseth,P.L. (1998) Promoter recognition by Escherichia coli RNA polymerase: effects of the UP element on open complex formation and promoter clearance. Biochemistry, 37, 18074–18080. [DOI] [PubMed] [Google Scholar]
- 19.Negishi T., Fujita,N. and Ishihama,A. (1995) Structural map of the alpha subunit of Escherichia coli RNA polymerase: structural domains identified by proteolytic cleavage. J. Mol. Biol., 248, 723–728. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi K., Fujita,N. and Ishihama,A. (1991) Identification of a subunit assembly domain in the alpha subunit of Escherichia coli RNA polymerase. J. Mol. Biol., 218, 1–6. [PubMed] [Google Scholar]
- 21.Zhang G. and Darst,S. (1998) Structure of the Escherichia coli RNA polymerase α subunit amino-terminal domain. Science, 281, 262–266. [DOI] [PubMed] [Google Scholar]
- 22.Niu W., Kim,Y., Tau,G., Heyduk,T. and Ebright,R.H. (1996) Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell, 87, 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busby S. and Ebright,R.H. (1999) Transcription activation by catabolite activator protein (CAP). J. Mol. Biol., 293, 199–213. [DOI] [PubMed] [Google Scholar]
- 24.Jeon Y.H., Negishi,T., Shirakawa,M., Yamazaki,T., Fujita,N., Ishihama,A. and Kyogoku,Y. (1995) Solution structure of the activator contact domain of the RNA polymerase α subunit. Science, 270, 1495–1497. [DOI] [PubMed] [Google Scholar]
- 25.Ishihama A. (1993) Protein–protein communication within the transcription apparatus. J. Bacteriol., 175, 2483–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kainz M. and Gourse,R.L. (1998) The C-terminal domain of the alpha subunit of Escherichia coli RNA polymerase is required for Rho-dependent transcription termination. J. Mol. Biol. 284, 1379–1390. [DOI] [PubMed] [Google Scholar]
- 27.Mah T.-F., Kuznedelov,K., Mushegian,A., Severinov,K. and Greenblatt,J. (2000) The α subunit of E. coli RNA polymerase activates RNA binding by NusA. Genes Dev., 14, 2664–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi K. and Ishihama,A. (1991) Bipartite functional map of the E. coli RNA polymerase α subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell, 65, 1015–1022. [DOI] [PubMed] [Google Scholar]
- 29.Busby S. and Ebright,R.H. (1994) Promoter structure, promoter recognition, and transcription activation in prokaryotes. Cell, 79, 743–746. [DOI] [PubMed] [Google Scholar]
- 30.Gaal T., Ross,W., Blatter,E.E., Tang,H., Jia,X., Krishnan,V.V., Assa-Munt,N., Ebright,R.H. and Gourse,R.L. (1996) DNA-binding determinants of the α subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev., 10, 16–26. [DOI] [PubMed] [Google Scholar]
- 31.Shao X. and Grishin,N.V. (2000) Common fold in helix-hairpin-helix proteins. Nucleic Acids Res., 28, 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross W., Ernst,A. and Gourse,R.L. (2001) Fine structure of E. coli RNA polymerase-promoter interactions: α subunit binding to the UP element minor groove. Genes Dev., 15, 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami K., Fujita,N. and Ishihama,A. (1996) Transcription factor recognition surface on the RNA polymerase α subunit is involved in contact with the DNA enhancer element. EMBO J., 15, 4358–4367. [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuno K., Yamazaki,T., Tanaka,Y., Kodama,T.S., Matsugami,A., Katahira,M., Ishihama,A. and Kyogoku,Y. (2001) Interaction of the C-terminal domain of the E. coli RNA polymerase α subunit with the UP element: recognizing the backbone structure in the minor groove surface. J. Mol. Biol., 306, 213–225. [DOI] [PubMed] [Google Scholar]
- 35.Ariyoshi M., Nishino,T., Iwasaki,H., Shinagawa,H. and Morikawa,K. (2000) Crystal structure of the Holliday junction DNA in complex with a single RuvA tetramer. Proc. Natl Acad. Sci. USA, 97, 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon Y.H., Yamazaki,T., Otomo,T., Ishihama,A. and Kyogoku,Y. (1997) Flexible linker in the RNA polymerase alpha subunit facilitates the independent motion of the C-terminal activator contact domain. J. Mol. Biol., 267, 953–962. [DOI] [PubMed] [Google Scholar]
- 37.Meng W., Savery,N.J., Busby,S.J.W. and Thomas,M.S. (2000) The Escherichia coli RNA polymerase α subunit: length requirements for transcription activation at CRP-dependent promoters. EMBO J., 19, 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita N., Endo,S. and Ishihama,A. (2000) Structural requirements for the interdomain linker of α subunit of Escherichia coli RNA polymerase. Biochemistry, 39, 6243–6249. [DOI] [PubMed] [Google Scholar]
- 39.Naryshkin N., Revyakin,A., Kim,Y., Mekler,V. and Ebright,R.H. (2000) Structural organization of the RNA polymerase-promoter open complex. Cell, 101, 601–611. [DOI] [PubMed] [Google Scholar]
- 40.Giladi H., Murakami,K., Ishihama,A. and Oppenheim,A.B. (1996) Identification of an UP element within the IHF binding site at the PL1-PL2 tandem promoter of bacteriophage λ. J. Mol. Biol., 260, 484–491. [DOI] [PubMed] [Google Scholar]
- 41.Giladi H., Koby,S., Prag,G., Engelhorn,M., Geiselmann,J. and Oppenheim,A. (1998) Participation of IHF and a distant UP element in the stimulation of the phage λ PL promoter. Mol. Microbiol. 30, 443–451. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Tang H., Severinov,K., Goldfarb,A. and Ebright,R.H. (1995) Rapid RNA polymerase genetics: one-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc. Natl Acad. Sci. USA, 92, 4902–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross W., Thompson,J.F., Newlands,J.T. and Gourse,R.L. (1990) E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J., 9, 3733–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker M.M., Gaal,T., Josaitis,C.A. and Gourse,R.L. (2001) Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol., 305, 673–688. [DOI] [PubMed] [Google Scholar]
- 46.Savery N.J., Belyaeva,T. and Busby,S.J.W. (1996) Protein–DNA interactions. In Docherty,K. (ed.), Gene Transcription: DNA Binding Proteins, Essential Techniques. Wiley/BIOS Press, Chichester, UK, pp. 1–43.
- 47.Miura A., Krueger,J.H., Itoh,S., de Boer,H.A. and Nomura,M. (1981) Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell, 25, 773–782. [DOI] [PubMed] [Google Scholar]
- 48.Gourse R.L., de Boer,H.A. and Nomura,M. (1986) DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell, 44, 197–205. [DOI] [PubMed] [Google Scholar]
- 49.Gaal T., Barkei,J., Dickson,R.R., deBoer,H.A., deHaseth,P.L., Alavi,H. and Gourse,R.L. (1989) Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J. Bacteriol., 171, 4852–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller J. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 51.Studier F.W. and Moffatt,B.A. (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol., 189, 113–130. [DOI] [PubMed] [Google Scholar]
- 52.Bullock W.O., Fernandez,J.M. and Short,J.M. (1987) XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. Biotechniques, 5, 376–378. [Google Scholar]
- 53.Guarente L., Lauer,G., Robert,T.M. and Ptashne,M. (1980) Improved methods for maximising expression of a cloned gene: a bacterium that synthesizes rabbit β-globin. Cell, 20, 543–553. [DOI] [PubMed] [Google Scholar]
- 54.Severinov K., Soushko,M., Goldfarb,A. and Nikiforov,V. (1993) Rifampicin region revisited—new rifampicin-resistant and streptolydigin-resistant mutants in the beta-subunit of Escherichia coli RNA-polymerase. J. Biol. Chem., 268, 14820–14825. [PubMed] [Google Scholar]
- 55.Zalenskaya K., Lee,J.Y., Gujuluva,C.N., Shin,Y.K., Slutsky,M. and Goldfarb,A. (1990) Recombinant RNA polymerase-inducible overexpression, purification and assembly of Escherichia coli rpo gene-products. Gene, 89, 7–12. [DOI] [PubMed] [Google Scholar]
- 56.Savery N.J., Lloyd,G.S., Kainz,M., Gaal,T., Ross,W., Ebright,R.H., Gourse,R.L. and Busby,S.J.W. (1998) Transcription activation at Class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase α subunit. EMBO J., 12, 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou C., Fujita,N., Igarashi,K. and Ishihama,A. (1992) Mapping the cAMP receptor protein contact site on the α subunit of Escherichia coli RNA polymerase. Mol. Microbiol., 6, 2599–2605. [DOI] [PubMed] [Google Scholar]
- 58.Gourse R.L., Gaal,T., Bartlett,M.S., Appleman,J.A. and Ross,W. (1996) rRNA transcription and growth rate dependent regulation of ribosomal RNA synthesis in Escherichia coli. Annu. Rev. Microbiol., 50, 645–677. [DOI] [PubMed] [Google Scholar]
- 59.Gaal T., Bartlett,M.S., Ross,W., Turnbough,C.L.,Jr and Gourse,R.L. (1997) Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science, 278, 2092–2097. [DOI] [PubMed] [Google Scholar]
- 60.Murakami K., Owens,J.T., Belyaeva,T., Meares,C.F., Busby,S.J.W. and Ishihama,A. (1997) Positioning of two alpha subunit carboxy-terminal domains of RNA polymerase along promoter DNA by two transcription factors. Proc. Natl Acad. Sci. USA, 94, 11274–11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tagami H. and Aiba,H. (1999) An inactive open complex mediated by an UP element at Escherichia coli promoters. Proc. Natl Acad. Sci. USA, 96, 7202–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belyaeva T., Rhodius,V., Webster,C. and Busby,S. (1998) Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organization of the RNA polymerase α subunits. J. Mol. Biol., 277, 789–804. [DOI] [PubMed] [Google Scholar]
- 63.Jinks-Robertson S., Gourse,R.L. and Nomura,M. (1983) Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell, 33, 865–876. [DOI] [PubMed] [Google Scholar]