Abstract
We compared same-day provider medical record documentation and interventions addressing depression and risk behaviors before and after delivering point-of-care patient-reported outcomes (PRO) feedback for patients who self-reported clinically relevant levels of depression or risk behaviors. During the study period (1/1/2006–10/15/2010), 2,289 PRO assessments were completed by HIV-infected patients. Comparing the 8 months before vs. after feedback implementation, providers were more likely to document depression (74% before vs. 87% after feedback, p=0.02) in patients with moderate-to-severe depression (n=317 assessments), at-risk alcohol use (41% vs. 64% p=0.04, n=155) and substance use (60% vs. 80% p=0.004, n=212). Providers were less likely to incorrectly document good adherence among patients with inadequate adherence after feedback (42% vs. 24% p=0.02, n=205). While PRO feedback of depression and adherence were followed by increased provider intervention, other domains were not. Further investigation of factors associated with the gap between awareness and intervention are needed in order to bridge this divide.
Keywords: patient reported outcomes, adherence, depression, sexual risk behavior, substance use, alcohol use
INTRODUCTION
Physicians routinely under-diagnose depression and suicidal ideation[1,2], substance use[3,4], poor medication adherence[5–7] and HIV transmission risk behaviors[8,9], all of which impact long-term clinical outcomes among people living with HIV (PLWH)[6,10,11]. Assessment of these conditions and behaviors relies on patient self-report. In routine practice, providers elicit this information from patients at the point of care. Improving elicitation and delivery of patient self-reported data may be critical for improving clinical care and outcomes.
Growing recognition of the value of systematic routine collection of patient-reported data in care has led to development and use of self-report assessments, referred to as patient-reported data, measures, or outcomes (PROs)[12]. PROs assess conditions and behaviors that might otherwise be overlooked or are difficult and time-consuming for providers to assess in brief clinical encounters.
Past efforts integrating PRO reports into clinical care have often shown disappointing results[13,14]. Potential explanations for this include time gaps between PRO completion and provider appointments rather than same-day assessments[15,16] and measurement of domains providers do not necessarily deem clinically relevant[13]. Recent technical advances can address several concerns, in particular, difficulty collecting data in real time in high-volume clinic settings without causing delays in patient throughput. Touch-screen computer technology now facilitates high completion rates, even by patients with low levels of computer literacy. Computerized PRO platforms permit time-saving skip patterns that minimize patient burden and facilitate real-time delivery of results to providers at the point-of-care[17–19]. These advances have allowed successful integration of PROs into routine care with minimal disruption to clinic flow[14,17].
We compared chart documentation from all patients who self-reported depression, inadequate HIV medication adherence, alcohol and substance use, and sexual risk behavior on the PRO assessment in the 8 months before and after we began to routinely deliver PRO reports to providers. We also compared provider documentation and actions taken from the subset of patients with depression or risk behaviors both before and after feedback initiation at any time during the study (not limited to 8-month windows). The purpose of this study was to determine the extent to which a well-integrated, clinically relevant, web-based, touch-screen PRO collection and feedback delivery system influenced provider documentation and actions in the care of PLWH.
METHODS
Study Setting
This study was conducted among patients in the University of Washington (UW) HIV Cohort, a longitudinal observational cohort of PLWA who receive primary care in the UW Harborview Medical Center HIV Clinic.
Study participants
PLWH ≥18 years of age who attended the clinic for a routinely scheduled appointment and completed the PRO assessment were eligible. Patients unable to complete the assessment, such as those with dementia or who did not speak English or Spanish, were excluded, as were those who declined to give informed consent. This study was approved by the UW’s institutional review board. All patients signed written informed consent.
PROs
Patients awaiting routine visits with their providers began completing PRO assessments as part of a research protocol in 2006. We used a web-based, open-source survey software application, developed specifically for PROs (http://cprohealth.org)[19–24]. Patients used tablet PCs with touch-screens. Questions were displayed in large, easy-to-read type with clearly labeled radio buttons to indicate responses. Patients completed the assessment approximately every ~4–6 months at the time of routine appointments.
PRO data were initially intended solely for research and were collected two days a week. As implementation of PRO collection was smooth and non-disruptive[19] this was expanded to daily collection. Based on discrepancies between PRO results and clinician documentation from the same visit date showing lower rates of risk behaviors in clinical documentation, clinic leadership supported delivery of PRO reports to clinicians as part of routine care processes beginning January, 2009[19]. Before initiating delivery of PRO reports to providers, we conducted a brief introduction and training session at the monthly provider meeting and sent out a summarizing email to clinic staff. Since then, assessment completion has automatically generated a brief printed report that is delivered to the provider immediately before the clinic visit with other visit paperwork. More recently, results for domains such as depression are also integrated into the electronic health record meeting a Meaningful Use objective[25]. This approach to PROs builds on the theoretical underpinnings of the Chronic Care Model including a clinical information system, delivery system design, and decision support to improve clinical care[26–29].
Instruments
We selected domains for the PRO assessment based on importance for clinical care and research. Using the Medical Outcomes Trust criteria[30], we considered instrument validity, reliability, responsiveness, efficiency in terms of patient burden, and interpretability when choosing instruments for each selected domain. When possible, we chose instruments with evidence of validity established among PLWH. The assessment contained 50 to 101 items depending on skip patterns generated by patient responses.
Instrument scoring
For these analyses, we focused on five clinically important domains. We measured depression symptoms using the 9-item Patient-Health Questionnaire (PHQ-9) from the Primary Care Evaluation of Mental Disorders (PRIME-MD)[31,32], with scores ≥10 indicating moderate-to-severe depression, considered at-risk. We also conducted sensitivity analyses requiring scores ≥10 and this score had to include elevated results for the items measuring depressed mood or anhedonia[32]. While feedback began 1/2009, even when PRO assessments were collected solely for research purposes (prior to 1/2009), providers and/or case managers always received an automated notification of suicidal ideation (PHQ-9 item 9).
We measured antiretroviral medication adherence using the 4-item Adult AIDS Clinical Trial Group (AACTG) instrument, a visual analogue scale, and a self-rating scale item[33–35]. Inadequate adherence for these analyses was defined as missing ≥1 dose in the prior four days. We measured substance use using the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST)[36,37]. At-risk substance use was defined as any non-prescribed use of opiates/heroin, crack/cocaine, or methamphetamine/crystal/speed within the prior 3 months. Alcohol use was measured using the Alcohol Use Disorders Identification Test consumption questions (AUDIT-C)[38,39]. At-risk alcohol use was defined as scores ≥5 for men and ≥4 for women[40]. Sexual risk behavior was measured using a modified version of the HIV Risk Assessment for Positives (HRAP)[41,42]. We defined sexual risk behavior as being sexual activity with one or more partners in the prior 6 months and reporting using condoms some of the time or never (in contrast to using condoms most of the time or always). The HRAP was not initially included in the assessment, it was added June, 2007.
Medical record review
We reviewed medical record documentation from provider clinic notes from the same day as the assessment among PLWH self-reporting clinically relevant levels of depression or risk behaviors. We determined whether depression or risk behaviors (i.e., inadequate adherence, substance use, at-risk alcohol use, sexual risk behavior) were identified by the provider and if any related actions were initiated by the provider. Documentation for a domain included in problem lists, discussion in the assessment and plan, or any other provider documentation for that day. Actions included referrals, prescriptions, or documentation of discussions. For example, if a patient had depression, potential provider actions could include a new prescription or dose change of anti-depressant medication, referral to psychiatry, referral to the Case Manager or Health Educator, or discussion with the patient regarding depression and available resources.
For patients with inadequate adherence, in addition to noting any acknowledgement of the issue in provider notes, we also identified notes in which providers inaccurately documented very good medication adherence (examples included “missed no doses,” “>95% adherence,” and “perfect adherence”). All medical record reviews were performed by chart reviewers blinded to study goals and the PRO results for each patient.
Analyses
Our primary analyses for each domain compared same-day problem identification and actions documented by providers in the eight months before vs. after the start of feedback among patients with depression or risk behaviors using chi-squared tests. We limited the time-windows for this analysis to minimize potential impact of changes in care guidelines or other period effects. This analysis focused on assessment results by visits before and after feedback initiation and did not require an individual patient to have an at-risk assessment in both time periods.
We conducted additional analyses for each domain that included patients with depression or risk behaviors both prior to and after feedback initiation. These secondary analyses differed from the primary analyses as they were within-person analyses not limited to 8-month windows. However, patients had to endorse depression or risk behaviors both before and after feedback initiation. To account for repeated measures and the matched quality of the data, we used generalized estimating equation (GEE) logistic models to verify that the statistical tests were robust to the issues of matching and repeated measures. We present the measures on the percentage scale, due to the easy clinical interpretability and consistency with other results. We compared provider-documented problem identification or actions before and after feedback initiation.
We repeated both analyses among the subset of patients with moderate-to-severe depression excluding those who indicated suicidal ideation, as this always resulted in automated notification even before feedback was routinely integrated. We conducted additional sensitivity analyses examining 6 and 10-month periods prior to and after initiation of provider feedback.
Finally, to address the possibility that changes in documentation were due to temporal trends or other period effects unrelated to the clinical assessment, we assessed documentation for four outcomes not included as part of the clinical assessment (diabetes, hypertension, pneumococcal vaccination, and hepatitis C virus screening) and compared rates of documentation in the 8 months before and 8 months after initiating provider feedback among a random selection of 300 individuals who had completed the assessment.
RESULTS
During the study period, 1/1/2006–10/15/2010, the assessment was completed 2,289 times by 1,083 PLWH, of these, 722 were completed by 381 PLWH in the 8 months before and after feedback initiation. Because of the initial ramp-up period during implementation, more assessments were completed in later than in earlier years. Refusal rates were ~1%. The mean number of assessments per patient was 1.9 (median 2, range 1–10). Providers received feedback from 99% of PROs completed after the initiation of provider feedback. Completion rates were high with minimal missing data. All 9 depression items were completed in 2,150 assessments (94%) and adherence data was available for 1,839 assessments (99% of patients receiving HIV medications). Patients not receiving HIV medications were not asked to complete adherence items. Information regarding alcohol use was available from 2,207 (96%) assessments, for substance use from 2,227 (97%) assessments and for sexual risk behavior from 1,778 (78%) assessments (note sexual risk behavior was added in 2007).
The mean age at initial assessment was 43 (SD 9); 85% were men; 60% were non-Hispanic white, 21% were African-American, and 12% were Hispanic. Demographic and clinical characteristics of study patients were similar to those of all patients receiving care at the clinic during the study period (data not shown).
Provider identification and action in the 8 months before and after PRO feedback
These analyses were based on at-risk assessments in the 8 months before or after feedback initiation from N=722 assessments.
Depression
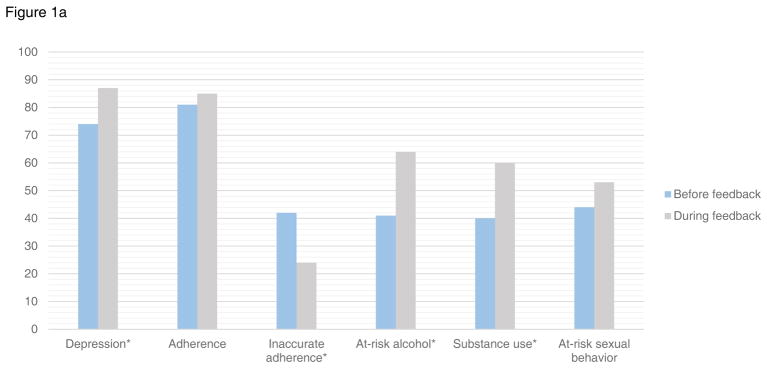
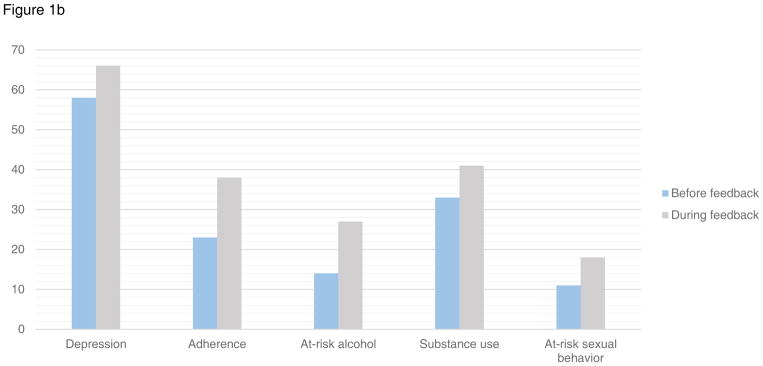
Patients reported moderate-to-severe depression symptoms in 317 assessments in the 8-month windows before and after provider feedback initiation. Prior to feedback, providers acknowledged depression in 74% (95%CI:62–85) of same-day clinic documentation vs. 87% (95%CI:82–91) after feedback (p=0.02) (Figure 1a) among patients who reported depression symptoms on the assessment. Providers took action (e.g. prescription for antidepressant medication, referral to mental health treatment) in response to moderate-to-severe depressive symptoms in 58% (95%CI:45–71) of patients prior to feedback, vs. 66% (95%CI:60–72) after feedback (p=0.3) (Figure 1b). Findings were similar in sensitivity analyses of patients with moderate-to-severe depression excluding those with suicidal ideation (documentation 70% vs. 85%, p=0.012, provider action 51% vs. 64%, p=0.09). Similar findings were also found in those with moderate-to-severe depression defined as a PHQ-9≥10 including depressed mood and/or anhedonia.
Figure 1.
Figure 1a. Provider documentation in the 8 months before and after initiation of provider feedback for patients with at-risk symptoms and behaviors
*p value <0.05
Figure 1b. Provider action in the 8 months before and after initiation of provider feedback for patients with at-risk symptoms and behaviors
Adherence
Patients reported inadequate adherence in 205 assessments in the 8-month windows before/after provider feedback initiation. Among these patients, providers acknowledged adherence in some way in 81% (95%CI:70–93) of same-day documentation in the 8 months prior to feedback vs. 85% (95%CI:80–91) after feedback (p=0.5) (Figure 1a). Providers documented action in response to inadequate adherence (i.e. adherence counseling, case management referral) in 23% (95%CI:11–36) prior to feedback and 38% (95%CI:31–46) after feedback (p=0.07) (Figure 1b).
Despite substantial provider documentation of adherence of some kind (good or bad) prior to feedback, we noted that adherence documentation in provider notes among patients with inadequate adherence was often inaccurate. Therefore, for provider notes that acknowledged adherence, we also tracked the percent of discrepant documentation when compared to PRO reports of inadequate adherence. Providers documented discrepant adherence (“perfect adherence, “missed no doses”, >95% adherence) for 42% (95%CI:27–57) of patients with PRO documented inadequate adherence in the 8 months prior to feedback vs. 24% (95%CI:17–31) in the 8 months after feedback was implemented (p=0.02) (Figure 1a).
At-risk alcohol use
Patients reported at-risk alcohol use in 155 assessments in the 8-month windows before/after provider feedback initiation. Providers documented alcohol use for 41% (95%CI:20–61) in the eight months prior to feedback vs. 64% (95%CI:56–72) after feedback (p=0.04) (Figure 1a). Providers documented action in response to alcohol use (e.g. health educator referral) in 14% (95%CI:0–28) of notes before vs. 27% (95%CI:20–35) after feedback (p=0.2) (Figure 1b).
Substance use
There were 212 assessments completed by patients reporting current substance use in the 8-month windows before/after provider feedback initiation. Among these patients, provider documentation acknowledged substance use 60% (95%CI:47–73) of the time in the 8 months prior to feedback, and 80% (95%CI:73–86) after feedback (p=0.004) (Figure 1a). Providers documented action (e.g. treatment referral) in response to substance use in 33% (95%CI:20–45) prior to feedback, and in 41% (95%CI:33–48) after feedback (p=0.3) (Figure 1b).
Sexual risk behavior
Patients reported sexual risk behavior in 344 assessments in the 8-month windows before/after provider feedback initiation. Among these patients reporting sexual risk behavior, providers documented risky sexual behavior in 44% (95%CI:31–56) of visits before vs. 53% (95%CI:47–59) after initiation of feedback (p=0.2) (Figure 1a). Providers documented action taken (i.e., safer sex counseling, referral to health educator) in 11% (95%CI:3–19) of notes before vs. 18% (95%CI:14–23) after feedback (p=0.2) (Figure 1b).
The pattern of findings across domains were similar using 6 and 10-month windows instead of 8 (data not shown).
Provider identification and action among patients with depression or at-risk behaviors both before and after PRO feedback initiation
These secondary analyses were based on at-risk assessments throughout the study period, not limited to the 8 month windows; however, patients had to report at-risk depression or behaviors both before and after PRO feedback initiation.
Depression
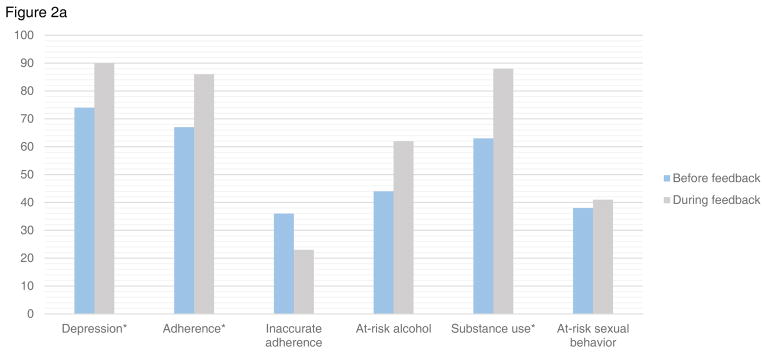
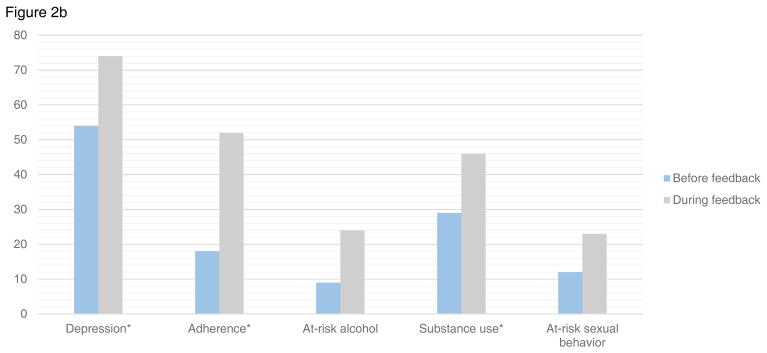
There were 156 PRO assessments completed by patients reporting moderate-to-severe depression symptoms both before and after initiation of provider feedback. Providers acknowledged depression in 74% (95%CI:63–84) of these patients prior to feedback vs. 90% (95%CI:84–97) after (p=0.008). Providers documented action in response to depression symptoms in 54% (95%CI:43–66) prior vs. 74% (95%CI:64–83) after feedback (p=0.01). Findings were similar in sensitivity analyses excluding those with suicidal ideation (acknowledged 71% vs. 89%, p=0.01, provider action 49% vs. 72%, p=0.009).
Adherence
There were 78 assessments reporting inadequate adherence both before and after provider feedback. Providers acknowledged adherence in 67% (95%CI:51–83) of patients before vs. 86% (95%CI:76–97) after feedback (p=0.05). Providers documented action in response to inadequate adherence in 18% (95%CI:5–37) before vs. 52% (95%CI:38–67) after feedback (p=0.003). Providers inaccurately documented good adherence in those with inadequate adherence in 36% (95%CI:20–53) before vs. 23% (95%CI:10–35) after feedback (p=0.2).
Alcohol use
There were 72 assessments completed by patients who reported at-risk alcohol use both before and after initiation of provider feedback. Providers documented alcohol use in 44% (95%CI:27–61) of notes prior to feedback, and in 62% (95%CI:47–78) after feedback (p=0.2). Providers documented action in response to at-risk alcohol use in 9% (95%CI:0–18) before vs. 24% (95%CI:10–38) of the same-day notes after feedback (p=0.1).
Substance use
There were 100 assessments completed by patients who reported substance use both before and after initiation of provider feedback. Substance use was acknowledged in 63% (95%CI:49–78) before vs. 88% (95%CI:80–96) of same-day notes after feedback (p=0.002). Providers documented action in response to substance use in 29% (95%CI:15–43) before vs. 46% (95%CI:33–58) after (p=0.03).
Sexual risk behavior
There were 100 assessments completed by patients reporting risky sexual behavior both before and after initiation of provider feedback. Providers documented sexual risk behavior in 38% (95%CI:22–55) of notes before vs. 41% (95%CI:29–53) after feedback (p=0.5). Providers documented action in response to sexual risk behavior in 12% (95%CI:1–23) before feedback and 23% (95%CI:13–33) after (p=0.1).
Control analyses
These analyses were for outcomes unrelated to the clinical assessment to ensure that changes seen in documentation were not due to temporal trends or other factors besides the clinical assessment.
We examined rates of documentation related to four outcomes unrelated to the clinical assessment: diabetes, hypertension, pneumococcal vaccination, and hepatitis C screening. Among 300 randomly selected patients who completed assessments in the 8 months prior to or after feedback initiation, there were no differences in documentation between these two time periods (p values 0.4–0.5).
DISCUSSION
The provision of PRO feedback to clinicians caring for PLWH was associated with improved provider awareness of depression, poor medication adherence, alcohol, and substance use as measured by documentation. A small, but not significant, increase in documentation of sexual risk behavior also occurred after implementing provider feedback. There was a decrease in documentation of ‘excellent adherence’ for patients who self-reported inadequate adherence. Across all domains, impact on provider action was smaller than impact on provider awareness, suggesting that PRO assessment and feedback is only the first step in addressing these complex issues.
Providers documented depression in a high proportion reporting depression even prior to PRO assessment feedback. Nevertheless, documentation was greater after feedback was provided. We were concerned that the automated follow-up to self-reported suicidal ideation would impact our results, but sensitivity analyses excluding those reporting suicidal ideation indicated similar results. These findings demonstrate that the PRO assessment and feedback not only identified a substantial number of patients with suicidal ideation (44 assessments in the 8 month windows before/after feedback), but also increased awareness of moderate-to-severe depressive symptoms among those without suicidal ideation.
PRO feedback did not impact overall adherence documentation for those who self-reported inadequate adherence in the 8 months after feedback initiation, although there was a significant difference in secondary analyses among those reporting inadequate adherence both before and after PRO feedback integration (67% vs. 86%, p=0.05). Furthermore, providers were more likely to take action addressing adherence after feedback was provided (18% vs. 52%, p=0.003). After PRO feedback started, providers documented excellent adherence less often for those who self-reported inadequate adherence (42 vs. 24%, p=0.02 in the primary analysis). While providers often acknowledged adherence in their documentation, the lack of accurate provider documentation of adherence is consistent with studies demonstrating provider-based assessments of adherence are poor[5,7]. Systematic implementation of PROs that include adherence may help address this issue. While increasing provider awareness of inadequate adherence is important, Wilson and colleagues reported that provider discussions of adherence tend to be directive, rather than problem-solving, and not particularly effective[43]. This suggests PRO feedback may be more effective when targeting the entire healthcare team (including case managers/health educators) rather than relying solely on providers and that additional interventions may be needed to improve the approach providers take to adherence counseling.
Providers acknowledgement of at-risk alcohol use (41% vs. 64%, p=0.04), and substance use (60% vs. 80%, p=0.004) increased significantly after feedback, however, there was not a significant increase in provider actions except in the secondary analyses for substance use. These findings underscore the urgent need for provider education and increased use of proven interventions to address all forms of substance use.
Providers did a poor job acknowledging risky sexual behaviors both before and after feedback (44% vs. 53%, p=0.2) and rarely documented action plans (11% vs. 18%, p=0.2). This is consistent with prior studies that have suggested providers are not necessarily comfortable discussing sexual risk behavior with patients and that there are many missed opportunities to do so [44–47]. This is concerning for prevention efforts, particularly considering the importance of sexual risk behavior as a means of HIV transmission.
There were substantial differences in the impact of PRO feedback on provider awareness and actions across domains. These cross-domain differences may be because providers prioritize certain clinical problems above others or perceive certain problems as more modifiable than others. Availability of interventions such as mental health services may impact provider actions[48]. Lower rates of identifying and addressing sexual risk behaviors, substance use, and at-risk alcohol use may be because providers believe these issues are more effectively addressed by other clinic staff, such as health educators or social workers.
Feedback regarding risky sexual behaviors had little impact on documented provider identification and actions. This may be due to the reasons mentioned or because providers are not comfortable discussing sexual risk behavior with patients, or may presume existing sexual partnerships are stable or exclusive.
Regardless of variations in feedback impact across domains, our results demonstrate PROs are a promising tool to supplement and enhance patient-provider communication. PRO integration was easily accomplished and well received by providers and staff, in part because of excellent feedback delivery rates (99%), on-site access to referral resources, and provider involvement and satisfaction with the PRO design, collection and feedback delivery process[17].
PROs may be especially useful when caring for patients with multiple morbidities and behavioral issues in a time-limited clinic visit. PROs may also be useful in reducing social desirability bias with potentially greater reporting of risky behaviors than would be reported directly to providers. Research is needed to understand the factors influencing differences in provider use across domains, changes in provider utilization of PROs over time, and the effect of PROs on outcomes.
Strengths
This study evaluated the impact of systematic integration of PROs into a clinical care setting and therefore may be more generalizable than findings from a clinical trial. Providers were involved in selecting domains clinically relevant to PLWH potentially increasing the usefulness and clinical impact. PRO assessments were completed the same day as clinical appointments using touch-screen tablets. Technological advances allowed for automatic scoring and generation of feedback reports, reducing staff burden compared with earlier studies and thereby improving feasibility for implementation in large busy clinics. In fact, the platform and assessment described here have now been integrated into multiple clinics across the U.S. as part of Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort with >70,000 PRO assessments completed to date as part of routine clinical appointments[49]. Additional information on CNICS and its data elements can be found at https://www.uab.edu/cnics/.
Limitations
First, our data relied on medical record documentation raising the possibility that providers identified or addressed issues, but forgot or chose not to document them. However, we have no reason to suspect that documentation completeness would have been systematically higher or lower during the two time periods.
Second, this was an observational study in a clinical care setting comparing documentation across time periods, rather than a randomized controlled trial. However, this is also a strength of this study in that the goal was to evaluate the impact of implementation in clinical care. Also, in secondary analyses where we addressed temporal trends by considering people with at-risk PRO results in both time periods, results were just as strong if not stronger. The within-patient secondary analyses confirmed that the PRO assessment feedback intervention’s success was not primarily driven by temporal differences in the patient population served. Furthermore, when we examined outcomes not included in the clinical assessment such as pneumococcal vaccination, there were no differences over time. This suggests that the differences we noted in provider documentation were in fact due to the feedback and not due to temporal trends.
Third, this study focused on the impact of PROs and feedback specifically on physician awareness and actions. Other health care team members, such as case managers and health educators, may also benefit from the use of PROs and the importance of non-medical providers will increase in the era of patient-centered medical homes. Furthermore, PRO feedback may have other system implications such as billing benefits with systematic documentation of review of system elements.
Fourth, patients filling out the assessment may have increased their awareness of depression or risk behaviors and communicated that to providers directly.
Finally, this study was conducted with only PLWH, limiting generalizability to other chronic disease settings, and was conducted at only one clinic so results may have been different elsewhere. PLWH may have higher rates of risk behaviors than other patient populations providing greater opportunities to intervene and potentially larger impact.
Future steps and ongoing studies
In addition to expanding PROs and their feedback to multiple clinics across CNICS, we are conducting studies on various approaches to optimize providing feedback including having providers sign paper-based feedback forms and implementing results directly into the electronic medical record in real time to facilitate clinical care visits. We are now testing the use of this approach as a building block combining PRO results with additional interventions integrated into clinical care targeting such domains as adherence and alcohol use. We are particularly interested in additional studies looking at the impact of PROs not just on provider behavior but on patient outcomes such as adherence and depression over time.
Conclusions
Implementing same-day PRO collection and feedback into HIV care improves care. Specifically, it improves provider awareness of depression, inadequate adherence, alcohol, and substance use as measured by documentation. PROs decrease how often providers inaccurately documented good adherence. However, PRO impact varies across domains and there is a much greater impact on provider awareness than on actions suggesting the need for additional interventions. Additional multi-disciplinary domain-specific interventions and trainings to effectively address risk behaviors may enhance the effectiveness of PROs and further improve clinical outcomes.
Figure 2.
Figure 2a. Provider documentation among patients with depression or at-risk behaviors both before and after PRO feedback initiation
*p value <0.05
Figure 2b. Provider actions among patients with depression or at-risk behaviors both before and after PRO feedback initiation
*p value <0.05
Acknowledgments
We wish to thank the patients and providers of the University of Washington Madison HIV clinic. This work was supported by grants from the NIH NIMH RO1 Grant (RO1MH084759), NIH PROMIS Grant (U01AR057954), NIH OBSSR PROMIS Supplement (U01AR057954), Mentored Patient-Oriented Research Career Development Award NIAID Grant (AI-60464), the University of Washington Center for AIDS Research NIAID Grant (AI-27757), the NIAAA ARCH grants (U01AA020793 and U24AA020801), the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) grant (AI-067039), and the Patient Centered Outcomes Research Institute (PCORI) PRO PROMIS grant (ME-1403-14081).
Footnotes
Findings were presented in part at the 6 th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, 2011.
Compliance with ethical standards: The authors have no conflicts of interest.
The funding sources are listed below.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
References
- 1.Staab JP, Datto CJ, Weinrieb RM, Gariti P, Rynn M, Evans DL. Detection and diagnosis of psychiatric disorders in primary medical care settings. Med Clin North Am. 2001 May;85(3):579–596. doi: 10.1016/s0025-7125(05)70330-8. [DOI] [PubMed] [Google Scholar]
- 2.Lowe B, Grafe K, Zipfel S, et al. Detecting panic disorder in medical and psychosomatic outpatients: comparative validation of the Hospital Anxiety and Depression Scale, the Patient Health Questionnaire, a screening question, and physicians’ diagnosis. J Psychosom Res. 2003 Dec;55(6):515–519. doi: 10.1016/s0022-3999(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 3.Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr. 2003 Aug 1;33(4):521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Messiah A, Loundou AD, Maslin V, Lacarelle B, Moatti JP. Physician recognition of active drug use in HIV-infected patients is lower than validity of patient’s self-reported drug use. J Pain Symptom Manage. 2001 Feb;21(2):103–112. doi: 10.1016/s0885-3924(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 5.Gross R, Bilker WB, Friedman HM, Coyne JC, Strom BL. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS. 2002 Sep 6;16(13):1835–1837. doi: 10.1097/00002030-200209060-00021. [DOI] [PubMed] [Google Scholar]
- 6.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000 Jul 4;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Hecht FM, Clague H, et al. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr. 2001 Apr 15;26(5):435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Marks G, Richardson JL, Crepaz N, et al. Are HIV care providers talking with patients about safer sex and disclosure?: A multi-clinic assessment. AIDS. 2002;16(14):1953–1957. doi: 10.1097/00002030-200209270-00013. [DOI] [PubMed] [Google Scholar]
- 9.Morin SF, Koester KA, Steward WT, et al. Missed opportunities: prevention with HIV-infected patients in clinical care settings. J Acquir Immune Defic Syndr (1999) 2004;36(4):960–966. doi: 10.1097/00126334-200408010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002 May 3;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 11.Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 x 10(9) cells/L. Ann Intern Med. 2003 Nov 18;139(10):810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 12.Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008 Mar;17(2):179–193. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 14.Schackman BR, Dastur Z, Rubin DS, et al. Feasibility of using audio computer-assisted self-interview (ACASI) screening in routine HIV care. AIDS Care. 2009;21(8):992–999. doi: 10.1080/09540120802657506. [DOI] [PubMed] [Google Scholar]
- 15.Wilson IB, Laws MB, Safren SA, et al. Provider-focused intervention increases adherence-related dialogue but does not improve antiretroviral therapy adherence in persons with HIV. J Acquir Immune Defic Syndr. 2010 Mar;53(3):338–347. doi: 10.1097/QAI.0b013e3181c7a245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fihn SD, McDonell MB, Diehr P, et al. Effects of sustained audit/feedback on self-reported health status of primary care patients. Am J Med. 2004 Feb 15;116(4):241–248. doi: 10.1016/j.amjmed.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Tufano JT, Fredericksen R, Schmidt S, et al. Evaluating integration of an HIV medication adherence computer-assisted self-administered interview (CASI) with routine patient care. 5th International Conference on HIV Treatment Adherence; 2010; Miami, Florida. [Google Scholar]
- 18.Schackman BR, Dastur Z, Rubin DS, et al. Feasibility of using audio computer-assisted self-interview (ACASI) screening in routine HIV care. AIDS Care. 2009 Aug;21(8):992–999. doi: 10.1080/09540120802657506. [DOI] [PubMed] [Google Scholar]
- 19.Fredericksen RJ, Crane PK, Tufano J, et al. Integrating a web-based patient assessent into primary care for HIV-infected adults. Journal of AIDS and HIV Research. 2012;4(2):47–55. doi: 10.5897/jahr11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res. 2007 Jan;5(1):109–118. doi: 10.2174/157016207779316369. [DOI] [PubMed] [Google Scholar]
- 21.Crane H, Tufano J, Fredericksen R, et al. Integration of an HIV medication adherence computer assisted self-administered interview (CASI) with routine patient care. 5th International Conference on HIV Treatment Adherence; 2010; Miami, Florida. [Google Scholar]
- 22.Berry DL, Blumenstein BA, Halpenny B, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011 Mar 10;29(8):1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry DL, Trigg LJ, Lober WB, et al. Computerized symptom and quality-of-life assessment for patients with cancer part I: development and pilot testing. Oncol Nurs Forum. 2004 Sep;31(5):E75–83. doi: 10.1188/04.ONF.E75-E83. [DOI] [PubMed] [Google Scholar]
- 24.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009 Jan;18(1):14–22. doi: 10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. [Accessed May 1, 2015];Introduction to Meaningful Use. http://www.cdc.gov/ehrmeaningfuluse/introduction.html.
- 26.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998 Aug-Sep;1(1):2–4. [PubMed] [Google Scholar]
- 27.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002 Oct 9;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 28.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001 Nov-Dec;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 29.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 30.Scientific Advisory Committee of the Medical Outcomes Trust. Assessing health status and quality-of-life instruments: attributes and review criteria. Quality of Life Research. 2002 May;11(3):193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000 Jun;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 34.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008 Jan;12(1):86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 35.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004 Mar-Apr;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 36.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug and Alcohol Review. 2005 May;24(3):217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 37.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002 Sep;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 38.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998 Sep 14;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 39.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003 Apr 14;163(7):821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 40.Gual A, Segura L, Contel M, Heather N, Colom J. AUDIT-3 and AUDIT-4: effectiveness of two short forms of the alcohol use disorders identification test. Alcohol and Alcoholism. 2002 Nov-Dec;37(6):591–596. doi: 10.1093/alcalc/37.6.591. [DOI] [PubMed] [Google Scholar]
- 41.Metraux S, Metzger DS, Culhane DP. Homelessness and HIV risk behaviors among injection drug users. J Urban Health. 2004 Dec;81(4):618–629. doi: 10.1093/jurban/jth145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg SD, Trumbetta SL, Mueser KT, et al. Determinants of risk behavior for human immunodeficiency virus/acquired immunodeficiency syndrome in people with severe mental illness. Compr Psychiatry. 2001 Jul-Aug;42(4):263–271. doi: 10.1053/comp.2001.24576. [DOI] [PubMed] [Google Scholar]
- 43.Wilson I, Lu M, Rogers W, et al. Results of a physician-focused intervention to improve antiretroviral medication adherence. 2nd International Conference on HIV Treatment Adherence; 2007; Jersey City, NJ. [Google Scholar]
- 44.Morin SF, Koester KA, Steward WT, et al. Missed opportunities: prevention with HIV-infected patients in clinical care settings. J Acquir Immune Defic Syndr. 2004;36(4):960–966. doi: 10.1097/00126334-200408010-00010. [DOI] [PubMed] [Google Scholar]
- 45.Metsch LR, Pereyra M, del Rio C, et al. Delivery of HIV prevention counseling by physicians at HIV medical care settings in 4 US cities. Am J Public Health. 2004 Jul;94(7):1186–1192. doi: 10.2105/ajph.94.7.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drainoni ML, Dekker D, Lee-Hood E, Boehmer U, Relf M. HIV medical care provider practices for reducing high-risk sexual behavior: results of a qualitative study. AIDS Patient Care STDS. 2009 May;23(5):347–356. doi: 10.1089/apc.2008.0063. [DOI] [PubMed] [Google Scholar]
- 47.Flickinger TE, Berry S, Korthuis PT, et al. Counseling to reduce high-risk sexual behavior in HIV care: a multi-center, direct observation study. AIDS Patient Care STDS. 2013 Jul;27(7):416–424. doi: 10.1089/apc.2012.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirl WF, Muriel A, Hwang V, et al. Screening for psychosocial distress: a national survey of oncologists. J Support Oncol. 2007 Nov-Dec;5(10):499–504. [PubMed] [Google Scholar]
- 49.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008 Oct;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]