Abstract
In adult mice with unilateral optic nerve crush injury (ONC), we studied visual response plasticity in the visual cortex following stimulation with sinusoidal grating. We examined visually evoked potentials (VEP) in the primary visual cortex ipsilateral and contralateral to the crushed nerve. We found that unilateral ONC induces enhancement of visual response on the side ipsilateral to the injury that is evoked by visual stimulation to the intact eye. This enhancement was associated with supranormal spatial frequency thresholds in the intact eye when tested using optomotor response. To probe whether injury-induced disinhibition caused the potentiation, we treated animals with the neurosteroid allopregnanolone, a potent agonist of the GABAA receptor, one hour after crush and on post-injury days 3, 8, 13, and 18.
Allopregnanolone diminished enhancement of the VEP and this effect was associated with the upregulated synthesis of the δ-subunit of the GABAA receptor. Our study shows a new aspect of experience-dependent plasticity following unilateral ONC. This hyper-activity in the ipsilateral visual cortex is prevented by upregulation of GABA inhibition with allopregnanolone. Our findings suggest the therapeutic potential of allopregnanolone for modulation of plasticity in certain eye and brain disorders and a possible role for disinhibition in ipsilateral hyper-activity following unilateral ONC.
Keywords: optic nerve crush, mouse visual cortex, neurosteroids, GABAA receptor, stimulus-selective response potentiation
Introduction
There is now good evidence that the adult visual cortex can undergo experience-dependent structural and functional modifications (Karmarkar and Dan, 2006; Gilbert and Li, 2012; Cooke and Bear, 2013; Kaneko and Stryker, 2017). One of the most extensively studied forms of experience-dependent changes is the effect of monocular visual deprivation (MD) on ocular dominance (OD) plasticity (Hofer et al., 2006; Gavornik and Bear, 2014). While normal mice show strong contralateral eye dominance, deprivation of visual input from the contralateral eye leads to weakening of the response from the deprived contralateral eye and strengthening of the response from the open ipsilateral eye (Frenkel and Bear, 2004; Smith and Bear, 2010).
Removal of visual input from an eye to the brain is induced not only by deprivation, but also by direct injury to the eye or optic nerve (Tagawa et al., 2005; Syken et al., 2006; Datwani et al., 2009; Nys et al., 2015a; Nys et al., 2015b). Most studies focus on changes in the visual cortex contralateral to the injured/enucleated/deprived eye, but it is important to note that a loss of visual input or damage to one hemisphere can also induce modifications of the other hemisphere through cortico-cortical (Van Brussel et al., 2011; Vasconcelos et al., 2011) and callosal connections (Restani et al., 2009; Laing et al., 2014).
A well-characterized animal model of optic nerve injury – optic nerve crush (ONC) – produces an acute insult that leads to retrograde degeneration of the vast majority of retinal ganglion cells and induces substantial functional reorganization in the brain (Sabel et al., 1999; Kreutz et al., 2004; Macharadze et al., 2012). The brain reorganization that accompanies optic nerve injury is more clinically relevant (optic neuropathies, glaucoma, optic nerve trauma) than monocular deprivation models, but it is understudied.
In this set of experiments we sought to determine how unilateral ONC injury affects both the contralateral and ipsilateral visual cortices of adult mice. We used a model of stimulus-selective response potentiation (SRP) to measure experience-dependent enhancement of cortical visually evoked potentials (VEP) on repeated presentation of a sinusoidal grating in a single orientation (Frenkel et al., 2006). We hypothesized that compared to MD, unilateral ONC will induce cortical enhancement of input from the intact eye, and consequently augmentation of cortical VEP ipsilateral to the intact eye (Sawtell et al., 2003; Frenkel et al., 2006). Since it was reported that OD plasticity is also associated with changes in visual behavior (Pruski et al., 2006; Iny et al., 2006), we measured the spatial frequency threshold following unilateral ONC with an optomotor test (Pruski et al., 2006; Tschetter et al., 2013).
Earlier studies on SRP and MD in adult rodents revealed that experience-dependent response enhancement in the visual cortex reflects strengthening of excitatory thalamo-cortical synaptic transmission in layer IV of the visual cortex (Frenkel et al., 2006; Coleman et al., 2010). To test whether post-ONC changes in VEP are also associated with increased excitatory transmission in thalamo-cortical synapses, we assessed expression of VGlut2, a vesicular glutamate transporter 2 specific to thalamo-cortical synapses (Nahmani and Erisir, 2004).
In contrast to the increased excitation observed after injury and in MD and SRP, cortical inhibition has been recently identified as a major determinant of normal sensory perception (Haider et al., 2013; Yazaki-Sugiuama et al., 2009; Smith and Bear, 2010; Chen et al., 2011; van Versendaal and Levelt, 2016). In this context, the role of GABA-active neurosteroids—positive modulators of GABAA receptors—in regulating inhibition has been established (Belelli and Lambert, 2005; Walker and Kullmann, 2012; Carver and Reddy, 2013). As an endogenous neurosteroid, allopregnanolone (ALLO) has been shown to increase inhibitory currents by activation of a wide range of GABAA receptors, particularly those containing the δ-subunit and considered responsible for tonic inhibition (Belelli and Lambert, 2005; Farrant and Nusser, 2005; Carver and Reddy, 2013; Reddy and Estes, 2016). ALLOhas also been shown to regulate expression and trafficking of the GABAA receptor subunits, leading to long-term inhibitory effects (Herd et al., 2007; Shen et al., 2005; Peng et al., 2009).
ALLO is one of the most potent positive modulators of cortical inhibition (Belelli and Lambert, 2005; Carver and Reddy, 2013; Crowley et al., 2016; Reddy and Estes, 2016) and has known neuroprotective properties (Djebaili et al., 2005; Van Landingham et al., 2006; Sayeed et al., 2009; Wang et al., 2010; Brinton, 2013; Irwin et al., 2014; Labombarda et al., 2013; Ishikawa et al., 2014; Guennoun et al., 2015). Given these properties, ALLO could be considered as a potential treatment for maladaptive cortical hyperexcitation. Pharmacological upregulation of GABAA-mediated inhibition by ALLO can also be used for mechanistic dissection of post-injury plasticity resulting from increased excitation and decreased inhibition. We further hypothesized that ONC-induced potentiation of cortical VEP can be altered by ALLO upregulation of GABAA-mediated inhibition. Reduction of potentiation by treatment with ALLO could suggest an involvement of GABAA-mediated disinhibition as one of the possible mechanisms of injury-induced potentiation in the visual cortex.
Materials and Methods
Animals
Young adult male C57BL/6 mice, 6 weeks of age, were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed on a 12:12 hour light:dark cycle with water and food access provided ad libitum. Procedures were approved by the Institutional Animal Care and Use Committee (Emory University protocol DAR-2003137-063018GN and Atlanta VA Medical Center protocol V008-13), and conformed to National Institutes of Health guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments are reported here in compliance with the ARRIVE guidelines.
Experimental design
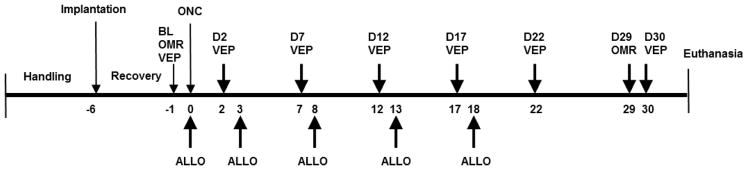
After a one-week acclimatization period to the laboratory environment with handling, the mice were randomly assigned to one of four groups (n = 16/group): Sham controls given vehicle (Sham/Veh), Sham controls given ALLO (Sham/ALLO), ONC given vehicle (ONC/Veh), ONC given ALLO (ONC/ALLO). Animals were implanted with electrodes over the primary visual cortex and allowed to recover for five days before baseline recording of VEP and an optomotor response (OMR) were performed. Mice were then subjected to ONC surgery or sham operation. Following ONC, VEP were recorded on post-injury days 2, 7, 12, 17, 22, and 30. OMR was assessed on post-injury day 29. Treatment with ALLO or vehicle was administered within one hour following ONC and on post-ONC days 3, 8, 13, and 18. At the end of the experiment, subgroups of mice in each experimental condition were euthanized for immunohistochemistry and western blot. The timeline for the experiment is presented in Fig. 1.
Figure 1.
Timeline for the experiment
Animals were implanted with electrodes over the primary visual cortex, allowed to recover for five days, and baseline VEP and optomotor tresponse (OMR) were recorded. The animals were then subjected to ONC surgery or sham procedure. Following ONC, VEP were recorded on post-injury days 2, 7, 12, 17, 22, and 30. OMR was assessed on post-injury day 29. Treatment with ALLO or vehicle was administered one hour following ONC and on post-ONC days 3, 8, 13, and 18. At the end of the experiment, subgroups of mice in each experimental condition were killed for immunohistochemistry and western blot. Abbreviations: BL – baseline; OMR – optomotor response; VEP – visual evoked potentials, D2 – D30 – experimental days post ONC; ALLO – allopregnanolone treatment.
All parts of the study were performed blind and third-party concealment of treatments with individually uniquely coded vials was applied. The order of treatments was randomized by drawing vial code numbers from a hat without replacement using a randomized block design.
Electrode implantation
Mice were anesthetized with 80 mg/kg ketamine and 16 mg/kg xylazine given intraperitoneally (i.p.), and then fixed in a stereotaxic frame. Body temperature was maintained at 37°C with a heating pad. The head was shaved and wiped with chlorhexidine solution. The topical anesthetic proparacaine 1% was applied to the scalp and a midline scalp incision was made. The skull was exposed, cleaned with saline and dried. Small burr holes were made in the skull overlying the binocular visual cortex (V1b: 0.0 mm lambda anteroposterior, ± 3.0 mm mediolateral), and stainless steel screws (Fine Science Tools, Heidelberg, Germany) were inserted over the V1b cortical surface in each hemisphere. A reference screw was placed over the frontal cortex. The electrodes were secured with cyanoacrylate and covered with dental cement. The animals were allowed to recover for five days before any further procedures were started.
Unilateral optic nerve crush
Mice were anesthetized with 80 mg/kg ketamine and 16 mg/kg xylazine given i.p. In the left eye, a lateral incision of the conjunctiva was performed and the optic nerve exposed by blunt dissection, leaving the dura and blood supply intact. The left optic nerves were crushed using Dumont self-closing forceps N7 (Fine Science Tools) customized with a calibrating micrometer screw made by the Emory University Physics Machine Shop from plans described in Sautter et al. (1991). The forceps were then used to apply pressure on the nerve for 30 sec with the jaws 0.04 mm apart, at a distance of 1 mm from the eye. At the end of the process, topical antibiotic eye ointment (Bausch and Lomb, USA) was applied to the wound. In mice subjected to the sham procedure, the nerves were exposed briefly but left intact.
VEP recording and analysis
Five days after the electrode implantations, the mice were anesthetized (80 mg/kg ketamine and 16 mg/kg xylazine, i.p.) and placed on a heating pad in front of a computer monitor. To prevent drying, eyes were lubricated with 0.9% saline drops. The signals were amplified and acquired with a bandpass filter set at 0.3 – 500 Hz using the UTAS-E3000 system and software (LKC, Gaithersburg, MD, USA).
Visual stimuli consisted of sinusoidal gratings of 100% contrast, 0.05 cycles/degree, reversal cycle 1 sec. They were presented independently to the left and right eyes in random order, in 300 stimuli per block, two blocks per eye. During the recordings a black plastic monocular patch was used to prevent stimulation of the opposite eye. To induce stimulus-selective response potentiation as described by Frenkel et al. (2006), each animal was stimulated throughout the experiment with gratings of a single orientation – vertical or horizontal – assigned randomly. Single evoked responses, 500 msec in length, were averaged for the 300-stimulus block, and peak-to-peak amplitude of the averaged VEP was calculated.
Measurement of optomotor behavior
Visual function was tested in a virtual optomotor system (OptoMotry system; Cerebral Mechanics, Lethbridge, AB, Canada) (Prusky et al., 2004; Douglas et al., 2005). For visual acuity assessment, the mice were exposed to moving vertical gratings (12°/s) of 100% contrast starting at a spatial frequency of 0.003 cycles per degree and increased stepwise until the response threshold was crossed three times as described earlier (Prusky et al., 2004; Douglas et al., 2005). The highest spatial frequency (the smallest gratings) to elicit movement of the head in the direction of the stimulus was determined for each eye separately and considered the spatial frequency threshold.
ALLO administration
The GABAA receptor-positive, allosteric modulator 3α-hydroxy-5α-pregnan-20-one, allopregnanolone (ALLO; Steraloids, Newport RI, USA), was dissolved in cyclodextrin (sterile water solution, 22.5%) in 1 mg/ml concentration and administered at a dose of 10 mg/kg. The first injection was made i.p. 1 hour after optic nerve crush to increase bioavailability. Subsequent injections were made subcutaneously on post-ONC days 3, 8, 13, and 18 to avoid immediate ALLO effects on VEP during the recording sessions. Cyclodextrin in the same volume was administered as the vehicle.
Western blot analysis
Mice were deeply anesthetized with isoflurane and then decapitated. Brains were quickly removed, and left and right primary visual cortices were dissected on a cold plate under a binocular microscope and the amount of protein was quantitated. Protein samples (40 μg) were separated on SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked in 5% non-fat milk probed with primary antibodies against VGlut2 (1:5000, AB2251, Millipore), GABAA receptor δ-subunit (1:500, AB9752, Millipore), NR2A (1:1000, AB1555P, Millipore). Beta-actin (1:10000, A5316, Sigma) was used as a loading control. The blots were incubated with appropriated peroxidase labeled secondary antibodies (1:5000, KPL) and visualized with ECL reagent followed by film exposure. Band densities were quantified using NIH ImageJ software and protein levels were normalized to β-actin. Data are presented as the fold change in protein levels relative to the Sham/Veh control group.
Immunohistochemistry and microscopy
Mice were deeply anesthetized (80 mg/kg ketamine and 16 mg/kg xylazine, i.p.) and perfused with 0.1 M PBS followed by 10% formalin. The brains were removed, post-fixed overnight and cryoprotected with 30% sucrose. Fresh-frozen 10-μm cross-sections through the primary visual cortices (V1) were permeabilized in 0.1M TBS – 30% Tween solution for 10 min, blocked with protein block serum-free (X0909; Dako, Carpinteria, CA, USA) for 30 min at room temperature and then incubated at 4°C overnight with the primary antibody raised in guinea pig against VGlut2 (AB2251: Millipore, Billerica, MA, USA) diluted 1:2000 in antibody diluent (S3022; Dako). Following three washes with 0.1M TBS – 30% Tween, sections were incubated for 1 hour at room temperature with goat anti guinea-pig secondary antibody conjugated with Alexa Fluor 568 (A-11075; Fisher Scientific, Waltham, MA, USA; 1:200). After washing, the slides were treated with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA) and coverslipped. The slides were scanned in a fluorescent microscope (Zeiss Axioscope, Carl Zeiss Microscopy, Germany).
Statistical analysis
An a priori power analysis determined sample size required to detect an effect size of ≥0.50 with a power of 0.8 at alpha 0.05, based on previous experience with the mouse ONC model and the principles of the three Rs (Replacement, Reduction and Refinement).
Statistical analysis was performed using IBM SPSS Statistics 23.0 for Windows. For VEP and OMR, the changes over time and differences among groups were evaluated with repeated and factorial measures ANOVA followed by the Greenhouse-Geisser correction for sphericity and post-hoc Tukey’s correction for multiple comparisons. T-tests were used for direct group comparisons. For analysis of imaging data and western blots, factorial ANOVA followed by Tukey’s post-hoc test for comparison among groups was used. Data are expressed as mean ± SEM. A value of p < 0.05 was considered significant.
Results
Experience-dependent enhancement of visual evoked response and enhancement of ipsilateral cortical function after unilateral ONC
Repetitive exposure to a stimulus with a single orientation induces potentiation of VEP amplitudes (known as the SRP) here operationally defined as a measure of experience-dependent plasticity in visual thalamo-cortical synapses (Frenkel et al., 2006).
Initially, because we were using chronically implanted skull screws instead of intracortical electrodes, we first validated our SRP model. We tested the VEPs recorded from skull screws over several recording days and clearly demonstrated the enhancement of VEP amplitude (Fig. 2; see also representative VEPs on the first and last day of recording) as well as the absence of enhancement on the stimulus of novel orientation (Fig. 3). This was in line with described SRP features (Frenkel et al., 2006). Therefore, although technically modified, our model provided stable recordings and was relevant to testing SRP.
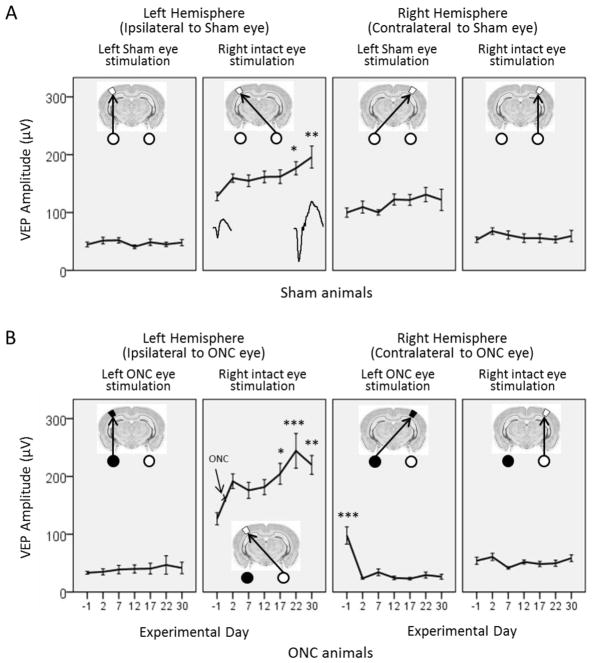
Figure 2.
Repetitive stimulation with sine gratings with a single orientation leads to potentiation of VEP amplitudes (SRP, stimulus-selective response potentiation) in the primary visual cortex of adult mice.
Average VEP amplitudes recorded in (A) Sham animals (no ONC, n = 6), and (B) animals with unilateral ONC (n = 8) are shown for the left (left panels) and right (right panels) hemispheres, evoked from stimulation of the eyes contralateral and ipsilateral to the recorded hemisphere on the days indicated by numerals. (A) In Sham animals, the responses were gradually potentiated: Day 22 *p = 0.017, Day 30 **p = 0.004. The representative VEPs are shown for the first and last day of recording. (B) In ONC animals in the left hemisphere, contralateral to the intact eye, the amplitude of VEP induced from the contralateral intact eye was even more potentiated: Day 17 *p = 0.055, Day 22 ***p < 0.001, Day 30 **p = 0.001. In the right hemisphere, contralateral to the damaged eye, the amplitude of VEP significantly dropped after ONC and barely changed throughout the remainder of the test days: all Days compared to Baseline Day -1, ***p < 0.001. Data are presented as mean ± SEM.
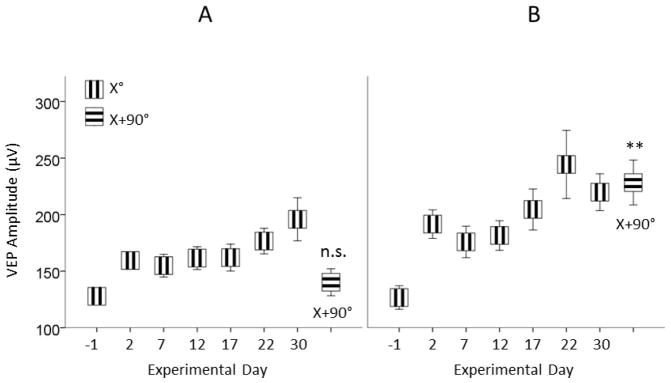
Figure 3.
Repetitive exposure to a grating stimulus of single orientation (X°) selectively potentiates the amplitude of VEP to this orientation.
(A) SRP does not transfer to a novel stimulus in intact mice: on Day 30, VEP amplitude to novel stimuli (X+90°) vs. Day -1 (X°) is not significant. However, (B) in ONC mice, contralateral VEP induced from the intact eye is potentiated even to stimuli of novel orientation: (X+90°) vs. Day -1 (X°) **p = 0.001. Data are presented as mean ± SEM.
As we predicted, Sham-operated animals (no ONC injury) demonstrated a gradual and statistically significant increase of VEP amplitude to stimuli of single orientation (X°) throughout the days of testing (Fig. 2A). One-way ANOVA with repeated measures and Greenhouse-Geisser correction showed a significant effect of testing day on increasing contralateral VEP amplitude when the intact eye was stimulated: F(3.40, 44.16) = 4.76, p = 0.004, ηp2 = 0.27. VEP amplitude increased from 127.79 ± 7.38 μV at baseline (Day -1) to 195.91 ± 19.05 μV at Day 30, p = 0.004.
After the left optic nerve was crushed, the amplitude of VEP in the right hemisphere contralateral to the damaged eye significantly dropped and barely changed throughout the remainder of testing (Fig. 2B, right panels). One-way ANOVA with repeated measures and Greenhouse-Geisser correction showed a significant effect of testing day: F(2.52,37.84) = 23.68, p < 0.001, ηp2 = 0.61. VEP amplitude decreased from 97.76 ± 14.91 μV at baseline (Day -1) to 26.48 ± 4.21 μV at Day 30, p < 0.001.
Interestingly, in the left hemisphere, ipsilateral to the ONC eye, the amplitude of VEP induced from the contralateral, intact eye showed significantly more potentiation than in Sham animals (Fig. 2B, left panels). One-way ANOVA with repeated measures and Greenhouse-Geisser correction showed a significant effect of testing day: F(3.32, 23.27) = 5.51, p = 0.004, ηp2 = 0.44. VEP amplitude increased from 126.70 ± 10.43 μV at Baseline to 219.91 ± 16.31 μV at Day 30, p = 0.001.
Analysis of variance between Sham and ONC groups showed significantly higher VEP amplitudes through the intact eye in ONC/Veh than in Sham/Veh animals (Fig. 2B, left panels): F(1,304) = 22.01, p < 0.001; correspondingly: Day 17 ONC/Veh (204.57 ± 18.14 μV) vs. Sham/Veh (162.00 ± 10.17 μV), Day 22 ONC/Veh (244.31 ± 30.07 μV) vs. Sham/Veh (176.63 ± 10.17 μV), Day 30 ONC/Veh (219.91 ± 16.31 μV) vs. Sham/Veh (195.91 ± 14.38 μV). The enhancement of VEP amplitude in animals with unilateral ONC compared to Shams suggests an additive effect of the injury on VEP enhancement.
Following induction of SRP on day 30, we presented stimuli of novel orthogonal orientation (X+90°). The SRP gained over previous days did not transfer to the novel stimuli and VEP amplitude was about the same as on the first day of stimulation: Day 30 (X+90°) (140.23 ± 14.97 μV) vs. Day -1 (X°) (127.79 ± 10.37 μV), and not statistically significant, p = 0.997 (Fig. 3A).
The potentiating effect of unilateral ONC was also confirmed with VEP amplitude induced in response to the stimulus of orthogonal orientation (X+90°). In contrast to Shams, the ONC animals demonstrated potentiated VEP to the novel stimuli presented on Day 30 (X+90°) (228.36 ± 19.84 μV) vs. Baseline (Day -1, X°) (126.70 ± 10.43 μV), p = 0.001 (Fig. 3B).
ALLO moderates injury-induced but not experience-induced potentiation of VEP
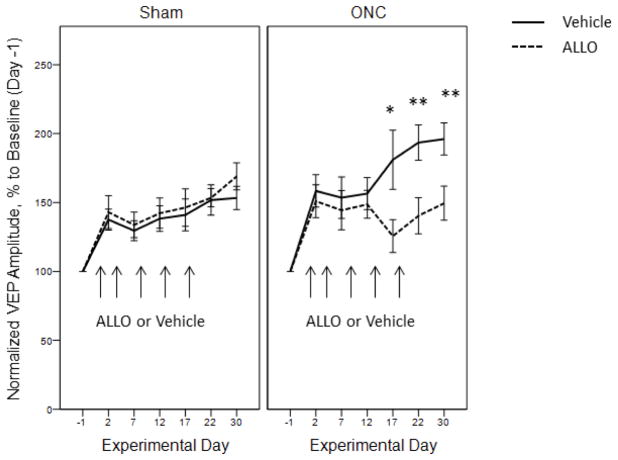
ALLO administration yielded persistent inhibitory effects as shown by attenuation of normalized VEP amplitudes in ONC animals on Days 17, 22 and 30 (Fig. 4, right panel; two-way ANOVA, effect of ALLO treatment: F(1,22) = 14.25, p < 0.001, effect of Day: F(6,22) = 6.97, p < 0.001, interaction of Treatment*Day: F(6,22) = 1.89, p = 0.084; post-hoc pairwise comparisons between vehicle-treated and ALLO-treated ONC animals: Day 17 ONC/Veh (180.99 ± 21.49 %) vs. ONC/ALLO (125.62 ± 11.95 %), t(30) = 2.25, p = 0.032; Day 22 ONC/Veh (193.51 ± 12.84 %) vs. ONC/ALLO (140.38 ± 13.13 %), t(27) = 2.89, p = 0.007; Day 30 ONC/Veh (196.10 ± 11.64 %) vs. ONC/ALLO (149.47 ± 12.37 %), t(43) = 2.75, p = 0.009). Increasing ALLO effects after the fourth injection reveals a possible cumulative effect of GABAA modulation on injury-induced plasticity.
Figure 4.
Attenuation of visual response potentiation with ALLO in animals with unilateral ONC but not in Sham
Pharmacological positive modulation of GABAA receptors with ALLO. Mice were systemically injected with ALLO (dashed line) or Vehicle (solid line). Arrows represent the time of administration. ALLO significantly diminished injury-induced potentiation in the ipsilateral visual cortex of mice with unilateral ONC: Day 17 *p = 0.032, Day 22 **p = 0.007, Day 30 **p = 0.009. In Sham animals ALLO did not affect experience-induced potentiation of VEP. Data are presented as mean ± SEM, n = 6–8/group.
In Sham animals ALLO did not attenuate VEP amplitudes (Fig. 4, left panel), indicating that alterations in GABAA-mediated inhibition exert greater effects in injury-induced potentiation of visual response than in experience-dependent potentiation alone.
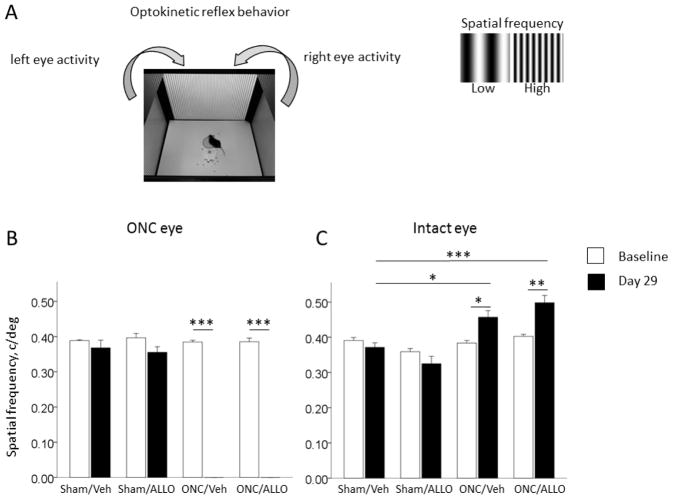
Unilateral ONC induces enhancement of the optomotor response in the intact eye
To examine whether the changes in cortical VEP following ONC were associated with changes in visual behavior, we tested optomotor response in mice before injury and then on day 29 post ONC. The optomotor reflex is a well-documented behavior used to measure visual spatial frequency threshold in mice (Fig. 5A). Optomotor responses in the ONC eye were not detectable after ONC–we observed zero spatial frequency thresholds (Fig. 5B; two-way ANOVA, interaction Group*Day: F(3,47) = 255.88, p < 0.001, ηp2 = 0.94. In ONC/Veh, Baseline (0.39 ± 0.01 c/deg) vs. Day 29 (0.00 ± 0.00 c/deg), t(14) = 78.66, p < 0.001. In ONC/ALLO, Baseline (0.39 ± 0.01 c/deg) vs. Day 29 (0.00 ± 0.00 c/deg), t(14) = 36.73, p < 0.001). In contrast, unilateral ONC induced enhancement of visual performance in the intact eye as indicated by increased spatial frequency thresholds in ONC mice on Day 29 post injury (Fig. 5C; two-way ANOVA, interaction Group*Day: F(3,51) = 10.117, p < 0.001, ηp2 = 0.37). Thus, in the ONC/Veh group, spatial frequency threshold on Day 29 (0.46 ± 0.02 c/deg) was significantly higher than at Baseline (0.38 ± 0.01 c/deg), t(14) = −3.68, p = 0.002, and significantly higher than on Day 29 in Sham/Veh (0.37 ± 0.01 c/deg), t(12) = −3.53, p = 0.004. Similar enhancement was observed in the ONC/ALLO group, Day 29 (0.50 ± 0.02 c/deg) vs. Baseline (0.40 ± 0.01 c/deg), t(14) = −4.43, p = 0.001, and vs. Day 29 in Sham/Veh (0.37 ± 0.01 c/deg), t(12) = −4.75, p < 0.001.
Figure 5.
Optic nerve crush injury-induced enhancement of visual performance in the intact eye
(A) Schematic of optomotor behavior. (B) Spatial frequency thresholds in the damaged eye were not detectable on Day 29 post-ONC in either ONC/Veh, ***p < 0.001, or ONC/ALLO, ***p < 0.001. (C) Unilateral ONC induced increased spatial frequency thresholds in the intact eye in ONC/Veh animals as measured on Day 29 post-ONC vs. Baseline, *p = 0.002, and vs. Day 29 in Sham/Veh, *p = 0.004; in ONC/ALLO, Day 29 vs. Baseline, **p = 0.001, and vs. Day 29 in Sham/Veh, ***p < 0.001. Data are presented as mean ± SEM, n = 6–8/group.
Enhancement of visual function is associated with VGlut2, NR2A, NR2B and GABAA receptor δ-subunit changes
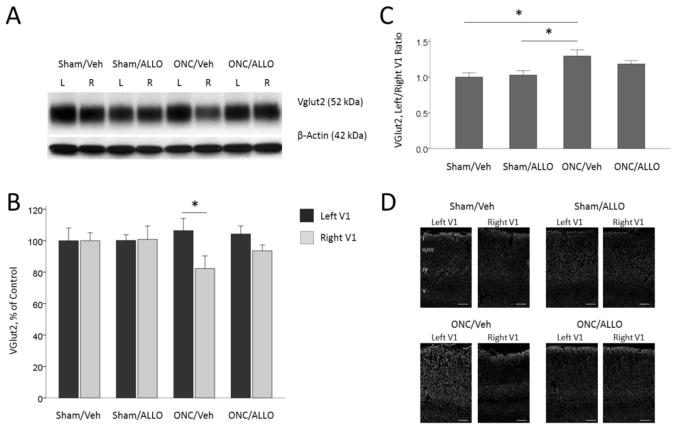
VGlut2 is a glutamate transporter which is specifically expressed in the thalamo-cortical synapses in layer IV of the cortex and localized in the membranes of the vesicles of glutamate synapses, where it transports glutamate into vesicles (Nahmani and Erisir, 2005). In ONC animals, VGlut2 levels in the right primary visual cortex contralateral to the damaged eye (crush projection area) were significantly down-regulated (82.2 ± 8.0 % to Sham/Veh control) vs. the left visual cortex (106.4 ± 7.7 %), p = 0.042 (Fig. 6A and 6B). The ratio of VGlut2 in the left and right cortices in ONC/Veh (129.66 ± 8.62 %) was significantly higher than in Sham/Veh (100.00 ± 6.08 %; post-hoc Tukey’s test: p = 0.013) or Sham/ALLO mice (118.44 ± 4.71 %; post-hoc Tukey’s test: p = 0.029; One-way ANOVA, F(3,44) = 4.50, p = 0.008; Fig. 6C). Representative immunofluorescence images show distribution of VGlut2 marker in the left and right visual cortex, with most of the staining in layer IV, where the thalamo-cortical synapses are located (Fig. 6D).
Figure 6.
VGlut2 expression in the left and right visual cortex
Representative blots (A) and densitometry data (B) for VGlut2 in the left and right primary visual cortex obtained from animals after ONC (% of Sham/Veh control). ONC induced up-regulation of VGlut2 in the left cortex ipsilateral to the ONC eye, and down-regulation in the right visual cortex contralateral to the ONC eye: *p = 0.042. (C) Left to right visual cortex ratio of VGlut2. ONC animals demonstrate a significantly higher ratio than sham controls: Sham/Veh vs. ONC/Veh, *p = 0.013; Sham/ALLO vs. ONC/Veh, *p = 0.029. Note that ALLO treatment in ONC animals show a reduced difference in VGlut2 levels between left and right hemispheres. Data are expressed as mean ± SEM, n = 12/group. (D) Representative immunofluorescence images showing distribution of VGlut2 marker in the left and right visual cortex. Scale bar: 100 μm.
Since the NR2A and NR2B subunits of the NMDA receptor were shown to be critical for experience-dependent plasticity in the visual cortex (Fagiolini et al., 2003; Cho et al., 2009), we assessed the levels of NR2A and NR2B expression in the left and right primary visual cortex. For NR2A, two-way ANOVA showed significant effect of Group (F(3,96) = 9.20, p < 0.001), and no significant effect of Hemisphere (F(1,96) = 0.05, p = 0.816, interaction Group*Hemisphere (F(3,96) = 0.55, p=0.653), suggesting that rearrangement of NMDR subunits following unilateral injury occurs in both hemispheres (Fig. 7A and 7B). Further post-hoc comparisons revealed that NR2A expression was significantly down-regulated following ONC: Sham/Veh (average of both hemispheres, 100.00 ± 5.62 %) vs. ONC/Veh (59.52 ± 3.90 %, post-hoc Tukey’s test p < 0.001), vs. ONC/ALLO (71.66 ± 3.46 %, post-hoc Tukey’s test p = 0.003; Fig. 7A and 7B).
Figure 7.
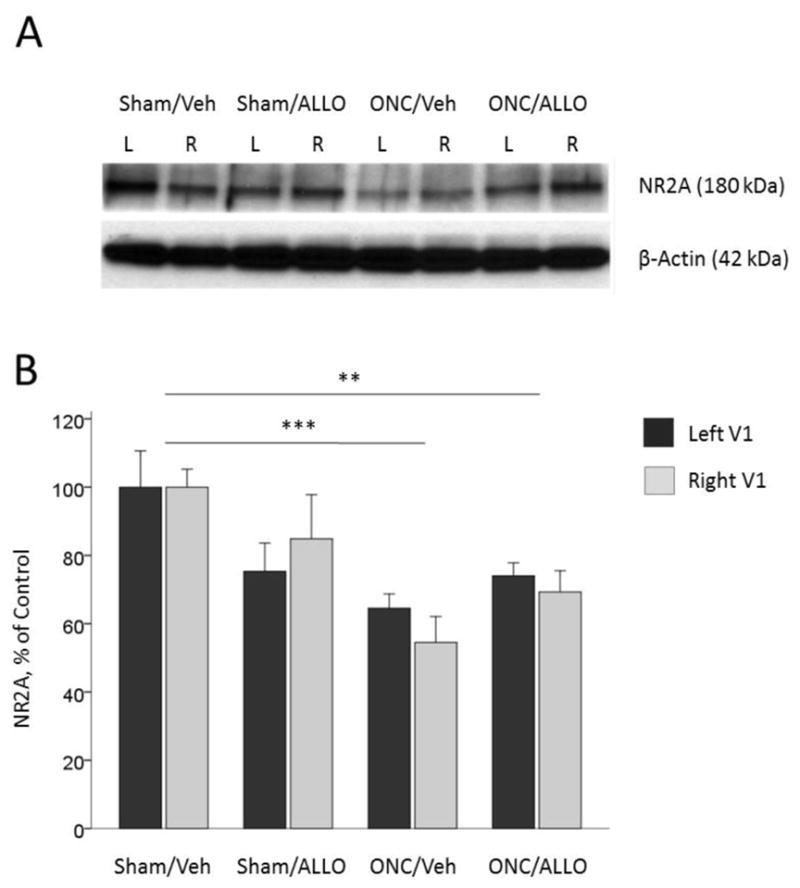
NR2A expression in the left and right visual cortex
Representative blots (A) and densitometry data (B) for NR2A in the left and right primary visual cortex obtained from animals after ONC (% of Sham/Veh control). Effect of hemisphere is not significant: p = 0.816. ONC-induced down-regulation of NR2A (post-hoc comparisons between groups): Sham/Veh vs. ONC/Veh, ***p < 0.001; Sham/Veh vs. ONC/ALLO, **p = 0.003. Data are expressed as mean ± SEM, n = 12/group.
For NR2B, two-way ANOVA showed a significant effect of Group (F(3,95) = 6.57, p < 0.001), but no significant effect of Hemisphere (F(1,95) = 3.49, p = 0.065, interaction Group*Hemisphere (F(3,95) = 0.42, p=0.736; Fig. 8A and 8B). Further post-hoc comparisons revealed that NR2B expression was significantly down-regulated following ONC: Sham/Veh (average of both hemispheres, 100.00 ± 7.66 %) vs. ONC/Veh (57.39 ± 7.49 %, post-hoc Tukey’s test p = 0.003), vs. ONC/ALLO (65.51 ± 7.49 %, post-hoc Tukey’s test p = 0.019; Fig. 8A and 8B).
Figure 8.
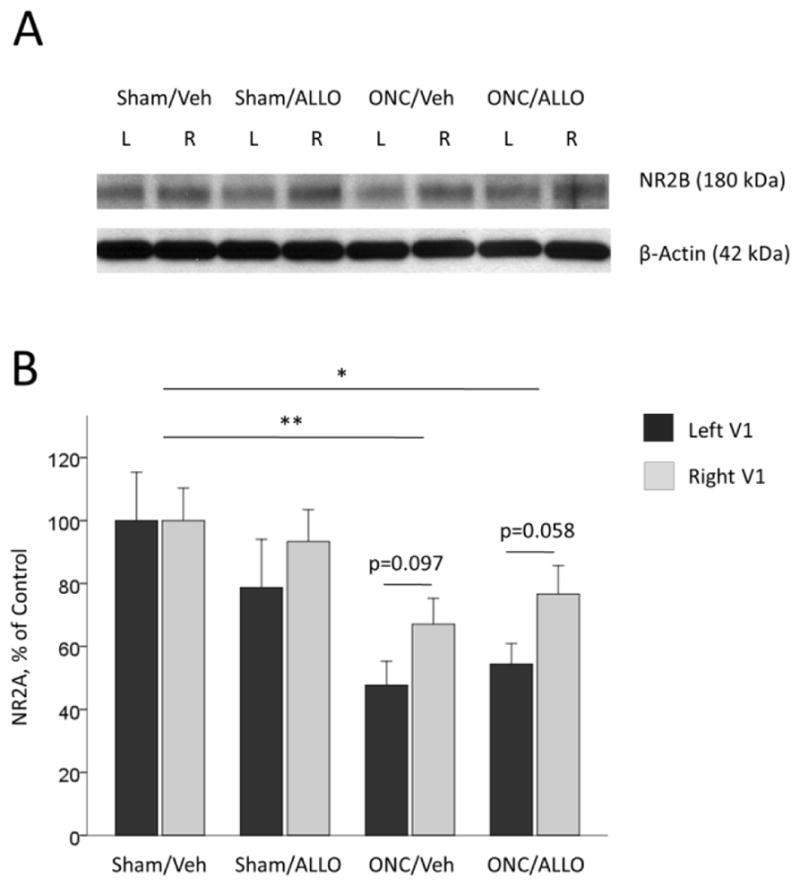
NR2B expression in the left and right visual cortex
Representative blots (A) and densitometry data (B) for NR2B in the left and right primary visual cortex obtained from animals after ONC (% of Sham/Veh control). ONC-induced down-regulation of NR2B (post-hoc comparisons between groups): Sham/Veh vs. ONC/Veh, **p = 0.003; Sham/Veh vs. ONC/ALLO, *p = 0.019. Difference between left and right hemispheres was not significant. Data are expressed as mean ± SEM, n = 12/group.
In previous studies ALLO was shown to be highly effective in the modulation of GABAA receptors containing the δ-subunit (Belelli and Lambert, 2005; Carver and Reddy, 2013). ALLO also increases δ-subunit expression and trafficking (Herd et al., 2007; Shen et al., 2005; Peng et al., 2009) which can underlie some of the chronic effects of the treatment. Here, we also analyzed the expression levels of the δ-subunit.
Two-way ANOVA showed a significant effect of Group on expression of the GABAA receptor δ-subunit (F(3,96) = 4.45, p = 0.006), with no differences between left and right visual cortex (F(1,96) = 0.01, p = 0.935, interaction Group*Hemisphere (F(3,96) = 0.01, p=0.998; Fig. 9A and 9B). Further post-hoc comparisons revealed that treatment with ALLO increased expression of the GABAA receptor δ-subunit following ONC: ONC/Veh (average of both hemispheres, 67.70 ± 7.73 %) vs. ONC/ALLO (106.77 ± 10.64 %, post-hoc Tukey’s test p = 0.044), vs. Sham/ALLO (120.44 ± 10.11 %, post-hoc Tukey’s test p = 0.003), but not in Sham animals (Sham/Veh vs. Sham/ALLO was not significant; Fig. 9A and 9B).
Figure 9.
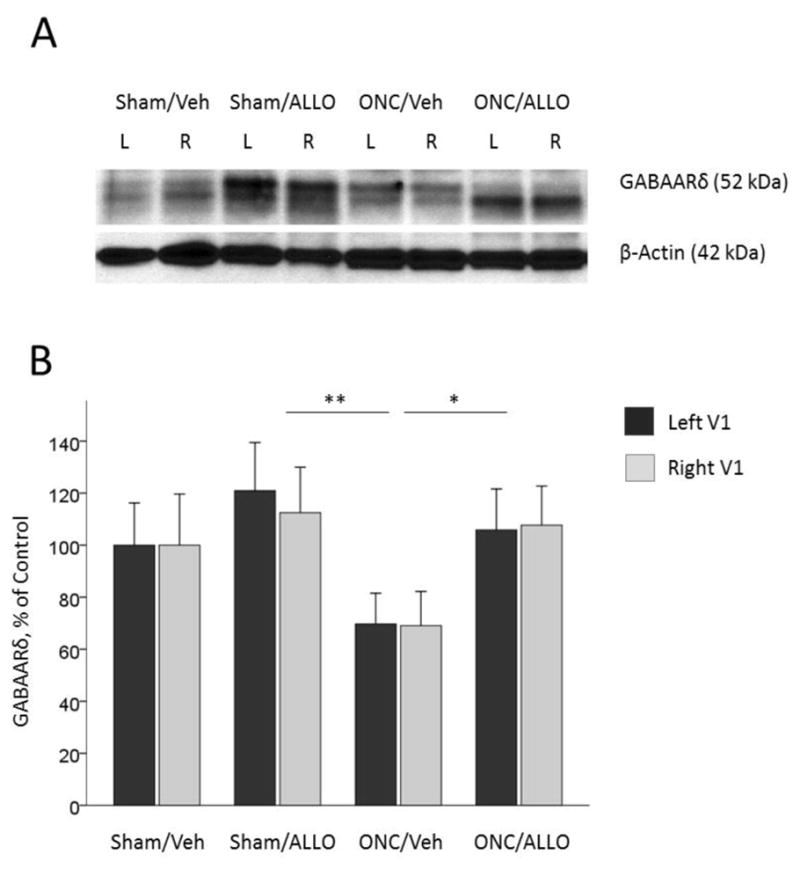
GABAA receptor δ-subunit expression in the left and right visual cortex
Representative blots (A) and densitometry data (B) for GABAA receptor δ-subunit in the left and right primary visual cortex obtained from animals after ONC (% of Sham/Veh control). Treatment with ALLO induced up-regulation of the δ-subunit (post-hoc comparisons between groups): ONC/Veh vs. ONC/ALLO, *p = 0.044; ONC/Veh vs. Sham/ALLO, **p = 0.003. Data are expressed as mean ± SEM, n = 12/group.
Discussion
In this study we observed an enhanced response in the visual cortex ipsilateral to the ONC eye that was induced by input from the intact eye. The injury-induced enhancement in the intact pathway substantially exceeded the level of SRP in sham-operated animals and, in contrast to normal SRP, was independent of stimulus orientation: gratings of both familiar and novel orientation evoked high-amplitude VEP, while in sham animals, a novel stimulus presented on day 30 showed no augmentation from baseline.
The potentiation of visual response we observed was associated with up-regulated expression of VGlut2 in the visual cortex ipsilateral to the ONC eye and down-regulated expression in the contralateral visual cortex. In contrast to MD, our finding suggests the clear involvement of both hemispheres in the plastic modifications of functional activity following unilateral ONC. In animals with MD, the reduction of input from the contralateral (initially dominant) deprived eye in the primary visual cortex lowers the “modification threshold” and subsequent visual experience yields cortical enhancement of the ipsilateral (initially weak) non-deprived eye (Bear et al., 1995; Kirkwood et al., 1995). Although the enhancement of non-deprived eye representation in the ipsilateral visual cortex is a well-known effect of MD (Bear et al., 1995; Kirkwood et al., 1996), the enhancement of representation of the non-deprived eye in its dominant, contralateral, hemisphere (as seen in animals with unilateral ONC) has not been observed in MD animals (Sawtell et al., 2003; Frenkel and Bear, 2004).
In the MD model, the absence of modifications in the hemisphere contralateral to the open eye was explained by an assumption that cortical activity is unlikely to be affected by the absence of the weak input from the deprived ipsilateral eye (Sawtell et al., 2003; Frenkel and Bear, 2004). The deprived eye produces spontaneous activity that is transferred via the lateral geniculate nucleus to the contralateral visual cortex and triggers Hebbian synaptic modifications – that is, long-term depression (LTD)-like loss of cortical response to the deprived eye and long-term potentiation (LTP) -like strengthening of synapses serving the open eye through a shift in LTP threshold (Bear, 2003; Cooke and Bear, 2010; Smith and Bear, 2010; Cooper and Bear, 2012).
In contrast to MD, unilateral ONC injury leads to elimination of most retinal activity in the affected eye and yields spontaneous synchronous thalamic bursts originating from cortical feedback, preventing LTD (Weliky and Katz, 1999). The absence of LTD may explain why, in contrast to the MD model, we did not observe the LTP-like enhancement of the ipsilateral intact eye input. However, the absence of LTD doesn’t explain why (again in contrast to the MD model) we observed potentiation in the opposite hemisphere, which is not seen following MD.
One possible explanation for this potentiation in the hemisphere ipsilateral to the ONC eye is suggested by studies demonstrating that the NR2A subunit of cortical NMDA receptors has roles both in establishing LTD, and in the shift in favor of the open eye in MD animals. Cho and colleagues reported that deletion of the NR2A subunit impaired the weakening of input from the deprived eye (LTD) and enhanced the activity-dependent strengthening of the open eye inputs (Cho et al., 2009). In the present study, long-term unilateral visual deafferentation induces reduction of NR2A subunit expression in both hemispheres and this reduction might then be permissive for potentiation of the intact visual pathway.
While feed-forward Hebbian mechanisms of eye dominance, discussed above, well describe the cortical modifications following MD, the mechanisms of feedback homeostatic plasticity—i.e., global synaptic scaling, a dynamic adjustment of synaptic strengths to promote network stability—suggest an alternative explanation for the potentiation following unilateral ONC (Turrigiano et al., 1998; Turrigiano and Nelson, 2004; Mrsic-Flogel et al., 2007). Chronic changes in visual experience have long been known to regulate both excitation and inhibition in the visual cortex (Hendry et al., 1994; Kilman et al., 2002; Sale et al., 2007; Maffei and Turrigiano, 2008; Kaneko et al., 2008; Versendaal et al., 2012). The blockade of activity from the eye, either with tetrodotoxin or by enucleation, leads to global scale-up of cortical excitatory currents (Kaneko et al., 2008), reduction of inhibitory currents (Kilman et al., 2002; Maffei and Turrigiano, 2008), elimination of functional inhibitory synapses (Versendaal et al., 2012), and down-regulation of the inhibitory receptors GABA (Hendry et al., 1994; Kilman et al., 2002) and neurotransmitter GABA (Sale et al., 2007). All these changes lead to a shift of the excitatory-inhibitory ratio (Beston et al., 2010) which then result in profound alterations of experience-dependent plasticity in the brain (Abraham, 2008). For instance, mice deficient in glutamic acid decarboxylase, GAD65, the enzyme responsible for GABA synthesis in synaptic terminals, showed altered OD plasticity in the MD paradigm (Hensch and Fagiolini, 2005). Disruption of excitatory-inhibitory balance due to excitotoxicity leading to increased excitation and attenuation of inhibition has been shown in a number of injury models including traumatic brain injury (Cantu et al., 2015), stroke (Schmidt et al., 2012), and global ischemia (Epsztein et al., 2006). Given all these findings, long-term deafferentation following ONC injury may lead to a scaling-up of excitatory synapses and scaling-down of inhibitory currents, with potentiation of the intact visual pathway, increase of VGlut2 ratio in the intact hemisphere, and down-regulation of the NR2A subunit.
We further tested for the involvement of disinhibition in ONC-induced reorganization in mouse visual cortex by pharmacological manipulation. Systemic administration of ALLO attenuated ONC-induced potentiation of visual evoked response towards a level observed in intact animals. Although there are no studies in the literature on the effect of neurosteroids on response potentiation in the visual system, there are several papers demonstrating the involvement of GABA-active neurosteroids in the mediation of inhibition in the processing of sensory stimuli. In line with our findings, Disney and Calford (2001) showed that blocking the synthesis of endogenous ALLO impaired habituation (that is, induced an opposite effect – potentiation) of the auditory response to repetitive stimulation in the auditory midbrain. Another study showed that progesterone and its metabolites (ALLO) produced inhibition of pain-related spinal reflex potentiation, and this decrease of potentiation was associated with GABAA receptor δ-subunit up-regulation (Peng et al., 2009). Although positive modulation of GABAA receptors is a major mechanism through which ALLO regulates the GABA-system (Belelli and Lambert, 2005; Carver and Reddy, 2013; Crowley et al., 2016; Reddy and Estes, 2016), the immediate enhancement of GABAA receptor efficacy by ALLO alone would not be likely to provide the sustainable long-term inhibitory effects on the visual potentiation observed in ONC mice. In our study, the long-term effects we observed were likely determined by ALLO-mediated up-regulation of the δ-subunit of the GABAA receptor. Our results are consistent with observations that ALLO can also regulate tonic inhibition through increased δ-subunit expression and trafficking (Herd et al., 2007; Shen et al., 2005; Peng et al., 2009).
Potentiation in the intact visual pathway after unilateral ONC is behaviorally significant. Similar to the gain in visual acuity through the non-deprived eye found in long-term MD, (Iny et al., 2006; Prusky et al., 2006; Tschetter et al., 2013), a novel finding in our study is that long-term deafferentation of the majority of visual inputs from one eye led to enhancement of visual acuity in the non-damaged eye. This gain in function in the intact eye was accompanied by the potentiation of visual evoked response through the same eye, indicating that the processes underlying VEP enhancement—thalamo-cortical synaptic strengthening and global synaptic scaling—may also account for the augmentation of visual acuity.
Interestingly, although ALLO administration decreased VEP enhancement, it did not influence visual acuity in the intact eye of ONC animals which demonstrated the same augmented spatial frequency threshold in the intact eye as ONC-vehicle-treated mice in three weeks post-ONC. This finding suggests that after optic nerve disruption there might be distinct mechanisms determining cortical potentiation and augmentation of optokinetic function. Although it is well established that experienced-enabled enhancement of adult visual acuity depends on the visual cortex (Prusky et al., 2006; Tschetter et al., 2013; Liu et al., 2016), optokinetic behavior is primarily a function of a subcortical accessory optic system (Sun et al., 2005), which may be differently affected by ALLO.
It is worth noting that progesterone and its metabolite ALLO given after brain injury can reduce inflammation, protect neurons against apoptosis and degeneration (Djebaili et al., 2005; Van Landingham et al., 2006; Sayeed et al., 2009; Labombarda et al., 2013; Guennoun et al., 2015; Allen et al., 2015, 2016), and promote regeneration (Wang et al., 2010; Brinton, 2013; Irwin et al., 2014). On the basis of this evidence it could be argued that by protecting the cells and axons along the damaged visual pathway, ALLO might increase visual input from the ONC eye and attenuate the differences between both eyes’ inputs by decreasing the potentiation of the intact eye. However, since we did not see any functional improvement in the ONC eye, this hypothesis is unlikely to be valid, at least with the regimen and dosage of ALLO administration we used here. From our perspective, the effects of ALLO treatment in our study are more likely to be caused by inhibition of the augmented response from the intact eye, and not by recovery of ONC eye input. In future studies it would be important to know whether other GABAergic agents, e.g. benzodiazepines and barbiturates, can mimic the observed effects of ALLO.
The present study provides new conceptual perspectives on ONC-induced plasticity (in contrast to MD), which is clinically relevant and not a rare condition (unilateral optic neuropathy, damage to the optic nerve axons due to advanced glaucoma, trauma, etc.). However, it is not yet known whether the potentiation of visual evoked response and the enhancement of visual acuity in the intact eye are functionally beneficial. On the one hand, the animals with ONC in one eye develop hyper-acuity in the other eye: they “could see better” with the intact eye. On the other hand, visual function in the ONC eye did not recover, as shown with VEP and behavioral testing (at least in the time frame we measured). If enhancement of the intact eye can act similarly to amblyopia, and the substantially strengthened intact pathway makes the animal more amblyopic and actually blocks potential recovery in the damaged pathway, this could put some constraints on regeneration and plasticity. Under such circumstances, attenuation of maladaptive ocular dominance plasticity, combined with other neuroprotective treatment, could be a therapeutic strategy to facilitate the recovery of visual function in the damaged eye. Pharmacological manipulation of excitatory-inhibitory balance with positive GABAA receptor modulators such as ALLO or other GABA-active neurosteroids could be exploited therapeutically to promote modulation of plasticity after damage to the eye or optic nerve or after brain injury.
Highlights.
Unilateral optic nerve crush induces ipsilateral potentiation of visual response
Injury-induced potentiation exceeds stimulation-induced potentiation
Unilateral optic nerve crush induces hyper-acuity in the intact eye
Allopregnanolone abolishes injury-induced potentiation
Injury-induced visual potentiation may result from cortical disinhibition
Acknowledgments
The authors thank Leslie McCann for her invaluable editorial assistance in the preparation of the manuscript.
This work was funded by unrestricted gifts in support of research from The Marcus Foundation, and Allen and Company, and in part by the Emory University Eye Center Core Facilities and the Microscopy in Medicine Core (Emory School of Medicine, Division of Cardiology). National Institutes of Health (grant number P30 EY006360); the Department of Veterans Affairs Rehabilitation R&D Service Merit Award (E0951-R) and Research Career Scientist Award (C9257) to MTP.
Abbreviations
- ALLO
allopregnanolone
- DAPI
4′,6-diamidino-2-phenylindole
- GABA
gamma-Aminobutyric acid
- GAD65
glutamate decarboxylase 65
- LTD
long-term depression
- LTP
long-term potentiation
- MD
monocular deprivation
- NMDA
N-methyl-D-aspartate
- OD
ocular dominance
- OKT
optokinetic tracking
- ONC
optic nerve crush
- PBS
phosphate buffered saline
- SRP
stimulus-selective response potentiation
- TBS
Tris-buffered saline
- TNF-α
tumor necrosis factor alpha
- V1
primary visual cortex
- VEP
visual evoked potential
- VGlut2
vesicular glutamate transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Allen RS, Olsen TW, Sayeed I, Cale HA, Morrison KC, Oumarbaeva Y, Lucaciu I, Boatright JH, Pardue MT, Stein DG. Progesterone treatment in two rat models of ocular ischemia. Invest Ophthalmol Vis Sci. 2015;56:2880–91. doi: 10.1167/iovs.14-16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RS, Sayeed I, Oumarbaeva Y, Morrison KC, Choi PH, Pardue MT, Stein DG. Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: progesterone receptor levels may play an important role. Restor Neurol Neurosci. 2016;34:947–963. doi: 10.3233/RNN-160672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. doi: 10.1016/0896-6273(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–55. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Beston BR, Jones DG, Murphy KM. Experience-dependent changes in excitatory and inhibitory receptor subunit expression in visual cortex. Front Synaptic Neurosci. 2010;2:138. doi: 10.3389/fnsyn.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–50. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, Dulla CG. Traumatic Brain Injury increases cortical glutamate network activity by compromising GABAergic control. Cereb Cortex. 2015;25:2306–2320. doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 2013;230:151–88. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–94. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KK, Khibnik L, Philpot BD, Bear MF. The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex. Proc Natl Acad Sci USA. 2009;106:5377–82. doi: 10.1073/pnas.0808104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30:9670–82. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–13. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex. Philos Trans R Soc Lond B Biol Sci. 2014;369:20140021. doi: 10.1098/rstb.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 2012;13:798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- Crowley T, Cryan JF, Downer EJ, O’Leary OF. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–277. doi: 10.1016/j.bbi.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–70. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney A, Calford MB. Neurosteroids mediate habituation and tonic inhibition in the auditory midbrain. J Neurophysiol. 2001;86:1052–6. doi: 10.1152/jn.2001.86.2.1052. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–18. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–84. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Epsztein J, Milh M, Bihi RI, Jorquera I, Ben-Ari Y, Represa A, Crépel V. Ongoing epileptiform activity in the post-ischemic hippocampus is associated with a permanent shift of the excitatory-inhibitory synaptic balance in CA3 pyramidal neurons. J Neurosci. 2006;26:7082–92. doi: 10.1523/JNEUROSCI.1666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003;100:2854–9. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–23. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Gavornik JP, Bear MF. Higher brain functions served by the lowly rodent primary visual cortex. Learn Mem. 2014;21:527–33. doi: 10.1101/lm.034355.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W. Adult visual cortical plasticity. Neuron. 2012;75:250–64. doi: 10.1016/j.neuron.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48–61. doi: 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Huntsman MM, Viñuela A, Möhler H, de Blas AL, Jones EG. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14:2383–401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–24. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Lifelong learning: ocular dominance plasticity in mouse visual cortex. Curr Opin Neurobiol. 2006;16:451–9. doi: 10.1016/j.conb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hu TT, Van den Bergh G, Thorrez L, Heylen K, Eysel UT, Arckens L. Recovery from retinal lesions: molecular plasticity mechanisms in visual cortex far beyond the deprived zone. Cereb Cortex. 2011;21:2883–92. doi: 10.1093/cercor/bhr079. [DOI] [PubMed] [Google Scholar]
- Iny K, Heynen AJ, Sklar E, Bear MF. Bidirectional modifications of visual acuity induced by monocular deprivation in juvenile and adult rats. J Neurosci. 2006;26:7368–74. doi: 10.1523/JNEUROSCI.0124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Solinsky CM, Brinton RD. Frontiers in therapeutic development of allopregnanolone for Alzheimer’s disease and other neurological disorders. Front Cell Neurosci. 2014;8:203. doi: 10.3389/fncel.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Neurosteroids are endogenous neuroprotectants in an ex vivo glaucoma model. Invest Ophthalmol Vis Sci. 2014;55:8531–41. doi: 10.1167/iovs.14-15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–80. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stryker MP. Homeostatic plasticity mechanisms in mouse V1. Philos Trans R Soc Lond B Biol Sci. 2017:372. doi: 10.1098/rstb.2016.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–85. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–37. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–8. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kreutz MR, Weise J, Dieterich DC, Kreutz M, Balczarek P, Böckers TM, Wittkowski W, Gundelfinger ED, Sabel BA. Rearrangement of the retino-collicular projection after partial optic nerve crush in the adult rat. Eur J Neurosci. 2004;19:247–57. doi: 10.1111/j.1460-9568.2004.03087.x. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Ghoumari AM, Liere P, De Nicola AF, Schumacher M, Guennoun R. Neuroprotection by steroids after neurotrauma in organotypic spinal cord cultures: a key role for progesterone receptors and steroidal modulators of GABA(A) receptors. Neuropharmacology. 2013;71:46–55. doi: 10.1016/j.neuropharm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Laing RJ, Turecek J, Takahata T, Olavarria JF. Identification of eye-specific domains and their relation to callosal connections in primary visual cortex of Long Evans rats. Cereb Cortex. 2015;25:3314–29. doi: 10.1093/cercor/bhu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Huberman AD, Scanziani M. Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour. Nature. 2016;538:383–387. doi: 10.1038/nature19818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macharadze T, Pielot R, Wanger T, Scheich H, Gundelfinger ED, Budinger E, Goldschmidt J, Kreutz MR. Altered neuronal activity patterns in the visual cortex of the adult rat after partial optic nerve crush--a single-cell resolution metabolic mapping study. Cereb Cortex. 2012;22:1824–33. doi: 10.1093/cercor/bhr256. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–84. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hübener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–72. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–73. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Nys J, Scheyltjens I, Arckens L. Visual system plasticity in mammals: the story of monocular enucleation-induced vision loss. Front Syst Neurosci. 2015a;28:60. doi: 10.3389/fnsys.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys J, Smolders K, Laramée ME, Hofman I, Hu TT, Arckens L. Regional specificity of GABAergic regulation of cross-modal plasticity in mouse visual cortex after unilateral enucleation. J Neurosci. 2015b;35:11174–89. doi: 10.1523/JNEUROSCI.3808-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lee SD, Lai CY, Chiu CH, Cheng CL, Chang YS, Hsieh MC, Tung KC, Lin TB. Neuroactive steroids inhibit spinal reflex potentiation by selectively enhancing specific spinal GABA(A) receptor subtypes. Pain. 2009;143:12–20. doi: 10.1016/j.pain.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci. 2006;26:11554–61. doi: 10.1523/JNEUROSCI.3396-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37:543–61. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Cerri C, Pietrasanta M, Gianfranceschi L, Maffei L, Caleo M. Functional masking of deprived eye responses by callosal input during ocular dominance plasticity. Neuron. 2009;64:707–18. doi: 10.1016/j.neuron.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Sabel BA. Restoration of vision I: neurobiological mechanisms of restoration and plasticity after brain damage - a review. Restor Neurol Neurosci. 1999;15:177–200. [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–81. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sautter J, Schwartz M, Duvdevani R, Sabel BA. GM1 ganglioside treatment reduces visual deficits after graded crush of the rat optic nerve. Brain Res. 1991;565:23–33. doi: 10.1016/0006-8993(91)91732-g. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–85. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009;1263:165–73. doi: 10.1016/j.brainres.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Bruehl C, Frahm C, Redecker C, Witte OW. Age dependence of excitatory-inhibitory balance following stroke. Neurobiol Aging. 2012;33:1356–63. doi: 10.1016/j.neurobiolaging.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–86. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Bear MF. Bidirectional ocular dominance plasticity of inhibitory networks: recent advances and unresolved questions. Front Cell Neurosci. 2010;4:21. doi: 10.3389/fncel.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, et al. Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron. 2015;86(4):971–84. doi: 10.1016/j.neuron.2015.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–8. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Tschetter WW, Alam NM, Yee CW, Gorz M, Douglas RM, Sagdullaev B, Prusky GT. Experience-enabled enhancement of adult visual cortex function. J Neurosci. 2013;33:5362–6. doi: 10.1523/JNEUROSCI.5229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Van Brussel L, Gerits A, Arckens L. Evidence for cross-modal plasticity in adult mouse visual cortex following monocular enucleation. Cereb Cortex. 2011;21:2133–46. doi: 10.1093/cercor/bhq286. [DOI] [PubMed] [Google Scholar]
- van Versendaal D, Levelt CN. Inhibitory interneurons in visual cortical plasticity. Cell Mol Life Sci. 2016;73:3677–91. doi: 10.1007/s00018-016-2264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, et al. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–85. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Vasconcelos N, Pantoja J, Belchior H, Caixeta FV, Faber J, Freire MA, Cota VR, Anibal de Macedo E, Laplagne DA, Gomes HM, Ribeiro S. Cross-modal responses in the primary visual cortex encode complex objects and correlate with tactile discrimination. Proc Natl Acad Sci USA. 2011;108:15408–13. doi: 10.1073/pnas.1102780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Kullmann DM. Tonic GABAA receptor-mediated signaling in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK50785/ [PubMed] [Google Scholar]
- Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:6498–503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–21. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]