Abstract
Background
Polychlorinated biphenyls (PCBs) are persistent organic pollutants that adversely affect human health. PCBs bio-accumulate in organisms important for human consumption. PCBs accumulation in the body leads to activation of the transcription factor NF-кB, a major driver of inflammation. Despite dietary exposure being one of the main routes of exposure to PCBs, the gut has been widely ignored when studying the effects of PCBs.
Objectives
We investigated the effects of PCB 153 on the intestine and addressed whether PCB 153 affected intestinal permeability or inflammation and the mechanism by which this occurred.
Methods
Mice were orally exposed to PCB 153 and gut permeability was assessed. Intestinal epithelial cells (IECs) were collected and evaluated for evidence of genotoxicity and inflammation. A human IEC line (SW480) was used to examine the direct effects of PCB 153 on epithelial function. NF-кB activation was measured using a reporter assay, DNA damage was assessed, and cytokine expression was ascertained with real-time PCR.
Results
Mice orally exposed to PCB 153 had an increase in intestinal permeability and inflammatory cytokine expression in their IECs; inhibition of NF-кB ameliorated both these effects. This inflammation was associated with genotoxic damage and NF-кB activation. Exposure of SW480 cells to PCB 153 led to similar effects as seen in vivo. We found that activation of the ATM/NEMO pathway by genotoxic stress was upstream of NF-kB activation.
Conclusions
These results demonstrate that oral exposure to PCB 153 is genotoxic to IECs and induces downstream inflammation and barrier dysfunction in the intestinal epithelium.
Keywords: Polychlorinated Biphenyl, PCB 153, intestinal inflammation, NF-кB, ATM, NEMO
Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous and persistent organic pollutants that adversely affect human health (Quinete et al. 2014). Although industrial production of PCBs has been discontinued, they remain a pressing environmental problem due to their slow biodegradation and high lipophilicity. These properties enable PCBs to bio-accumulate in food chains leading to high levels of PCBs in the tissues of economically important foodstuffs, especially fish. PCB 153, a non-coplanar PCB, has been shown to be especially prevalent in the environment and in fish consumed by humans (Fitzgerald et al. 2007). Indeed, the people at the highest risk of adverse PCB related effects are those from high fish-consuming populations (Barone et al. 2014). PCB 153 constitutes the bulk of PCB congeners found in humans—making it a vital PCB to study (Kraft et al. 2017). Once in the body, PCBs accumulate in various organs leading to inflammation (Sipka et al. 2008). These populations are at risk for a variety of health concerns associated with inflammation; such as cancer, metabolic syndrome, and endocrine dysfunction (Arrebola et al. 2014; Kashima et al. 2015). Despite these risks, and the fact that dietary exposure is one of the main routes of exposure to PCBs, the human gastrointestinal tract has been widely ignored when studying the effects of PCBs. One notable exception to this oversight is the reported increase in intestinal permeability after oral PCB exposure in mice (Choi et al. 2010).
Previous work by our group has shown that when acutely exposed to PCBs, including PCB 153, intestinal barrier function is compromised allowing for lipopolysaccharide (LPS) to leak into the systemic circulation (Choi et al. 2012). In vitro experiments have also shown that when exposed to PCBs, intestinal epithelial cell (IEC) line monolayers increase their permeability and alter regulation of junctional proteins (Choi et al. 2010). Disruption of the intestinal barrier is seen in many inflammatory diseases such as inflammatory bowel disease, metabolic syndrome, celiac disease and multiple sclerosis (Arrieta et al. 2006). This association between inflammation and intestinal barrier dysfunction, along with reports of PCB-induced inflammation in other tissues led us to suspect PCBs may have pro-inflammatory effects on the intestinal epithelium.
IECs are rapidly dividing, line the gastrointestinal tract, and are some of the first cells exposed to toxins released from foods. Research on other rapidly dividing cell lines, such as gonadal fibroblast from trout, have shown that PCB 153 causes damage to DNA (Marabini et al. 2011). In other settings, genotoxic damage can activate the transcription factor NF-κB, a major driver of inflammation, through ataxia telangiectasia mutated (ATM), a kinase activated by DNA damage, and the NF-κB essential modulator (NEMO) (Wu et al. 2006). PCB 153 has been shown to increase NF-κB nuclear localization and DNA binding in the liver of mice following intraperitoneal exposure (Lu et al. 2004). Additionally, PCB 153 has been shown to upregulate inflammatory genes, such as interleukin (IL) −6, and tumor necrosis factor-α (TNF-α), in an NF-κB dependent manner in mast cells (Kwon et al. 2002). Taken together, this evidence led us to hypothesize that PCB 153 causes DNA damage, leading to the activation of NF-κB, which drives inflammation and barrier dysfunction in the intestine.
The aim of the current study was to characterize the response of IECs to PCB 153. Although it is known that PCB exposure occurs through the intestine, there is a dearth of information on the effect of acute oral exposure of PCB153 on the intestinal epithelium. Specifically, we wanted to explore if PCB 153 causes inflammation in IECs. We then wanted to elucidate if genotoxic activation of NF-κB could be one mechanism for the inflammation and barrier dysfunction associated with PCB 153 exposure. In a series of in vitro and in vivo studies, we show that PCB 153 causes genotoxic damage to IECs, leading to the activation of NF-κB, upregulation of inflammatory cytokines, and an increase in intestinal permeability.
Materials and Methods
Chemicals and reagents.
PCB 153 (2,2´,4,4´,5,5´-hexachlorobiphenyl) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in dimethyl sulfoxide (DMSO, EMD Millipore Corp., Billerica, MA) to make the stock solution (10mM). For in vivo use, PCB 153 stock solution was diluted in safflower oil. The same amount of DMSO diluted in safflower oil was used a negative control. Etoposide was purchased from Sigma-Aldrich (St. Louis, MO) and LPS was purchased from Invivogen (San Diego, CA). Etoposide, a topoisomerase II inhibitor, was used as a positive control for both genotoxic damage and for genotoxic activation of NF-κB via ATM and NEMO, as etoposide was the agent first used to elucidate this mechanism (Wu et al. 2006). LPS was used as a positive control for NF-κB activation by the canonical, non-genotoxic pathway. The free radical scavenger, N-acetylcysteine (NAC), the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), and the inhibitor of genotoxic NF-κB activation, Clostridium difficile Toxin B, were all purchased from Sigma-Aldrich (St. Louis, MO). The ATM inhibitor, KU-55933 was purchased from Abcam (Cambridge, United Kingdom).
Cell culture and PCB 153 Treatment.
The human intestinal cell line SW480 (ATCC CCL-228) was used for all in vitro experiments. SW480s were chosen because of their high level of responsiveness to other activators of NF-κB such as LPS. Cells were grown at 37°C in Dulbecco's Modified Eagle Medium (DMEM, Corning, Corning, NY) supplemented with 10% fetal bovine serum (Gemini Bio Products, West Sacramento, CA) and 1% Penicillin-Streptomycin (Sigma-Aldrich, St. Louis, MO). For experiments, DMEM without FBS was used. For inhibition experiments, cells were pretreated for 1 hour with either 5mM NAC or 10μM of KU-55833; or for 2 hours with 100 ng/mL or 50 ng/mL of C. difficile Toxin B, before PCB 153 exposure. Cells were exposed to either 50ng/mL LPS, 10μM or 1μM Etoposide, 100μM, 50μM, or 10μM PCB 153, or DMSO. Dosages of PCB 153 were chosen based on the range of possible concentrations that intestinal epithelial cells would be exposed to after a meal high in PCBs (Desvignes et al. 2015). Cells were exposed for 3 hours and then collected for analysis. Treatments at these dosages did not affect cell growth or viability of the cells as determined by an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (ATCC, Manassas, VA) performed as per manufacturer’s instructions (supplemental figure 1).
Transfection and NF-κB Reporter Assay.
Transfections were performed in Opti-MEM, Reduced-Serum Medium (Thermo-Fisher, Waltham, MA). Lipofectamine 2000 (Thermo-Fisher) was used as per manufactures protocol. For the NF-κB reporter assay, cells were transfected with two plasmids: pGL4.32[luc2P/NF-κB- RE/Hygro] vector containing five copies of an NF-κB response element and pRL Renilla Luciferase Control Reporter Vector to normalize for number of cells, both from Promega (Madison, WI). The Dual-Luciferase Reporter Assay System from Promega (Madison, WI) was used to measure luciferase activity and read on a luminometer (Promega GloMax). Data are presented as fold change in luminescence as compared to the DMSO exposed controls. Each treatment group was normalized to its respective treated, DMSO exposed control.
Animals and PCB 153 Exposure.
Male, C57BL/6 mice between 8–12 weeks of age were obtained from Jackson Laboratory (Bar Harbor, ME) and housed under 12:12 hour light/dark conditions and given access to food (Teklad Global 18% Protein Rodent Chow; Envigo, Madison, WI) and water ad libitum. Animals were exposed to 300 μmol/kg of PCB 153 stock solution dissolved in 100μL stripped safflower oil (Alfa Aesar, Ward Hill, MA) and administered via oral gavage. This dosage was chosen based on typical amounts of PCBs found in fish meals of a highly polluted area and meant to represent the high end of reported daily intake of PCBs in a single day (Desvignes et al. 2015; Fromberg et al. 2011). Control animals received an equal volume of DMSO as the PCB 153 stock solution, also dissolved in 100μL safflower oil (<1% DMSO by volume) For inhibition experiments, mice were intraperitoneally injected with PDTC (120mg/kg dissolved in 100uL of PBS) or vehicle (PBS), 30 minutes prior to PCB 153 exposure based on previously reported effective and nontoxic dosages (Lauzurica et al. 1999). Mice were exposed once per day for two days and then euthanized on the third day. Upon euthanasia, samples of intestinal tissue were collected and processed as outlined below. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Miami Miller School of Medicine and followed National Institutes of Health guidelines (National Research Council 2011).
Intestinal Permeability
Intestinal epithelial integrity was determined using a FITC-dextran permeability assay as previously described (Dheer et al. 2016). Briefly, mice were orally gavaged with 4 kDa FITC- dextran (Sigma-Aldrich, St Louis, MO) at a concentration of 60mg/100g body weight 4 hours prior to sacrifice. Blood was collected via cardiac puncture and centrifuged to isolate serum. The concentration of FITC-dextran in the serum was measured by Gemini EM fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) at 490/525 nm.
Mesenteric lymph node isolation and bacterial culture
Mesenteric lymph nodes (MLN) were aseptically isolated at the time of euthanasia. MLNs were homogenized in sterile PBS and serial dilutions were plated onto Tryptic Soy Agar (TSA) supplemented with 5% sheep’s blood (Hardy Diagnostics, Santa Maria, CA). Cultures were incubated at 37°C for 24–48hrs under both aerobic and anaerobic conditions. Quantity of translocated bacteria was expressed as colony-forming units (CFU) per mg of MLN tissue.
Intestinal epithelial cell isolation.
Sections of proximal and distal small intestine, and colon were removed following euthanasia. Small intestinal samples were cut longitudinally and transferred to 10mL of cold HBSS with gentle agitation to remove debris. The small intestinal samples were then cut into 1cm pieces and transferred to cold HBSS containing 3mM ethylenediaminetetraacetic acid (EDTA) for 30 minutes on ice with 200rpm agitation on an orbital shaker. Colons were processed similarly to small intestines, but were incubated in 60mM EDTA in HBSS for one hour. The pieces of intestine were then transferred to cold HBSS and shaken vigorously at 450rpm on an orbital shaker for 5 minutes. Released epithelial cells in the supernatant were filtered through 70μm strainer. IECs were washed twice with 1% FBS in HBSS to remove any residual EDTA and then pelleted for further analysis.
Western Blotting and RT-qPCR.
Total cell lysate from cultured cells or IECs were obtained by using M-PER protein extraction reagent (Thermo Scientific, Waltham, MA) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, Waltham, MA). Protein lysates were loaded into precast, NuPAGE 4%−12% Bis-Tris Gels (Novex by Life Technologies, NY), electrophoresed (200V, 1h) and then transferred (30V, 1.25h for anti-γH2A.X; 30V, 16hr for ATM) onto polyvinylidene fluoride microporous membranes. Membranes were blocked for 1 hour in 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO) and incubated with their respective antibody (1:1000 dilution) overnight at 4°C. Mouse anti-γH2A.X, mouse anti-NEMO, mouse anti-phospho NEMO (s85), mouse anti-ATM, and mouse anti-phospho-ATM (s1981) antibodies were obtained from Abeam (Cambridge, United Kingdom). Membranes were washed and incubated with either goat anti-mouse (anti-γH2A.X and anti-phospho-ATM), or goat anti-rabbit antibody (anti-ATM, anti- NEMO, and anti-phospho NEMO), both conjugated to horseradish peroxidase (1:10,000 dilution; Invitrogen, MA) for 30 minutes. Anti- β-Actin conjugated to horseradish peroxidase (1:10,000 dilution; Sigma, St Louis, MO) was used as the loading control. Membranes were developed using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) as per manufacturer instructions and imaged on a myECL Imager (Thermo Scientific). Total RNA was extracted from IECs or cultured cells using RNA Bee (Tel-test, Friendswood, TX) and Direct-zol RNA MiniPreps (Zymo Research, Irvine, CA). RNA was reverse transcribed using transcriptor reverse transcriptase enzyme and random hexamers (Roche life science, Indianapolis, IN) and qPCR was performed using SYBR Premix Ex Taq (Clontech Laboratories, Mountain View, CA) on a Roche LightCycler 480 (Roche, Indianapolis, IN). See supplemental table 1 for primer pairs. Relative expression levels were calculated using the ΔΔCt method (Livak and Schmittgen 2001) normalizing to Gapdh or β-Actin as the housekeeping genes for mouse or human cell culture samples, respectively.
Nuclear fractionation and NF-κB Transcription Factor Assay.
IECs, isolated from mice, were pelleted after isolation. In order to extract the nuclear contents of the cells, pellets were resuspended in buffer containing 10mM 4-(2-hydroxyethyl)-l- piperazineethanesulfonic acid (HEPES), 1.5mM MgCl2, 10mM KCl, 0.5mM Dithiothreitol (DTT), 0.05% NP40, and brought to a pH of 7.9. After addition of the buffer, cells were incubated on ice for 10 min to allow for lysis then spun down at 3000rpm for 10 min. The supernatant was removed and the pellet was resuspended in buffer containing 20mM HEPES, 400mM NaCl, 1mM EDTA, 1mM DTT, and supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, Waltham, MA). This was homogenized using a dounce homogenizer and left on ice for 30 minutes. The solution was then centrifuged for 30min at 12,000g and the supernatant containing the nuclear fraction of proteins was collected for analysis. NFκB p65 Transcription Factor Assay Kit (Abcam, Cambridge, United Kingdom) was used as per manufacturer’s instructions to assess DNA binding activity of NF-κB in the nuclear fraction.
Alkaline comet assay.
Alkaline comet assay was performed utilizing the CometAssay Kit from Trevigen (Gaithersburg, MD) as per manufacturer’s instructions. Briefly, following exposure, SW480 cells from four biological replicates were collected and suspended in LMagarose and plated onto slides in duplicate. Slides were immersed in lysis solution and then placed in the CometAssay ES Tank for alkaline electrophoresis at 21V for 30 minutes. Slides were then fixed and left to dry overnight. Slides were then stained with SYBR Gold (ThermoFisher) and photographed utilizing the FITC filter on a Keyence BZ-X710 All-in-one Fluorescence Microscope (Okasaka, Japan). Fifty, randomly selected cells were analyzed on for each slide (total of 100 cells per condition) and data are presented as “tail moment”. Images were analyzed using ImageJ with the OpenComet software (Gyori et al. 2014).
Gene silencing.
SW480 cells were grown to approximately 60–70% confluence and were transfected using Lipofectamine RNAiMAX reagent (Invitrogen, MA) with either scrambled (Silencer Negative Control No. 2, Thermo-Fisher Scientific) or ATM-specific siRNA (s1708, Thermo-Fisher). Cells were incubated with the transfection mixtures for 12 hours, allowed to recover in complete medium for 48hr, and subsequently exposed to PCB 153, etoposide, or DMSO.
Statistics.
Data are expressed as mean ± standard error of the mean. Experiments were powered to show significance based on prior published data both from our laboratory and others utilizing the method outlined in Eng 2003 (Eng 2003). Multiple fully powered in vivo experiments were conducted to collect necessary amounts of tissue for all assays conducted with markers of inflammation collected from each trial. Further details on power calculations can be found in the supplement. Normality was assessed using the Shapiro-Wilk test. Statistical significance for data determined to be parametric was assessed using student T test, for comparison of two means and one-way analysis of variance (ANOVA) for comparison of more than two means with Dunnett’s test for multiple comparisons. For non-parametric data involving more than two groups we used the Kruskal-Wallis H test with Dunn’s test for multiple comparisons. For experiments examining the influence of two different independent variables, two-way ANOVAs were used with Tukey’s multiple comparison test. All comparisons of means were made a priori. All analyses were performed in GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Differences were considered statistically significant when P<0.05.
Results
PCB 153 activates NF-κB leading to inflammation and epithelial barrier dysfunction
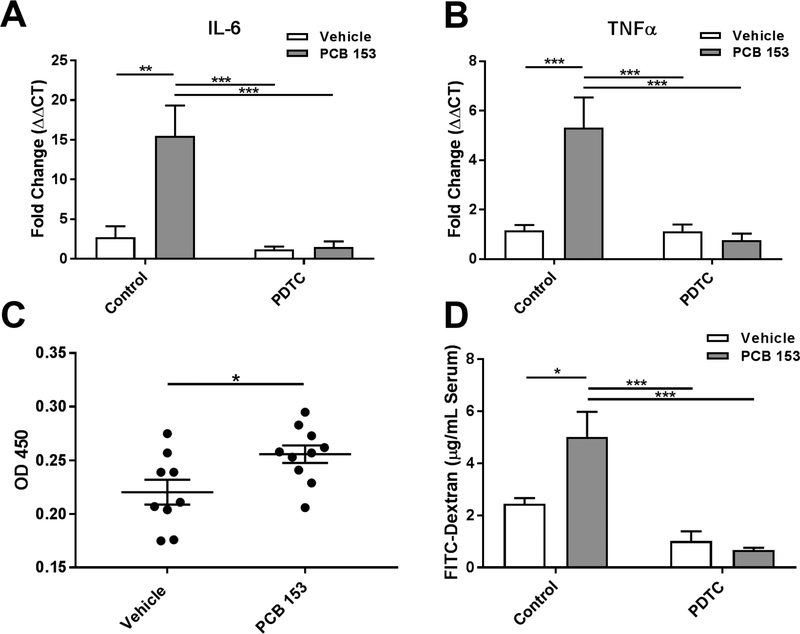
To test the hypothesis that PCB 153 causes intestinal inflammation, mice were exposed to 300 μmol/kg of PCB 153 dissolved in safflower oil, delivered via oral gavage once per day for 2 days. This dosage was chosen based on typical amounts of PCBs found in fish meals of a highly polluted area and meant to represent the upper end of reported daily intake of PCBs (Desvignes et al. 2015; Fromberg et al. 2011). On the third day, mice were euthanized and IECs from the proximal small intestine, distal small intestine, and the colon were isolated. RT-qPCR was performed looking at the expression of the inflammatory cytokines IL-6 and TNFα. These were chosen based on their importance in inflammatory diseases and based on prior work on the inflammatory effects of PCB 153 (Kwon et al. 2002). Following oral exposure to PCB 153, transcription of these inflammatory cytokines increased (figure 1a and 1b). Expression of IL-6 was upregulated (15.5 fold, P<0.01) in the IECs of the proximal small intestine, as was TNFα (6.5 fold, P<0.001). There was no increase in inflammatory cytokine production in the distal small intestine and colon (supplemental figure 2).
Figure 1. PCB 153 causes NF-κB mediated intestinal inflammation and increases gut permeability.
Mice were orally exposed once per day to PCB 153 (300μmol/kg) or vehicle for 2 days, following IP injections of the NF-κB inhibitor PDTC or control (PBS).
(A.) IECs were isolated, RNA was extracted and RT-qPCR run for IL-6 and (B.) TNFα. N=5 mice per group. Two-way AVOVA with Tukey’s multiple comparison test. **P<0.01, ***P<0.001
(C.) IECs were isolated and the nuclear fraction was extracted. A NF-κB binding assay was performed. (N=9–10) Unpaired, two-tailed T test. *P<0.05
(D.) 4 hours prior to sacrifice, mice were orally gavaged with 60mg/100g body weight of FITC- dextran. At the time of euthanasia, serum was collected and levels of FITC-dextran were quantified. N=5 mice per group. Two-way AVOVA with Tukey’s multiple comparison test. *P<0.05, ***P<0.001
Since transcription of IL-6 and TNFα is known to be under the control of NF-κB, we next isolated nuclear extracts from the IECs and tested for DNA binding activity of NF-κB. In the proximal small intestine of PCB 153 exposed mice, there was a subtle but statistically significant increase (P<0.05) in the DNA binding activity of NF-κB compared to the control mice, suggesting an increase in NF-κB activity in response to PCB 153 exposure (figure 1c).
To test if the observed increase in NF-κB activity was responsible for the increase in inflammatory cytokines, mice were injected with PDTC to inhibit NF-κB activation prior to PCB 153 exposure. Blockade of NF-κB dramatically inhibited induction of inflammatory cytokines (figure 1a and 1b). Both IL-6 and TNFα were significantly reduced in mice after administration of the NF-κB inhibitor, PDTC (IL-6: P<0.001; TNFα: P<0.001).
In order to interrogate the effects of PCB 153 on intestinal permeability, FITC-dextran was orally administered 4 hours prior to sacrifice and then measured in the serum. Consistent with previous reports (Choi et al. 2010), oral administration of PCB 153 significantly increased (P<0.05) intestinal permeability compared to vehicle-treated controls (figure 1d). Interestingly, the inhibition of NF-κB via PDTC significantly ameliorated (P<0.001)the observed increase in serum FITC-dextran. In order to establish a more functional readout of this barrier dysfunction, we aseptically isolated the mesenteric lymph nodes (MLN) from mice at the time of sacrifice in order to quantify bacterial translocation out of the intestines. Lymphatic vessels from the intestines primarily drain to the MLNs thus making them a likely location to isolate bacteria that have translocated out of the intestinal lumen. Consistent with the increase in serum FITC- dextran, there were a great deal more viable bacteria cultured from the MLNs of PCB 153- exposed mice compared to vehicle-treated controls with increases in both anaerobic and aerobic bacteria. This increase in bacterial translocation was prevented in mice that had been pretreated with the NF-κB inhibitor, PDTC (supplemental figure 3). Taken together, these data suggest that the observed increase in NF-κB activation is likely responsible for the barrier dysfunction observed after oral exposure to PCB 153.
Free-radical scavenger blocks NF κB activation in vitro
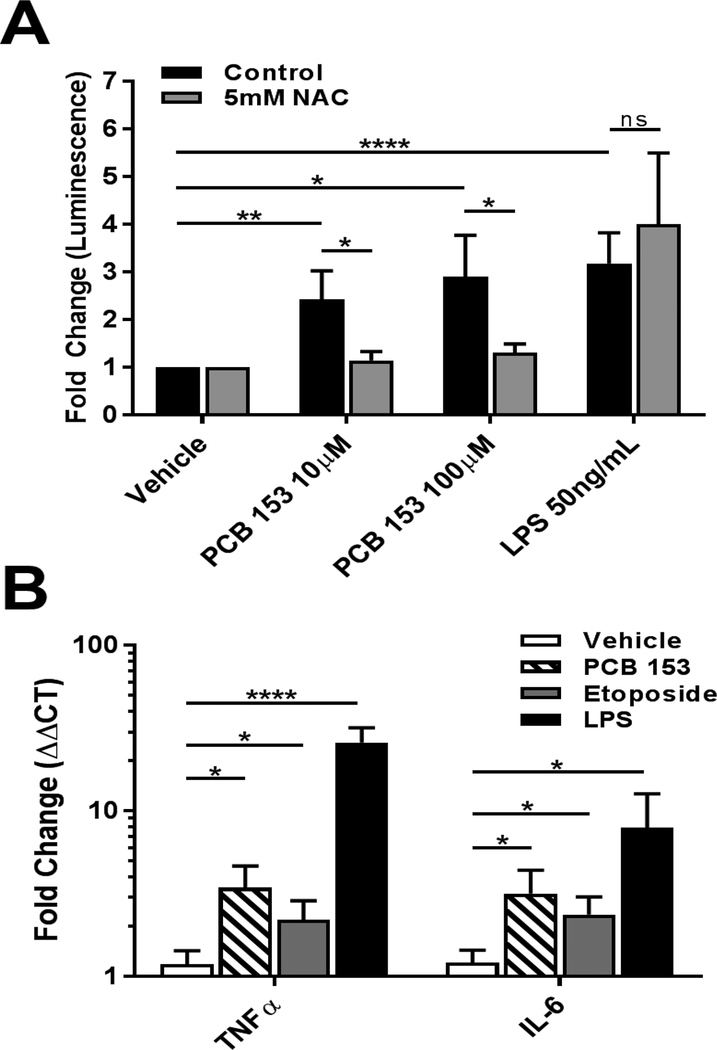
To interrogate the mechanism of NF-κB induced inflammation, an in vitro model was established using the human intestinal cell line, SW480. Cells were transfected with an NF-κB reporter plasmid, which expresses firefly luciferase under the control of NF-κB-dependent transcription. Cells were also transfected with a control plasmid, which constitutively produce Renilla luciferase as a means of normalizing the transfection efficiency. Twenty-four hours after transfection, cells were exposed to PCB 153, LPS as a positive control, or DMSO for three hours. In agreement with the in vivo data, we observed a significant increase in luciferase production in cells exposed to PCB 153 at both the 100μM (2.8 fold, P<0.05) and 10μM (2.4 fold, P<0.01) doses as compared to the DMSO control (figure 2a). Similar significant increases in inflammatory cytokines, as seen in vivo, were also observed with a 3.4 fold increase in TNFα (P<0.05) and a 3.2 fold increase (P<0.05) in IL-6 (figure 2b).
Figure 2. In vitro activation of NF-κB by PCB 153 is ameliorated by free radical scavenger.
(A) SW480 cells were transfected with a NF-κB reporter plasmid. Cells were pretreated for 1 hr with 5mM N-Acetyl-Cysteine (NAC), a free radical scavenger, or control (PBS). Cells were subsequently exposed to LPS, PCB153, or vehicle (DMSO) for 3 hrs. (N=5) Two-way AVOVA with Tukey’s multiple comparison test. *P<0.05, **P<0.01 ****P<0.0001
(B) SW480 cells were exposed to, LPS (50ng/mL), Etoposide (10uM), PCB153 (100uM) or vehicle (DMSO) for 3 hrs. RNA was collected and RT-qPCR run for IL-6 and TNFα. (N>4) Kruskal-Wallis with Dunn's multiple comparisons test. *P<0.05, ****P<0.0001
PCBs and PCB-like chemicals have been shown produce free radicals, which are known to activate NF-κB (Dong et al. 2015). In order to see if the production of free radicals was involved in the activation of NF-κB in the intestinal epithelium, we pretreated the SW480 cells with N-acetyl cysteine (NAC), a free radical scavenger shown to be effective in preventing oxidative damage from PCB-like chemicals in other cell lines (Dong et al. 2014). Following a 1 hour pretreatment with 5mM NAC, cells were exposed to PCB 153 in the same manner as described above. NAC pretreatment attenuated the PCB 153 increase in NF-κB activation, significantly decreasing (P<0.05) the amount of luciferase produced by the cells (figure 2a). This suggests that the production of free radicals is a driving factor in the inflammatory effects of PCB 153 on intestinal epithelial cells.
PCB 153 causes genotoxic damage
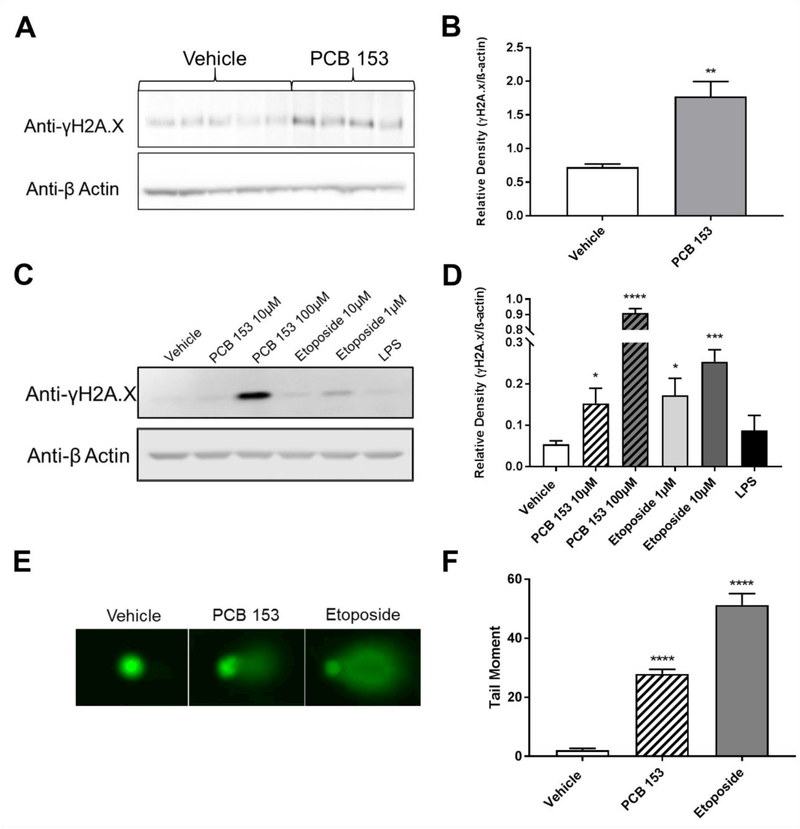
While there are many pathways that can activate NF-κB, our data suggests that free radicals are involved in the NF-κB activation by PCB 153. This led us to investigate the possibility that genotoxic effects of PCB 153 may be involved in the observed activation of NF-κB. To measure DNA damage, we used γ-H2A.X, a phosphorylated variant of the H2A histone, which serves as a sensitive biomarker of DNA damage. This protein serves as the first step in recruiting DNA repair proteins to the site of the DNA damage. As such it is one of the earliest marks of DNA damage and thus a sensitive protein biomarker of DNA damage both in vitro and in vivo (Keogh et al. 2006). In addition, it has been used in past studies to assess the genotoxicity of PCB related chemicals (Dong et al. 2015). Utilizing the same exposure scheme used to assess permeability and inflammatory cytokine expression, we saw a significant increase in γ-H2A.X in the proximal small intestinal IECs of mice exposed to PCB 153 (P<0.01) indicating DNA damage (figure 3a and b). As seen with the increases in inflammatory cytokine expression, there was no DNA damage in the distal small intestine and colon suggesting PCB 153-induced toxicity is localized to the proximal small intestine (supplemental figure 2). After 3 hours of exposure to PCB 153 in vitro, the SW480 cells also showed a significant increase (10μM: P<0.05, 100μM: P<0.0001) in γ-H2A.X indicating that PCB 153 also causes DNA damage in vitro in the absence of other responding cells or a microbiome (figure 3c and d). To further verify the genotoxicity of PCB 153, an alkaline comet assay was performed. Cells were exposed to either the positive control etoposide, PCB 153 (50μM), or the negative control DMSO. Cells from 4 biological replicates for each exposure condition were pooled and 100 random cells (50 per slide) were assessed for DNA damage. There was a significant increase (P<0.0001) in the tail moment in cells exposed to PCB 153 as compared to cells exposed to DMSO (figure 3e). Tail moments were comparable to past studies that have reported genotoxicity of PCB 153 (Marabini et al. 2011).
Figure 3. PCB 153 causes DNA damage.
(A.) IECs were isolated from mice after 2 days of exposure to 300μmol/kg of PCB 153 or vehicle via oral gavage. Protein was extracted and westerns run for γ-H2A.X.
(B.) Densitometry quantification of 3A. N=4–5 mice per group. Unpaired, two-tailed T test. **P<0.01
(C.) Protein was extracted from SW480 cells after exposure to vehicle (DMSO), PCB 153, Etoposide, or LPS. Western blots were run for γ-H2A.X, (representative blot shown).
(D.) Densitometry quantification of 3C. N=4 experiments. One way ANOVA with Dunnett’s multiple comparison test. *P<0.05, ***P<0.001. ****P<0.0001
(E.) Alkaline comet assay to determine genotoxicity. Representative comets from SW480 cells exposed to vehicle (DMSO), PCB 153 (50μM) and Etoposide (10μM).
(F.) Tail Moment taken from comet assay. Four biological replicates for each exposure condition were pooled and 100 random cells (50 per slide) were assessed for DNA damage. Unpaired, two- tailed T test. ****P<0.0001
PCB 153 activates NF-κB via the ATM/NEMO genotoxic response pathway
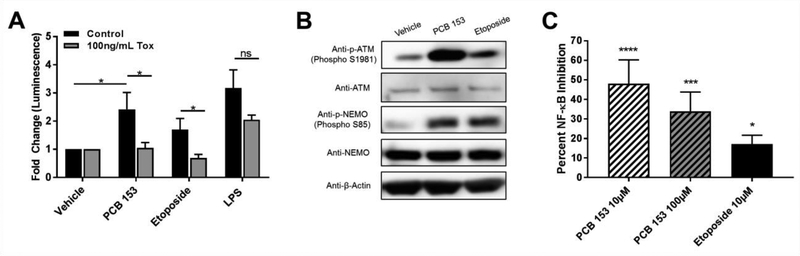
We next wished to address if the observed NF-κB activation is driven by the genotoxic response pathway. To answer this question, we used the NF-κB reporter assay system and Clostridium difficile Toxin B as an inhibitor of genotoxic activation of NF-κB. Clostridium difficile Toxin B has been shown to specifically inhibit the genotoxic activation of NF-κB, while not effecting the canonical activation of NF-κB (Gnad et al. 2001). The day after transfection with NF-κB and renilla reporter plasmids, cells were pretreated for 2 hours with Clostridium difficile Toxin B (lOOng/mL or 50ng/mL) and then subsequently stimulated for 3 hours. Pretreatment with lOOng/mL of Clostridium difficile Toxin B significantly decreased NF-κB driven luciferase production in cells stimulated with etoposide and PCB 153 as compared to controls (P<0.05), while not affecting cells exposed to LPS (figure 4a). While non-significant, a similar trend could be seen after pretreatment of cells with 50ng/mL of Clostridium difficile Toxin B (supplemental figure 4).
Figure 4. PCB 153 activates NF-κB via the genotoxic response pathway and ATM/NEMO.
(A.) SW480 cells were transfected with a NF-κB reporter plasmid and were pretreated for 2hrs with 100 ng/mL of C. difficile Toxin B (Tox) to specifically inhibit genotoxic activation of NF-κB while allowing LPS-induced activation. They were then exposed to vehicle (DMSO), PCB 153 (10μM), etoposide (10μM), or LPS (50 ng/mL) for 3 hrs. (N>4) Two-way AVOVA with Tukey’s multiple comparison test, ns = not significant. *P<0.05
(B.) Protein was extracted from SW480 cells after exposure to etoposide (10μM), PCB153 (100uM), or vehicle (DMSO). Westerns were run for phosphorylated-ATM (Phospho S1981), ATM, phosphorylated-NEMO (Phospho S85), NEMO and β-Actin. Blots shown are representative of at least 3 biological replicates.
(C.) SW480 cells were transfected with a NF-κB reporter plasmid and were pretreated for 1hr with 10μM of KU-55833, an inhibitor of ATM. They were then exposed to etoposide and PCB153 for 3 hrs. (N=5) One way ANOVA with Dunnett’s multiple comparison test. *P<0.05, ***P<0.001, ****P<0.0001
The genotoxic response pathway links DNA damage to NF-κB activation through phosphorylation of several key intermediaries (Wu et al. 2006). First, the DNA damage causes the phosphorylation of ATM. ATM then phosphorylates NEMO, also known as inhibitor of nuclear factor kappa-B kinase subunit gamma (IKK-γ), which is subsequently transported out of the nucleus. NEMO is then responsible for the activation of NF-κB. Using the same exposure scheme described above, we extracted protein from the SW480 cells after exposure to etoposide, PCB 153, and DMSO. Western blots for phosphorylated ATM (Phospho S1981) showed an increase in phosphorylation after exposure to PCB 153 (figure 4b). Next we measured the phosphorylated form of NEMO (Phospho-S85) and once again found an increase in the cells exposed to PCB 153 (figure 4b). Taken together these data show that PCB 153 is activating ATM and NEMO, two key components of the genotoxic response pathway.
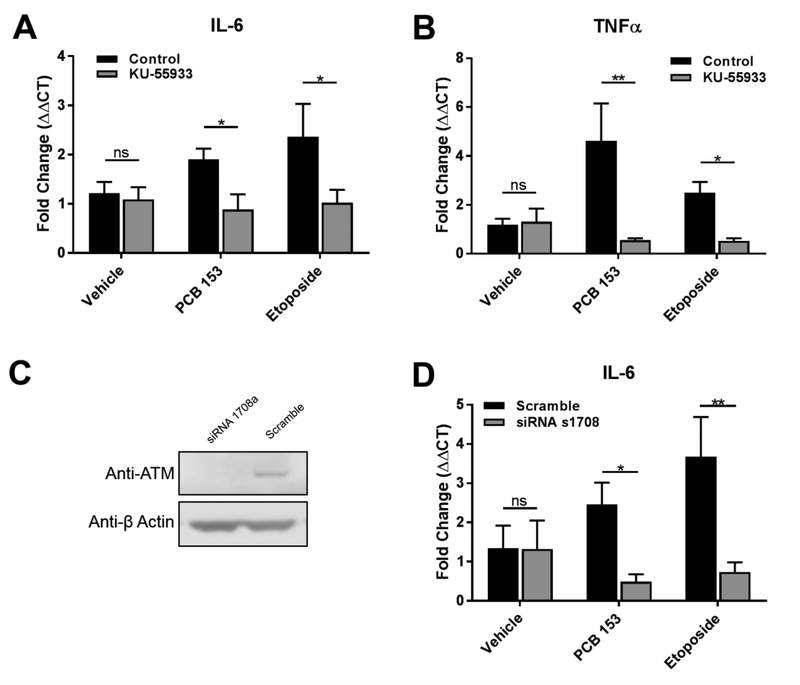
Finally, to test if the activation of ATM/NEMO by PCB 153 was indeed activating NF-κB, we specifically inhibited ATM utilizing the competitive ATM inhibitor, KU-55933.KU- 55933 has been shown to exhibit great specificity and a lack of known off target effects, even on ATM’s most closely related enzyme, ATM-Rad3-related (ATR)(Hickson et al. 2004). Utilizing the NF- κB reporter assay, cells were pretreated for 1 hour with 10μM of KU-55933 before being exposed to the same exposure scheme used above. ATM inhibition significantly inhibited NF-κB activation in both the high (34% inhibition, P<0.001), and the low dosages (48% inhibition, P<0.0001) of PCB 153 (figure 4c). Additionally, the induction of the inflammatory cytokines IL- 6 and TNFα were significantly reduced after the treatment of cells with the ATM inhibitor (P<0.05 and P<0.01 respectively, figure 5a and 5b). To further strengthen the involvement of ATM in PCB 153-induced inflammation, siRNA was used to silence the expression of ATM in SW480 cells (figure 5c). Following exposure to PCB 153, the increase in expression of IL-6 was significantly down-regulated in cells lacking ATM (P<0.05, figure 5d). Similar trends were seen in expression of TNFα after exposure to PCB 153 (supplemental figure 5). These data strongly support that PCB 153 activates NF-κB through the genotoxic response pathway utilizing ATM/NEMO and that this activation leads to inflammation.
Figure 5. ATM is required for PCB 153-induced inflammation.
(A.) SW480 cells were pretreated for 1hr with 10μM of KU-55833. They were then exposed to etoposide (10μM) and PCB153 (100μM) or vehicle (DMSO) for 3 hrs. RNA was collected and RT-qPCR run for IL-6 and (B.) TNFα. (N=4) Two-way AVOVA with Tukey’s multiple comparison test. *P<0.05, **P<0.01
(C.) SW480 cells transfected with siRNA to silence ATM, protein was extracted, and western blots for ATM were performed to determine the efficacy of the silencing procedure.
(D.) Following the silencing of ATM, SW480 cells were exposed to etoposide (10μM) and PCB153 (100μM) or vehicle (DMSO) for 3 hrs. RNA was collected and RT-qPCR run for IL-6 (N=4). Two-way AVOVA with Tukey’s multiple comparison test, ns = not significant. *P<0.05, **P<0.01
Discussion
After the industrial production of PCBs stopped in the early 1980s, oral exposure through contaminated foods became the primary route of human exposure to PCBs. This makes the intestinal epithelial cells of the gastrointestinal tract one of the first sites of PCB exposure.
Despite this, our knowledge of the effects of PCBs on the intestine is lacking. In the present study, we demonstrate that PCB 153, the most common PCB in the environment, causes inflammation in the intestinal epithelium. We further go on to show that PCB 153 activates ATM and NEMO leading to the genotoxic activation of NF-κB in IECs. This activation is required for both the inflammatory effects of PCB 153 and the epithelial barrier dysfunction it has been shown to cause. This knowledge is important as increases in intestinal permeability are associated with a variety of diseases and can lead to the leakage of bacteria and pro- inflammatory substances out of the intestinal lumen, which in turn can cause systemic inflammation. In addition, low level intestinal inflammation caused by PCB 153 could impair the mucosal immune system in the gut and could lead to negative systemic metabolic effects.
We showed PCB 153 led to an increase in γ-H2A.X, a sensitive biomarker for DNA damage. When the DNA is damaged, this histone variant is phosphorylated by kinases such as ATM or ATR (Kuo and Yang 2008). Once phosphorylated, γ-H2A.X allows for DNA damage repair proteins to be recruited to the site of the break. The ubiquity of γ-H2A.X in the presence of DNA damage makes it a sensitive and reliable marker of multiple types of DNA damage. Our results clearly show an increase in γ-H2A.X, and thus DNA damage, in proximal IECs after PCB 153 exposure in vivo. We further went on to demonstrate that PCB 153 causes DNA damage in vitro, utilizing both the protein biomarker γ-H2A.X and by directly measuring DNA strand breakage via alkaline comet assay. These data are consistent with published results of PCBs and PCB-related chemicals in other rapidly dividing cell lines (Dong et al. 2015; Marabini et al. 2011) but is the first documentation of PCB 153 induced DNA damage in IECs. Interestingly, while there was significant DNA damage in response to both etoposide and PCB 153, the extent of the damage diverged with etoposide causing more damage as measured by the comet assay, and PCB 153 causing more dramatic increases in γ-H2A.X. These data potentially reflect a wider repertoire of molecular mechanisms for PCB 153 to damage DNA than etoposide. Etoposide primarily damages DNA through strand breaks (Hande 1998), a type of damage specifically measured in the comet assay (Collins 2004). Cells use γ-H2A.X in response to a wider range of DNA damage, including oxidative damage and the formation of DNA adducts (Audebert et al. 2010; Watters et al. 2009; Zhou et al. 2006). PCB 153 has been shown to form DNA adducts (Jeong et al. 2008) and increase the production of reactive oxygen species (ROS), which can lead to oxidative DNA damage (Zhu et al. 2009). Additionally, PCB 153 has been shown to induce the activation of NADPH oxidase, a major source of ROS within cells (Choi et al. 2010). Both of these PCB 153-mediated genotoxic effects, in addition to its ability to directly break the DNA, could increase the levels of γ-H2A.X. Taken together, these results demonstrate that PCB 153 causes DNA damage in IECs. While our results demonstrate the genotoxicity of PCB 153, the mechanism of this genotoxicity, whether direct or via its breakdown products, in IECs has yet to be determined.
An interesting result to emerge from the in vivo mouse exposures to PCB 153 was the specific localization of the genotoxic and inflammatory effects. The increases in γ-H2A.X, and subsequent NF-κB activation and inflammation, were only seen in the proximal small intestine while the distal small intestine and colon were spared from the genotoxic effects of PCB 153.
This makes anatomical sense as PCBs, due to their lipophilicity, are primarily absorbed in the second segment of the proximal small intestine, the jejunum, much like fat soluble vitamins (Jandacek and Genuis 2013). Additionally, the location of the phenotype also makes physiological sense. The main enzymes responsible for this initial metabolism of PCBs into more metabolically active forms are the cytochrome P450 (CYPs) enzymes. CYPs are found in a much higher quantity in the proximal small intestine, specifically the jejunum (Paine et al. 1997).
CYPs in the intestine are also found in the highest concentration in the mature enterocytes of the villus tip, where orally administered compounds such as PCBs would be absorbed (Thelen and Dressman 2009). CYP3 A, which makes up approximately 80% of the total immunoquantified intestinal CYPs (Thelen and Dressman 2009), has also been reported to be highest in the proximal small intestine (Paine et al. 1997) and multiple studies have linked these enzymes to PCB 153 (Al-Salman and Plant 2012; Grans et al. 2015). Taken together, the observed geographic specificity of the DNA damage and subsequent inflammation induced by PCB 153 in our studies could be explained by the location of intestinal absorption and subsequent metabolism into metabolically active, and potentially genotoxic, metabolites.
Despite our evidence demonstrating the genotoxic effects of PCB 153 on the small intestine, there is no evidence, epidemiological or otherwise, that PCB exposure increases risk of small intestinal cancers. One possible reason for this is the relative resistance of the small intestine to cancer. While the exact reason the small intestine is resistant to cancers is not known, several hypotheses have been suggested including rapid turnover of IECs, the alkaline environment, lower density of bacteria, and faster luminal transit times (Kariv 2003). After absorption by the small intestine however, PCBs are transported to the liver where they have been shown to cause DNA damage and have been linked both experimentally and epidemiologically to hepatocellular carcinoma (Dong et al 2015).
The pretreatment of cells with the free radical scavenger, N-acetyl cysteine also reduced NF-κB activation suggesting a role of free radicals in PCB 153-mediated activation ofNF-κB. Indeed, PCB-induced free radical production has been reported in the literature and while further studies need to be done, supplementation of antioxidants may represent a therapeutic target to combat PCB 153 induced genotoxicity and inflammation (Amaro et al. 1996; Oakley et al. 1996).
NF-κB is vital for cell survival but is also a major driver of inflammation and has been linked to a variety of inflammatory conditions (Lawrence 2009). PCB 153 has been previously shown in the literature to increase nuclear localization of NF-κB in the liver of mice and to induce the expression of NF-κB-controlled inflammatory cytokines in a mast cell line (Kwon etal. 2002; Lu et al. 2004). These findings are consistent with the outcomes ofPCB 153 exposure we observed in the intestinal epithelium. Following oral exposure to PCB 153 there was an increase in the expression of the inflammatory cytokines IL-6 and TNFα in the IECs. NF-KBis a major driver of these cytokines’ transcription (Lawrence 2009) and indeed, following its inhibition, the upregulation of IL-6 and TNFα was ameliorated. In humans, increased expression of these cytokines has been linked to pathogenesis of a variety of inflammatory diseases and, in the intestine, NF-KB-mediated inflammation has been linked to inflammatory bowel disease. Studies performed on a variety of cell types have also implicated NF-κB and NF-κB-induced cytokine production as a key event in the early pathogenesis of diabetes mellitus (Patel and Santani 2009). Epidemiological data has also linked exposure to PCB 153 to diabetes and the development of metabolic syndrome (Rylander et al. 2005). Additionally, intestinal inflammation and increases in the same cytokines seen in this study have been experimentally linked to obesity and insulin resistance in mouse models (Ding et al. 2010). While our data clearly show increases in NF-KB-induced intestinal inflammation caused by PCB 153, further studies should investigate if there is a causal link between PCB exposure, intestinal inflammation, and the onset of diabetes.
Disruption of the intestinal barrier is seen in many inflammatory diseases such as diabetes mellitus, inflammatory bowel disease, metabolic syndrome, celiac disease and multiple sclerosis (Arrieta et al. 2006). Previous work has shown that oral exposure to PCB 153 can cause an increase in intestinal permeability in mice, allowing for the leakage of bacterial products such as LPS (Choi et al. 2010). We build on this finding showing that not only is there an increase in permeability, but that it can lead to the increase of bacterial translocation to the MLNs. Additionally we link the activation of NF-κB to this breakdown of the intestinal barrier suggesting that inflammation may play a role in the increased permeability caused by PCB 153. NF-κB could be altering permeability in a number of ways. Oral exposure to PCB 153 in mice has been shown to upregulate NF-κB and may induce expression of matrix metalloproteinases (Sipka et al. 2008) and alter the expression of tight junctional proteins such as zonula occludens- 1 and occludin, both of which have been shown to be under the control of NF- kB (Chen et al. 2011; Choi et al. 2010; Ma et al. 2004). Alternatively, the TNFa produced by IECs could be acting in an autocrine fashion to increase intestinal permeability as well (Ma et al. 2004). Further research is needed to fully elucidate the mechanism behind the NF-κB dependent increases in intestinal permeability. Epithelial barrier dysfunction can have profound health consequences as the leakage of bacterial products can cause systemic inflammation. This chronic, low level, systemic inflammation has been shown to impair the adaptive immune system and decrease responsiveness to vaccinations (Frascaetal. 2016). High serum levels of PCBs in humans have also been shown to impair the humoral immune response (Heilmann et al. 2006). Whether the leakage of pro-inflammatory molecules from the intestinal lumen is to blame for this immunosuppression has yet to be investigated, but the breakdown in the intestinal barrier after exposure to PCBs could be responsible for far reaching health consequences.
One of the major limitations of this study is reconciling the differences between PCB exposure in the environment and PCB exposure in the laboratory. In the environment, PCBs exist as complex mixtures of multiple congeners, each with unique chemical properties that can exert different biological effects. Additionally, it is extremely challenging to accurately establish levels of dietary exposure to PCBs (Binnington et al. 2016). Because of the mechanistic nature of this study, we chose to focus on only one congener, PCB 153, and levels of exposures that intestinal epithelial cells would encounter after a single meal high in PCBs (Desvignes et al. 2015). While this dosage only represents a subset of the PCB exposed population, it was chosen in order to glean insight into the mechanism of the initial physiological response of IECs to an oral exposure to PCB 153.
While this study focused exclusively on PCB 153 induced activation of NF-κB, this represents only one of many ways exposure to PCB 153 can cause inflammation and toxicity. PCB 153 has been shown in various cell types and conditions to activate constitutive androstane receptor (CAR), pregnane x receptor (PXR), Activator Protein-1 (AP-1), and NADPH oxidase (NOX). The lull scope of PCB 153 induced toxicity on the intestinal epithelial cells most likely involves multiple mechanisms and a diverse array of transcription factors.
Conclusions
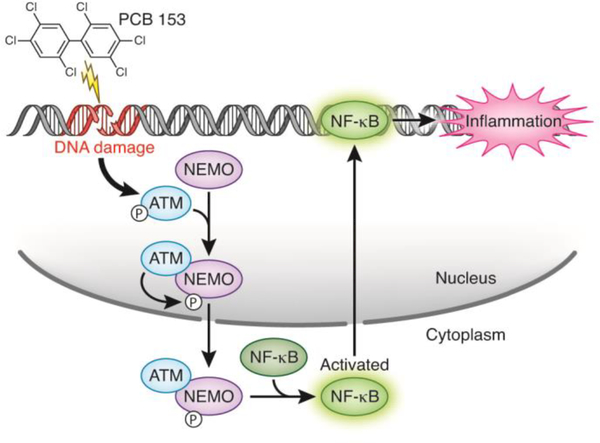
The results of this study present evidence that PCB 153 causes DNA damage, driving inflammation and barrier dysfunction in the intestinal epithelium. Our data suggest that the driver of this inflammation and increase in permeability is the transcription factor NF-κB, which can become activated through ATM and NEMO. A summary of the proposed mechanism can be found in figure 6. While further studies need to be done, these data suggest that inflammation in the intestinal epithelium may be an important driver for pathologies that have been epidemiologically linked to PCB exposure in humans and could represent an attractive therapeutic target to combat PCB-induced inflammation.
Figure 6.
Proposed mechanism
Supplementary Material
Highlights.
PCB 153 causes an increase in inflammatory cytokines in intestinal epithelial cells
PCB 153 increases intestinal permeability
PCB 153 activates the transcription factor NF-κB to cause these effects
PCB 153 is genotoxic to intestinal epithelial cells
PCB 153 activates NF-κB through the ATM/NEMO pathway
Acknowledgements
This study was supported in part by grants F30ES026797 from the NIEHS and 2R01DK099076– 07A1 from the NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential competing financial interests.
References
- Al-Salman F, Plant N. 2012. Non-coplanar polychlorinated biphenyls (pcbs) are direct agonists for the human pregnane-x receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicology and applied pharmacology 263:7–13. [DOI] [PubMed] [Google Scholar]
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. 1996. Metabolic activation of pcbs to quinones: Reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chemical research in toxicology 9:623–629. [DOI] [PubMed] [Google Scholar]
- Arrebola JP, Fernandez MF, Martin-Olmedo P, Molina-Molina JM, Sanchez-Perez MJ, Sanchez-Cantalejo E, et al. 2014. Adipose tissue concentrations of persistent organic pollutants and total cancer risk in an adult cohort from southern spain: Preliminary data from year 9 of the follow-up. The Science of the total environment 500-501:243–249. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Bistritz L, Meddings JB. 2006. Alterations in intestinal permeability. Gut 55:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert M, Riu A, Jacques C, Hillenweck A, Jamin EL, Zalko D, et al. 2010. Use of the gammah2ax assay for assessing the genotoxicity of polycyclic aromatic hydrocarbons in human cell lines. Toxicology letters 199:182–192. [DOI] [PubMed] [Google Scholar]
- Barone G, Giacominelli-Stuffler R, Garofalo R, Castiglia D, Storelli MM. 2014. Pcbs and pcdd/pcdfs in fishery products: Occurrence, congener profile and compliance with european union legislation. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 74:200–205. [DOI] [PubMed] [Google Scholar]
- Binnington MJ, Curren MS, Quinn CL, Armitage JM, Arnot JA, Chan HM, et al. 2016. Mechanistic polychlorinated biphenyl exposure modeling of mothers in the canadian arctic: The challenge of reliably establishing dietary composition. Environment international 92-93:256–268. [DOI] [PubMed] [Google Scholar]
- Chen F, Hori T, Ohashi N, Baine AM, Eckman CB, Nguyen JH. 2011. Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure. Hepatology 53:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Choi YJ, Chen L, Zhang B, Eum SY, Abreu MT, et al. 2012. Lipopolysaccharide potentiates polychlorinated biphenyl-induced disruption of the blood-brain barrier via tlr4/irf-3 signaling. Toxicology 302:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, et al. 2010. Polychlorinated biphenyls disrupt intestinal integrity via nadph oxidase-induced alterations of tight junction protein expression. Environmental health perspectives 118:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR. 2004. The comet assay for DNA damage and repair: Principles, applications, and limitations. Molecular biotechnology 26:249–261. [DOI] [PubMed] [Google Scholar]
- Desvignes V, Volatier JL, de Bels F, Zeghnoun A, Favrot MC, Marchand P, et al. 2015. Study on polychlorobiphenyl serum levels in french consumers of freshwater fish. The Science of the total environment 505:623–632. [DOI] [PubMed] [Google Scholar]
- Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. 1994. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. The American journal of physiology 266:L612–619. [DOI] [PubMed] [Google Scholar]
- Dheer R, Santaolalla R, Davies JM, Lang JK, Phillips MC, Pastorini C, et al. 2016. Intestinal epithelial toll like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infection and immunity 84:798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. 2010. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PloS one 5:e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Xu D, Hu L, Li L, Song E, Song Y. 2014. Evaluation of n-acetyl-cysteine against tetrachlorobenzoquinone-induced genotoxicity and oxidative stress in hepg2 cells. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 64:291–297. [DOI] [PubMed] [Google Scholar]
- Dong H, Shi Q, Song X, Fu J, Hu L, Xu D, et al. 2015. Polychlorinated biphenyl quinone induces oxidative DNA damage and repair responses: The activations of nhej, ber and ner via atm-p53 signaling axis. Toxicology and applied pharmacology 286:10–16. [DOI] [PubMed] [Google Scholar]
- Eng J 2003. Sample size estimation: How many individuals should be studied? Radiology 227:309–313. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Hwang SA, Jansing RL, Hicks HE. 2007. Environmental exposures to polychlorinated biphenyls (pcbs) among older residents of upper hudson river communities. Environmental research 104:352–360. [DOI] [PubMed] [Google Scholar]
- Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. 2016. Obesity decreases b cell responses in young and elderly individuals. Obesity 24:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromberg A, Granby K, Højgård A, Fagt S, Larsen J. 2011. Estimation of dietary intake of pcb and organochlorine pesticides for children and adults. Food chemistry 125:1179–1187. [Google Scholar]
- Gnad R, Kaina B, Fritz G. 2001. Rho gtpases are involved in the regulation of nf -kappab by genotoxic stress. Experimental cell research 264:244–249. [DOI] [PubMed] [Google Scholar]
- Grans J, Wassmur B, Fernandez-Santoscoy M, Zanette J, Woodin BR, Karchner SI, et al. 2015. Regulation of pregnane-x-receptor, cyp3a and p-glycoprotein genes in the pcb-resistant killifish (fundulus heteroclitus) population from new bedford harbor. Aquatic toxicology 159:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV. 2014. Opencomet: An automated tool for comet assay image analysis. Redox biology 2:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande KR. 1998. Etoposide: Four decades of development of a topoisomerase ii inhibitor. European journal of cancer 34:1514–1521. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jorgensen E. 2006. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS medicine 3:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, et al. 2004. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase atm. Cancer research 64:9152–9159. [DOI] [PubMed] [Google Scholar]
- Jandacek RJ, Genuis SJ. 2013. An assessment of the intestinal lumen as a site for intervention in reducing body burdens of organochlorine compounds. TheScientificWorldJournal 2013:205621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YC, Walker NJ, Burgin DE, Kissling G, Gupta M, Kupper L, et al. 2008. Accumulation of m1dg DNA adducts after chronic exposure to pcbs, but not from acute exposure to polychlorinated aromatic hydrocarbons. Free radical biology & medicine 45:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima S, Yorifuji T, Tsuda T, Eboshida A. 2015. Cancer and non-cancer excess mortality resulting from mixed exposure to polychlorinated biphenyls and polychlorinated dibenzofurans from contaminated rice oil: "Yusho". International archives of occupational and environmental health 88:419–430. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, et al. 2006. A phosphatase complex that dephosphorylates gammah2ax regulates DNA damage checkpoint recovery. Nature 439:497–501. [DOI] [PubMed] [Google Scholar]
- Kraft M, Rauchfuss K, Sievering S, Wockner M, Neugebauer F, Fromme H. 2017. Quantification of all 209 pcb congeners in blood-can indicators be used to calculate the total pcb blood load? International journal of hygiene and environmental health 220:201–208. [DOI] [PubMed] [Google Scholar]
- Kuo LJ, Yang LX. 2008. Gamma-h2ax - a novel biomarker for DNA double-strand breaks. In vivo 22:305–309. [PubMed] [Google Scholar]
- Kwon O, Lee E, Moon TC, Jung H, Lin CX, Nam KS, et al. 2002. Expression of cyclooxygenase-2 and pro- inflammatory cytokines induced by 2,2',4,4',5,5'-hexachlorobiphenyl (pcb 153) in human mast cells requires nf-kappa b activation. Biological & pharmaceutical bulletin 25:1165–1168. [DOI] [PubMed] [Google Scholar]
- Lauzurica P, Martinez-Martinez S, Marazuela M, Gomez del Arco P, Martinez C, Sanchez-Madrid F, et al. 1999. Pyrrolidine dithiocarbamate protects mice from lethal shock induced by lps or tnf-alpha. European journal of immunology 29:1890–1900. [DOI] [PubMed] [Google Scholar]
- Lawrence T 2009. The nuclear factor nf-kappab pathway in inflammation. Cold Spring Harbor perspectives in biology 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real -time quantitative pcr and the 2(-delta delta c(t)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lu Z, Lee EY, Robertson LW, Glauert HP, Spear BT. 2004. Effect of 2,2',4,4',5,5' hexachlorobiphenyl (pcb- 153) on hepatocyte proliferation and apoptosis in mice deficient in the p50 subunit of the transcription factor nf-kappab. Toxicological sciences : an official journal of the Society of Toxicology 81:35–42. [DOI] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. 2004. Tnf-alpha-induced increase in intestinal epithelial tight junction permeability requires nf-kappa b activation. American journal of physiology Gastrointestinal and liver physiology 286:G367–376. [DOI] [PubMed] [Google Scholar]
- Marabini L, Calo R, Fucile S. 2011. Genotoxic effects of polychlorinated biphenyls (pcb 153, 138, 101, 118) in a fish cell line (rtg-2). Toxicology in vitro : an international journal published in association with BIBRA 25:1045–1052. [DOI] [PubMed] [Google Scholar]
- Moreau A, Vilarem MJ, Maurel P, Pascussi JM. 2008. Xenoreceptors car and pxr activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Molecular pharmaceutics 5:35–41. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Robertson LW, Gupta RC. 1996. Analysis of polychlorinated biphenyl-DNA adducts by 32p- postlabeling. Carcinogenesis 17:109–114. [DOI] [PubMed] [Google Scholar]
- Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, et al. 1997. Characterization of interintestinal and intraintestinal variations in human cyp3a-dependent metabolism. The Journal of pharmacology and experimental therapeutics 283:1552–1562. [PubMed] [Google Scholar]
- Patel S, Santani D. 2009. Role of nf-kappa b in the pathogenesis of diabetes and its associated complications. Pharmacological reports : PR 61:595–603. [DOI] [PubMed] [Google Scholar]
- Quay JL, Reed W, Samet J, Devlin RB. 1998. Air pollution particles induce il-6 gene expression in human airway epithelial cells via nf-kappab activation. American journal of respiratory cell and molecular biology 19:98–106. [DOI] [PubMed] [Google Scholar]
- Quinete N, Schettgen T, Bertram J, Kraus T. 2014. Occurrence and distribution of pcb metabolites in blood and their potential health effects in humans: A review. Environmental science and pollution research international 21:11951–11972. [DOI] [PubMed] [Google Scholar]
- Rylander L, Rignell-Hydbom A, Hagmar L. 2005. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environmental health : a global access science source 4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, et al. 2008. Oral administration of pcbs induces proinflammatory and prometastatic responses. Environmental toxicology and pharmacology 25:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen K, Dressman JB. 2009. Cytochrome p450-mediated metabolism in the human gut wall. The Journal of pharmacy and pharmacology 61:541–558. [DOI] [PubMed] [Google Scholar]
- Watters GP, Smart DJ, Harvey JS, Austin CA. 2009. H2ax phosphorylation as a genotoxicity endpoint. Mutation research 679:50–58. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. 2006. Molecular linkage between the kinase atm and nf -kappab signaling in response to genotoxic stimuli. Science 311:1141–1146. [DOI] [PubMed] [Google Scholar]
- Zhou C, Li Z, Diao H, Yu Y, Zhu W, Dai Y, et al. 2006. DNA damage evaluated by gammah2ax foci formation by a selective group of chemical/physical stressors. Mutation research 604:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, et al. 2009. Polychlorinated-biphenyl- induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free radical biology & medicine 47:1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.